Three-dimensional aspects of formulation excipients in drug discovery:a critical assessment on orphan excipients,matrix effects and drug interactions
2021-01-21VijyhskrVeervlliHnumnthSriknthCheruvuPrtimSrivstvLkshmiMohnVmsiMdgul
Vijyhskr Veervlli,Hnumnth Sriknth Cheruvu,Prtim Srivstv,Lkshmi Mohn Vmsi Mdgul,*
aSyngene International Limited,Biocon Park,SEZ,Bommasandra Industrial Area-Phase-IV Bommasandra-Jigani Link Road,Bangalore,560099,India
bGVK BIO Pvt Ltd,Nacharam,Hyderabad,Telangana,500076,India
ABSTRACT
ARTICLEINFO
Article history:
Keywords:
Formulation excipients
Preclinical
Drug discovery
Matrix effects
Drug interactions
Bioanalysis
Pharmacokinetics
Formulation development
1.Introduction
The word excipient is derived from the Latin word excipere,meaning ‘to except',which could be simply explained as ‘other than'[1,2].Formulation/pharmaceutical excipients are basically everything other than the active ingredient.“Formulation excipients”terminology is often used in preclinical research space,as an alternate to “pharmaceutical excipients”termed in clinical arena.They are nothing but a refined list of pharmaceutical excipients used in preclinical in vivo studies.Pharmaceutical excipients are used to prepare a wide variety of dosage forms including tablets,capsules,oral liquids,ointments,creams,gels,transdermal patches,injectable products,implants,suppositories and inhalers[3-9].On the other hand,formulation excipients are used to prepare either suspensions or solutions for the ease of administration of drug candidates to the preclinical species(i.e.,mice,rat,rabbits,dogs,pigs and monkeys)in order to evaluate their efficacy,safety,toxicity and pharmacokinetic disposition.For preparation of clinical dosage forms,various pharmaceutical excipients such as diluents,disintegrants,glidants,lubricants,co-solvents,binders,granulating agents,compression aids,plasticizers,preservatives,complexing agents,lipids,polymers,surfactants,emulsifying agents,sweeteners,preservatives,thickeners,viscosity modifiers and formulation-dependent pH buffers are used.However,in the case of preclinical formulations,various excipients such as surfactants,emulsifying agents,co-solvents,complexing agents,lipids,polymers and formulation-dependent pH buffers are routinely used.Apart from these routine formulation excipients,there are many pharmaceutical excipients which are not extensively used in developing preclinical formulations despite their prerogative in the clinical setting.Taking the above facts into consideration,wecoined the term “orphan excipients”for this category of pharmaceutical excipients with the objective of bringing them to limelight and providing broader applications in drug discovery.Our review will focus on the key attributes of these orphan excipients,which may help in strategizing the current approaches being followed in preparing preclinical formulations.
In pharmacokinetic studies,following the administration of drug candidates to preclinical species,concentration of the analyte of interest is measured in the biological matrices(blood,plasma or tissues)using liquid chromatography/tandem mass spectrometry(LC-MS/MS).Introduction of highly sensitive and specific LC-MS/MS instruments has revolutionized the bioanalytical methodologies by offering high-throughput sample analysis[10-12].Even though there exist different ionization techniques in LC-MS/MS,electrospray ionization(ESI)and atmospheric pressure chemical ionization(APCI)are the most preferred ones because of the obvious advantages of robustness,speed,specificity and sensitivity[13].However,ion suppression/enhancement effects that are frequently encountered in the analysis of biological samples outweigh these advantages.Despite being a detection technique superior to HPLC,the issues encountered with ion suppression/enhancement effects raise questions on the authenticity of data generated using LC-MS/MS.Ion suppression/enhancement effects observed while using LC-MS/MS for bioanalysis can be broadly termed as“matrix effects”.The “matrix”refers to all components in the sample other than analyte(s)of interest[14].Matrix effects are defined as the“interference from matrix components that are unrelated to the analyte”and result in significant deviation in bioanalytical data which in turn questions the reliability of corresponding pharmacokinetic parameters of drug candidate.Matrix effect alters the sensitivity and reproducibility and challenges the reliability of analytical techniques.Although matrix effects occur because of various exogenous and endogenous components,one major area of concern is formulation excipients(an exogenous component)used in the preparation of preclinical formulations.In this review,we will discuss the impacts of formulation excipients on the ionization of analytes,mechanisms by which formulation excipients cause matrix effects and possible alternatives to mitigate these effects.
Drug-drug interaction is defined as“a substance(perpetrator drug)which impacts the disposition of a second drug(victim)when administered together”.Interactions can be synergistic or antagonistic cause new effects that neither produces.Drug-drug interactions(DDIs)are not desired as they increase the risk of adverse events and inevitably result in increased/decreased clearance or absorption of the affected drugs[15].These changes can alter the safety and efficacy profile of a drug or its active metabolites in important ways.The science of DDIs involves the drug transporters and drug metabolizing enzymes that are ubiquitously present in major clearance organs(intestine,liver,brain and kidney).Drug-drug interactions can also be food-drug,formulationdrug and herb-drug type.As the subject of this review is focused on formulation excipients,we emphasize more on formulation-drug interactions.In general,excipient-drug interactions occur when excipients inhibit/induce enzymes that are actively involved in metabolism and inhibit/induce transporters involved in the uptake/efflux transport mechanism[16-23].In this review,we will comprehensively discuss the excipient mediated drug interactions shown by commonly used excipients,their impacts on drug disposition and finally care to be taken while using these excipients.Even though excipient-drug interactions can be numerous,we focus on the impacts of excipients on drug disposition modulated by drug metabolizing enzymes and drug transporter proteins.
Overall,to our knowledge,this will be a unique review that proposes the uncovered pharmaceutical excipients(defined here as orphan excipients)to be used in preclinical drug discovery;describes mechanism by which excipients cause matrix effects and offers possible remedies;and presents excipient-drug interactions caused by the commonly used excipients and precautionary measures to be followed while choosing these excipients.
2.Scope
We conducted a review of published formulation/pharmaceutical excipients,matrix effects caused by these excipients and excipient mediated drug interactions.Since there is ambiguity on the pharmaceutical excipients(privileged in a clinical setting)that can be used in preclinical formulations,this review primarily focuses on these excipients which we believe will help drug discovery scientists to understand the holistic picture of their applications in the preparation of these formulations.Additionally,knowledge on matrix effects and possible excipient-mediated drug interactions will also help with key decision making while choosing formulation excipients.The literature review was conducted using PubMed®search(NCBI 2016),SCIFINDER®and Google Scholar databases with specific key words such as preclinical,formulation excipients,excipient-drug interactions,cytochrome P(CYP)450 interactions,pharmaceutical excipients,and matrix effects to collect the related full-length articles and abstracts.The literature search covers the period until January 2019.
3.Preclinical safety and tolerability of orphan excipients
Formulation selection in preclinical drug discovery comes with a broad range of options.Based on the route of administration,test articles must be either in solution or suspension form.Solution formulations are more desired for parenteral route(esp.intravenous)of administration,whereas suspension formulations are handy for parenteral(except intravenous)/enteral routes.The objective of formulation vehicle selection is to provide the desired availability/bioavailability of test article and should be as simple as possible with low toxicity.Formulation vehicle selection in early pharmacokinetics should also consider its suitability for late stage developments and strengths used should be within generally recognized as safe(GRAS)limits.Various conventional formulation selection approaches include pH adjustment,low concentrations of polymers/suspending agents(methyl cellulose,carboxymethyl cellulose,hydroxypropyl methyl cellulose),low concentrations of solubilizing agents(cyclodextrins,polysorbate80,cremophor EL,solutol HS15),cosolvents(Dimethyl sulfoxide,dimethylacetamide,propylene glycol,ethanol,N-methyl-2-pyrrolidone,propylene glycol,PEG400,and PEG300),lipid based excipients(medium chain glycerides),nanosuspensions and solid dispersions(hydroxypropyl methylcellulose acetate succinate,PEG6000,polyvinylpyrrolidone)[24].Irrespective of various combinations available,the simplest formulations comprise primarily aqueous solutions and suspensions.Solution and suspension formulations can be prepared using pH buffers,suspending agents,cosolvents and solubilizing agents.
For compounds insoluble in simple solution and suspensionbased formulations,alternate strategies such as lipid,solid dispersion and nanosuspension based approaches might be considered.Lipid-based vehicles are primarily helpful in solubilizing highly lipophilic compounds[25-28].Even though it is challenging to use these excipients when it comes to late stage development,these formulation approaches will help in making early insights to decide the fate of discovery projects.Solid dispersion and nano suspension approaches are effective in solubilizing highly lipophilic compounds;however,they are much difficult and labor intensive to be adopted in early drug discovery stages.Advantages of both of these strategies are extensively reported in the literature[29-33].
Pharmaceutical applications of commonly used pharmaceutical excipients are reported in the literature[34].Few of these excipients are very rarely used as formulation excipients in drug discovery,termed here as “orphan excipients”.If used in preclinical formulations,these orphan excipients can enhance druggability of new compounds[35].Hence,we presume this review article will extend the vision of preclinical researchers to make the best use of these orphan excipients.Listed orphan excipients include cyclodextrins(captisol),cosolvents(glycerin),non-ionic surfactants(dα-tocopherol,alpha-tocopherolpolyethylene glycol 1000 succinate(Vit E-TPGS),sorbitan monoesters,poloxamers,Softigen 767,polyoxylglycerides,Lauroglycol and Plurol),and water insoluble lipids(labrafac/labrafac lipophile).A brief overview of these orphan excipients is given below:
·Captisol is a polyanionic beta-cyclodextrin derivative with a sodium sulfonate salt separated from the lipophilic cavity by a butyl ether spacer group,or sulfobutylether.It helps in solubilizing neutral,cationic and anionic compounds.Captisol exhibits limited plasma protein binding,distributes to extracellular fluid and does not produce any pharmacological effects on the cardiovascular system;autonomic or somatic functions;respiratory capacity;orfluid or electrolyte excretion following intravenous administration.It is relatively safer than other cyclodextrins[36]and can be used at a concentration up to 40%(m/V)for both enteral and parenteral routes inpreclinical as well as clinical species[34,37].
·Glycerin is present naturally in animal and vegetable fats and oils that are consumed as part of a normal diet.It is mainly obtained asa by-productfrom oilsand fatsused in manufacturing of soaps and fatty acids.It may also be obtained from natural sources by fermentation.Synthetic glycerin is prepared by the chlorination and saponification of propylene[38].Glycerin is used in a wide variety of pharmaceutical formulations including oral,ophthalmic,parenteral,and topical preparationsasa solvent/cosolvent,humectant/emollient,sweetening agent,plasticizer,antimicrobial preservative and viscosity enhancer[39].Higher LD50values(4.1 g/kg in mouse,12.6 g/kg in rats)in rodents make it a suitable vehicle for usage in preclinical formulations[40].
·Alpha-tocopherol is the orally bioavailable alpha form of the naturally-occurring fat-soluble vitamin E,with potent antioxidant and cytoprotective activities.It is a highly lipophilic compound,and is an excellent solvent for many poorly soluble drugs[34,41,42].It is used as a non-ionic surfactant in oral and injectable formulations and as a plasticizer.The reported LD50value for tocopherol is 7.5 g/kg in rats[43].In general,tocopherols are well tolerated;however,excessive oral intake of tocopherol may cause headache,fatigue,weakness,digestive disturbance,and nausea[38].
·Alpha-tocopherol polyethylene glycol 1000 succinate(Vit ETPGS):TPGS is the esterified product of vitamin E succinate with polyethylene glycol(PEG).1000 denotes the molecular weight of PEG,and the final product is referred to as TPGS1000,or simply TPGS.Vit E-TPGS is a nontoxic,non-ionic surfactant that is used in many drug delivery systems[38].TPGS is used as a P-glycoprotein(P-gp)inhibitor,solubilizer,and absorption/permeation enhancer.Vitamin E TPGS has been hypothesized to increase the bioavailability of certain drugs byenhancing the solubility of the active pharmaceutical ingredient(API)and by acting as a weak P-gp inhibitor[35].LD50value of Vitamin E-TPGS is reported to be>7 g/kg in rats[35].
·Sorbitan monoesters are a series of mixtures of partial esters of sorbitol and its mono-and dianhydrides with fatty acids.Sorbitan monooleate is a pharmaceutical excipient that has been used in cyclosporine formulations,Gengraf and Sandimmune[34].Sorbitan esters are widely used in cosmetics,food products,and pharmaceutical formulations as lipophilic nonionic surfactants.They are mainly used as emulsifying agents in the preparation of emulsions,creams and ointments.Sorbitan esters produce stable water-in-oil emulsions and microemulsions.However,when used in combination with varying proportions of a polysorbates,they produce water-in-oil or oil-in-water emulsions,and their applications include self-emulsifying drug delivery systems for poorly soluble compounds[38].Sorbitan esters are generally considered as nontoxic,nonirritant excipients.Various sorbitan monoesters used as excipients include sorbitan di-isostearate,sorbitan dioleate,sorbitan monoisostearate,sorbitan monolaurate,sorbitan monooleate,sorbitan monopalmitate,sorbitan monostearate,sorbitan sesquiisostearate,sorbitan sesquioleate,sorbitan sesquistearate,sorbitan tri-isostearate,sorbitan trioleate and sorbitan tristearate.LD50values of sorbitan monolaurate and sorbitan monooleate in rats are reportedly>33.6 g/kg and>31 g/kg,respectively[38].
·Poloxamers are nonionic polyoxyethylene-polyoxypropylene copolymers used primarily in pharmaceutical formulations as emulsifying or solubilizing agents[44-51].Poloxamer polymers are prepared by reacting propylene oxide with propylene glycol to form polyoxypropylene glycol.Ethylene oxide is then added to form the block copolymer.The polyoxyethylene segment is hydrophilic while the polyoxypropylene segment is hydrophobic.Poloxamers are used as emulsifying,solubilizing,wetting and stabilizing agents.Poloxamers are used in a variety of oral,parenteral,and topical pharmaceutical formulations,and are generally regarded as nontoxic and nonirritant excipients.Various poloxamers used as excipients include Poloxamer 124,Poloxamer 188,Poloxamer 237,Poloxamer 338,and Poloxamer 407.LD50value of Poloxamer 188 is>15 g/kg in mice and>9.4 g/kg in rats[38].
·Softigen 767(PEG-6 caprylic/capric glycerides)is the ethoxylation product of medium chain partial glycerides whose fatty acids are derived from coconut and palm kernel oil.Softigen acts as surfactant,viscosity reducing,solubilizer,wetting and refatting agent.Softigen 767 was dosed at 120 mg/kg in male Sprague-Dawley(SD)rats[52].However,information on LD50values is not available.
·Polyoxylglycerides are mixtures of monoesters,diesters,and triesters of glycerol,and monoesters and diesters of PEG.Polyoxylglycerides act as dissolution enhancer,nonionic surfactants,and emulsifying/penetration/solubilizing/sustained-release agents[34].They are nontoxic and nonirritant materials[38].Various polyoxylglycerides include caprylocaproyl polyoxylglycerides(labrasol),lauroyl polyoxylglycerides(gelucire 44/14),linoleoyl polyoxylglycerides(labrafil M2125CS),oleoyl polyoxylglycerides(labrafil M1944CS)and stearoyl polyoxylglycerides(gelucire 50/13).LD50values of gelucire 44/14,labrasol,and labrafil M1944CS in rats are 20 g/kg,22 g/kg and>20 mL/kg,respectively[38,43].
·Lauroglycol is a nonionic water-insoluble surfactant used a cosurfactant in oral lipid-based formulations.It consists of propylene glycol mono-and di-esters of lauric(C12)acid,mainly composed of monoesters and a small fraction of diesters.Lauroglycol acts as a solubilizer of highly lipophilic compounds and enhances bioavailability.LD50value of lauroglycol in rats is 2.003 g/kg/day[53].
·Plurol(Polyglyceryl-3-diisostearate)is a nonionic waterinsoluble surfactant used as a co-surfactant in oral lipid-based formulations.It consists of polyglyceryl-3 esters of stearic(C18)acid,with the diester fraction being predominant.It acts as a solubilizer for highly lipophilic compounds and enhances bioavailability.The LD50values of polyglyceryl-3-diisostearate in mice and rats are>2 g/kg,and>5 g/kg,respectively[54].
·Labrafac/Labrafac lipophile(medium chain triglycerides)consist of a mixture of triglycerides of saturated fatty acids,mainly of caprylic acid and of capric acid.It acts as emulsifying agent,solvent,suspending and therapeutic agent.Mediumchain triglycerides have been used in a variety of pharmaceutical formulations including oral,parenteral,and topical preparations which are essentially nontoxic and nonirritant materials[38].Reported LD50value in rats after oral administration is>10 mL/kg[43].
In summary,these orphan excipients have been extensively used as pharmaceutical excipients in clinical drug formulations.However,they have not been rigorously considered in preclinical formulations.With higher LD50values of most of these excipients and also their diverse applications,they broaden the scope of formulatability of highly lipophilic compounds in a preclinical setting.A summary of the list of orphan excipients and their LD50values is presented in Table 1.
4.Excipients causing bioanalytical matrix effects
Matrix components that exist in extracts/supernatants after sample preparation alter the ionization of compounds in mass spectrometry.This could be due to ion suppression or ion enhancement.However,more typically ion suppression is encountered than ion enhancement.The process of ion suppression/enhancement is in general referred to as matrix effect and is the main subject of various published reviews[55-60].Matrix effects show major impact on the bioanalytical results when extracted components are differentially present between calibration and study samples.They occur from endogenous components,which include phospholipids;exogenous components such as mobile phase additives,co-administered drugs,metabolites,internal standards,formulation vehicles and plastic tubes[58-67].Asthe current context is on formulation excipients,we discuss briefly the nature/mechanism/impact of formulation excipient-mediated matrix effects and possible strategies to counter these effects.
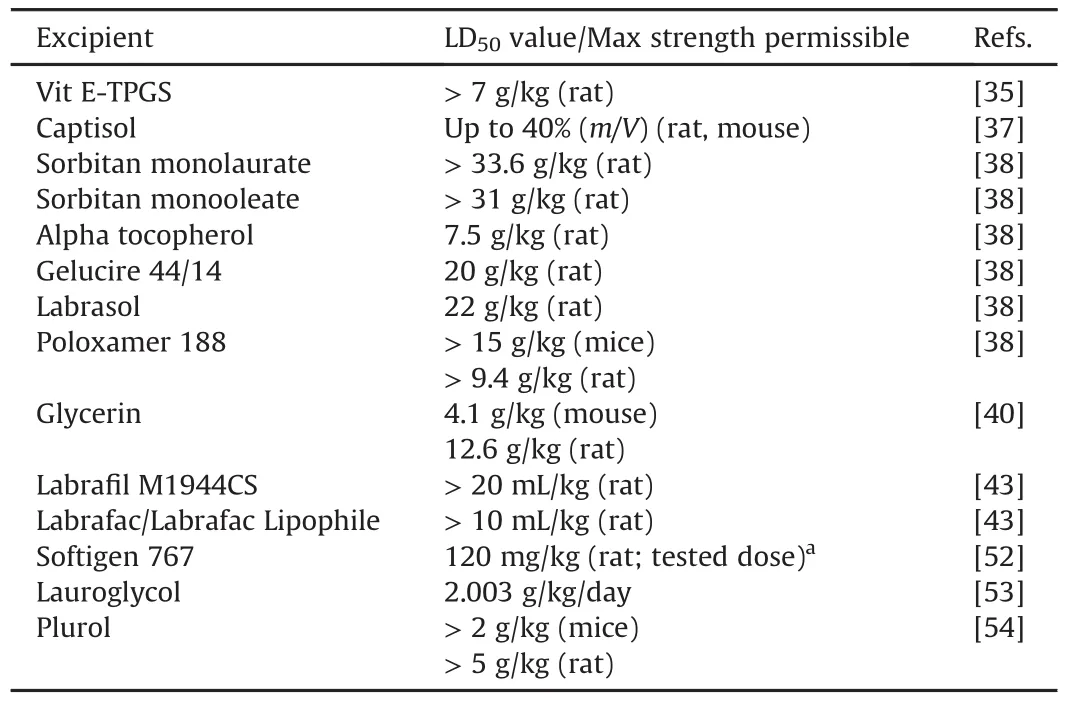
Table 1Summary of a list of orphan excipients and their LD50values.
Dosing vehicles are generally used at high concentrations to solubilize highly lipophilic test articles[63,64,68].This in turn can be instrumental in causing matrix effects,thereby weakening the reliability of preclinical pharmacokinetic parameters.This phenomenon has been reported by us for various excipients such as PEG400,cremophor EL and solutol HS15[58-60,69-72].Formulation vehicles could cause 80%-90% ion suppression when administered in both oral and intravenous administration routes[63,64,73,74].Xu et al.[73]did not find any matrix effects following either intravenous or oral dosing of drug candidates formulated using 20% hydroxypropyl-β-cyclodextrin or 0.4% methyl cellulose;however,50%-80% ion suppression for early eluting compounds was observed in the presence of 0.1% Tween 80.PEG400 resulted in 30%-50% ion suppression for early eluting compounds in oral formulations[75].In other instance,polymeric vehicles such as Tween 80 and PEG400 were reported to cause significant ion suppression>50%,when sample clean-up was minimal and analytes co-eluted with the vehicles[64].In a separate study,PEG was reportedly present in large quantities in the blood collection tubes used for pharmacokinetic studies[76].The effects of dosing vehicle excipients such as PEG400,propylene glycol,Tween 80,and hydroxypropyl-β-cyclodextrin on the accuracy of LC-MS/MS measurements used in pharmacokinetic studies were reported in the literature[63].
Significant ion suppression in general is noticed a with early sampling points in intravenous/oral route of administration.This is due to a higher concentration of excipients in the early sampling points.As excipients are eliminated from the body in due course of time,impact of ion suppression gradually reduces[63,64,76].Also,matrix effects are more pronounced when samples are analyzed using ultrafast gradients and shorter elution times.These analytical conditions typically cause co-elution of analytes with excipients and imparts ion suppression.Interference from coeluting excipients is more difficult to address as polymeric components elute across a wide retention window,hovering in the intermediate retention time range[77].When both analyte and excipient coelute,ion suppression can occur;however,the exact mechanism by which matrix components causes matrix effects is not known.Matrix effects occur at the interface between the MS system and LC system[78].These mechanisms are discussed in detail in the subsequent sections.The U.S Food and Drug Administration(US FDA)guidance for industry on bioanalytical method validation insists upon the assessment of matrix effects during method validation for quantitative LC-MS/MS methods[79].Schematic representation of the matrix effects and their impact on physiological concentrations of drug candidates is shown in Fig.1.
When it comes to ionization source,it is generally understood that ESI is more prone to ion suppression effects than atmospheric pressure chemical ionization(APCI)[80].In ESI,ion formation happens through a multistep process which involves both liquid and gas phases.Ionization occurs in liquid phase as LC effluent passes through a needle maintained at high voltage[81].High voltages along with nebulizer gas assist the formation of charged droplets that undergo coulombic explosion and solvent evaporation[82,83].It is in the process of ionization and evaporation,matrix components and analyte of interest compete with each other for charge and result in ion suppression[84].Response saturation at higher concentrations of the analyte is also a result of competition for charge mechanism[85,86].Furthermore,high concentrations of matrix components can limit access of the analyte to the surface of the droplet.In addition,species with hydrophobic moieties such as lipids as well as dosing additives such as Tween 80 and PEG 400 have high surface activity and can thereby limit the number of analyte ions reaching the surface of the droplet,further suppressing the ionization efficiency of the analyte[73].These matrix components can also affect the viscosity and surface tension of the droplet,resulting in less efficient spray formation and subsequent solvent evaporation,leading to a decreased number of ions reaching the gas phase.Ion suppression is highly experienced with very polar compounds.Polar compounds tend to accumulate in the aqueous phase of droplet instead of surface,which eventually results in lower surface activity and higher ion suppression[87].When mobile phase consists of ion pairing agents,it results in the formation of neutral complexes with the charged analytes and causes ion suppression[88].
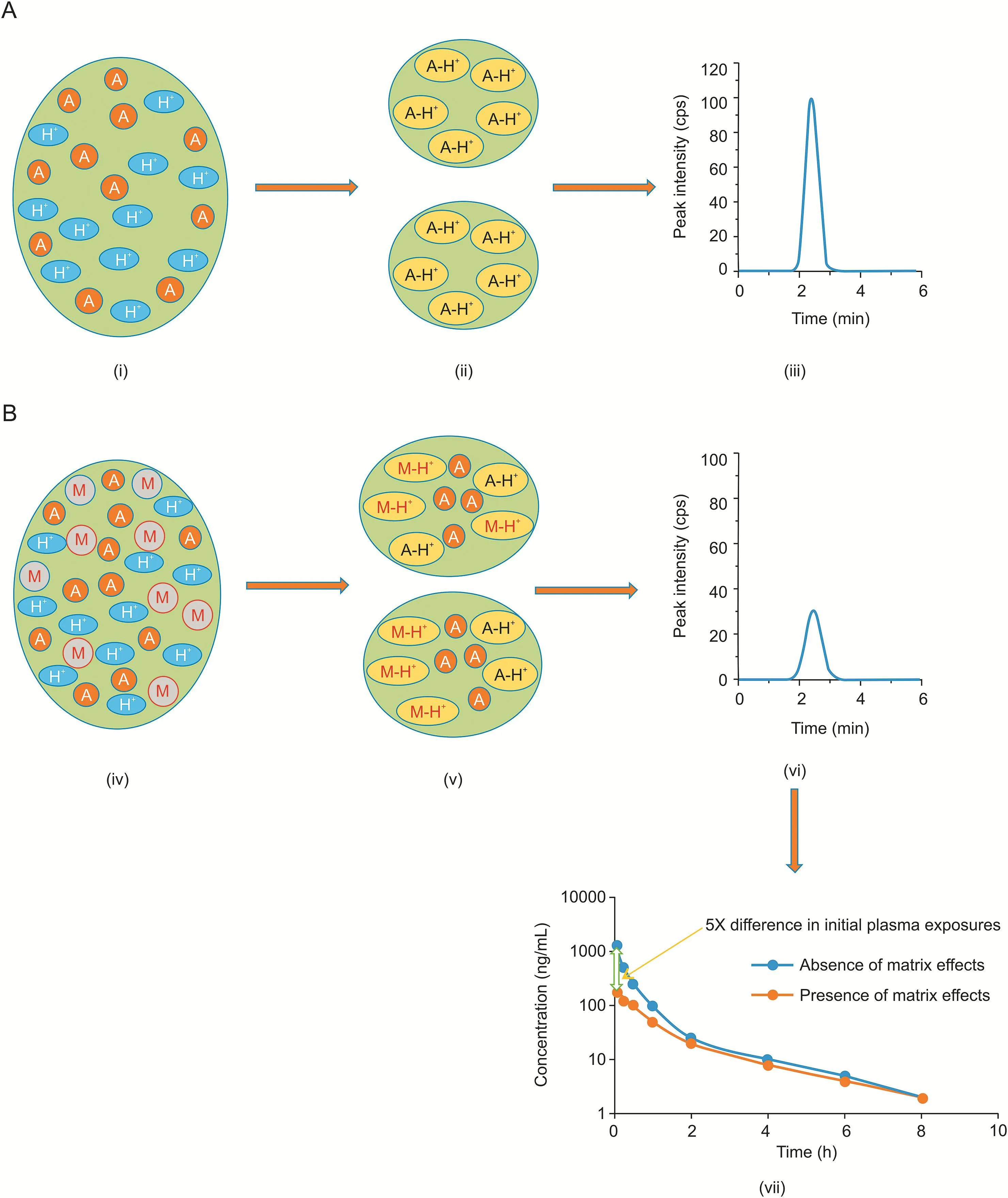
Fig.1.Schematic representation of matrix effects and their impact on physiological concentrations of drug candidates.(A)In the absence of interfering components,conventional droplet is formed with only analyte ions acquiring charge(i),which further breaks down to mist of droplets(ii)and results in optimal peak response(iii);(B)in the presence of interfering components,droplet formation is altered with only few analyte ions acquiring charge due to competition(iv,v),finally resulting in compromised peak response(vi)and eventually impacting the exposures(vii).“A”represents analyte; “M”represents matrix components;"A-H+"represents ionized analyte;"M-H+"represents ionized matrix components.
Once in the gas phase,the analyte ions are still susceptible to the influence of matrix components that also exist in the gas phase.Neutral matrix species may then compete for protons from the charged analyte based on their relative gas phase basicity through proton transfer reactions.Those components with higher gas phase basicity will remove a proton from the analyte,neutralizing its charge and causing decreased signal intensity[89].APCI ionization consists of formation of ions from neutral species in gaseous phase,through reagent ions(which is termed as chemical ionization)generated from corona discharge needle.Hence,matrix effects that exist in liquid phase do not occur in APCI.Overall based on the principle of ionization,matrix effects are more predominant in ESI source than in APCI source.
Several mechanisms have been proposed to explain matrix effects,but the exact process remains uncertain[78,90].Various mechanisms by which matrix components cause ion suppression are as follows:
·Charge competition between analyte and matrix components[91,92].
·Change in droplet surface tension leading to formation of large droplets and insufficient desolvation[78,87].
·Preferential ion evaporation due to matrix components gathering at droplet surface[14].
·Change in mass of analyte ion due to ion pairing and adduct formation[14].
·Co-precipitation with non-volatile matrix components[93].
·Gas phase deprotonation[14].
Reduction of matrix effects can be achieved by various strategies which include decreasing the level of matrix components,improving chromatographic separation of interfering materials from the analyte,various sample preparation strategies,lowering injection volumes,and even simple dilution of samples to reduce the overall concentrations of both analyte and co-extracted materials[65,74].Switching ionization sources will also help in mitigating the matrix effects.Matrix effects occurring in the early time point samples can be monitored,using another aliquot of the early time point samples analyzed at a higher dilution[74,94].If the two measurements with different dilution factors agree with each other,it indicates matrix effect is insignificant.If the two measurements for the early time points differ by more than a certain threshold(e.g.,30%),one may need to improve the method and reanalyze samples[74].Additionally,it is worthwhile to use a combination of ultrafast liquid chromatography and microbore columns.This analytical feature helps in reducing matrix effects by increasing the resolution of analytes of interest from the interfering components[92].Overall,these are various strategies that can be employed to mitigate the impacts of matrix effects caused by excipients and a careful choice should be made based on the nature of matrix effects.
5.Excipients driven drug interactions
Ideal excipient should not interfere with the pharmacological activity of test compound of interest.However,most of the excipients used in drug discovery produce some sort of interactions.These interactions could be due to modulation of drug metabolizing enzymes/drug transporters.For example,clinically achievable concentrations of PEG-300 caused almost complete inhibition of P-gp activity in both Caco-2 and MDR1-MDCK cell monolayers[95].P-gp inhibition caused by polyethoxylated pharmaceutical excipients was also reported in the literature[96-98].
Similarly,cremophor EL enhanced the bioavailability of a P-gp substrate doxorubicin which was considered desirable from an efficacy perspective and resulted in the increased antitumor activity.On the contrary,the enhanced doxorubicin exposure resulted in higher cardiotoxicity[99].Excipients such as PEG fatty acid esters,PEG stearates,poloxamers and polysorbates showed higher P-gp inhibition potential.Researchers should be aware of this bearing on overall exposures and cautiously monitor for safety/toxicity concerns[100].The interaction potential of excipients(Polysorbate 80,cremophor EL,and Solutol HS15 on the intrinsic clearance(CLint)of midazolam(MDZ)was investigated in rat microsomes and hepatocytes.The above excipients caused a decrease in the intrinsic clearance of CYP3A substrate MDZ with the increase in concentrations[101].This case study presented excipient-mediated inhibition of CYP3A isozyme and altered clearance.
Impact of nine excipients(lactose,sodium lauryl sulfate,Tween 80,HPMC,docusate sodium,EDTA,propylene glycol,PEG400 and anhydrous cherry flavor)on the Caco-2 permeability of seven low permeable compounds was studied by Bhagwant et al.[102].Sodium lauryl sulfate and Tween 80 increased apical to basolateral permeability of low permeable compounds.However,the rest of excipients did not show considerable impact on the overall permeability of these compounds.On the other hand,PEG-cholecalciferol,polyethylene glycol succinate and TPGS increased the uptake of P-gp substrates by inhibiting the P-gp efflux process[96].Increased permeability results in higher exposures and also triggers toxicity concerns.As discussed above,researchers should be careful in understanding the modulation of exposures caused by these excipients and develop effective correlations to safety/toxicity.
Anderberg et al.[103]studied the effects of synthetic,anionic and nonionic surfactants on the monolayer integrity of epithelial cells,permeability,intracellular enzyme activity and cell morphology.All surfactants exhibited concentration-dependent effects on intracellular enzyme activities,permeability,and morphology.The effects of the anionic surfactants were more pronounced than those of the nonionic surfactants.In a different study,selected excipients(imwitor 742,labrasol,cremophor EL,softigen 767,miglyol,solutol HS15,sucrose monolaurate,TPGS,polysorbate 20,and polysorbate 80)were screened for their abilities to enhance the absorption of digoxin and celiprolol in vitro[52].It was concluded that these excipients/surfactants can modify the pharmacokinetics of orally administered drugs that are P-gp substrates.
Apart from P-gp inhibition,which results in enhanced cell permeability,excipients also alter the permeability by increasing elasticity and reducing the membrane viscosity[104,105].This altered morphology in Caco-2 cell model enhances the absorption of compounds by both paracellular and transcellular routes.Cremophor RH40 inhibits both CYP3A and P-gp in a concentrationdependent manner which explains the increase in bioavailability of P-gp substrates in vivo[20].Similarly,Tween 20 and pluronic P85 increased the mitoxantrone uptake in breast cancer resistance protein(BCRP)expressing cells,but these effects disappeared on the absence of excipients[106].
With regard to effect of excipients on drug metabolizing enzymes,when male SD rats were fed 20% corn oil for 4 days following 2 days of fasting,the hepatic P450s 1A2,2B2,2E1,and 3A were regulated positively but the level of pulmonary P450 2B1 was suppressed.This enhanced/suppressed enzyme levels altered the drug disposition[107].Impacts of various excipients(dimethyl sulfoxide(DMSO),ethanol,propylene glycol,PEG,dimethyl acetamide,cyclodextrins,glycofurol,cremophor,solutol HS15)on altered drug disposition(metabolism,pharmacokinetics,renalelimination,absorption,distribution,hepatic blood flow)were extensively discussed in published reports[108].In a separate study,researchers evaluated the effects of 23 commonly used excipients on CYP450 isoforms using recombinant CYP enzymes.It was concluded that several excipients have the potential to modify the pharmacokinetics of administered drugs[109].
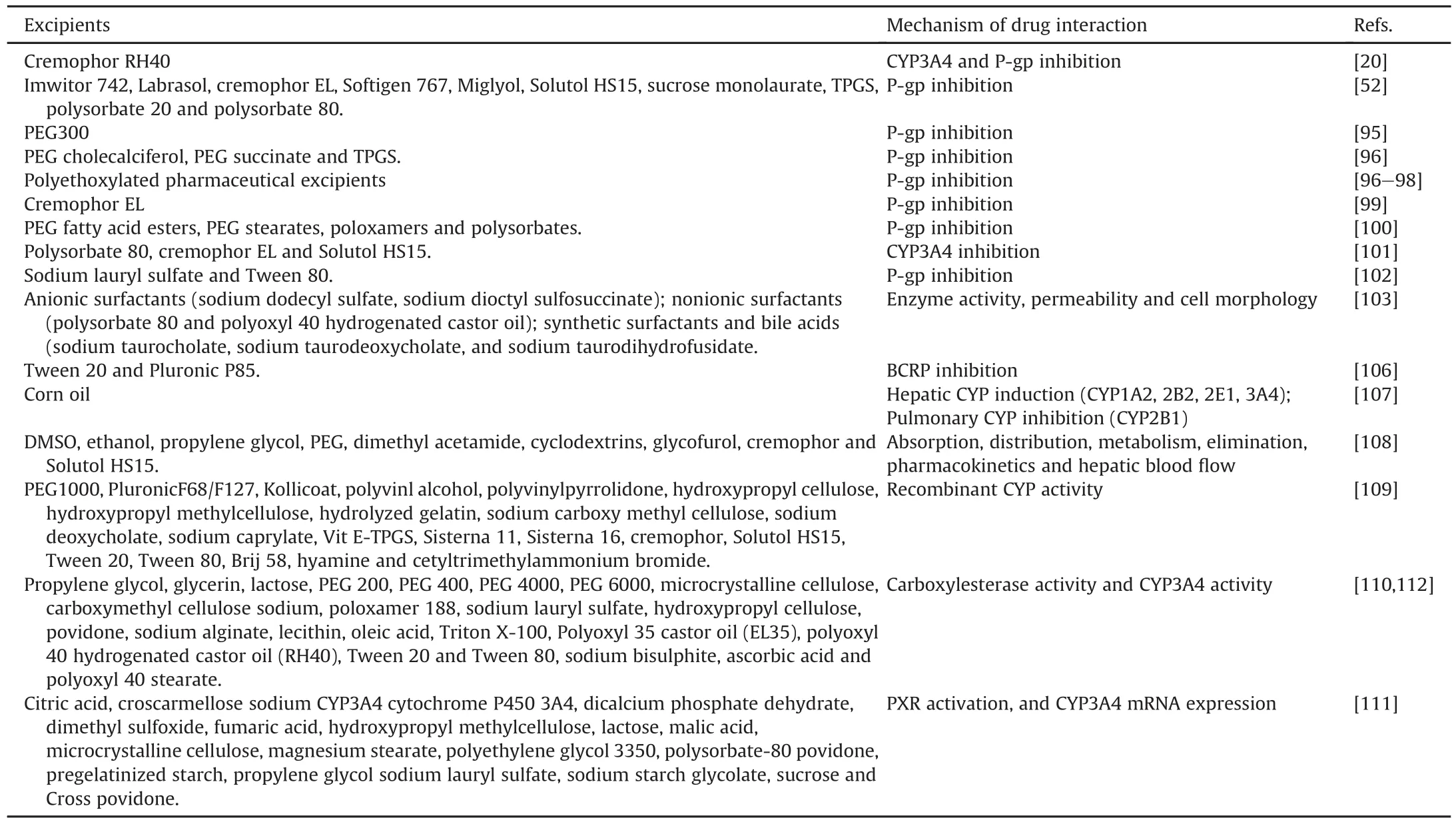
Table 2Summary of formulation excipients-mediated drug interactions.
Apart from CYP isoforms,impacts of 25 excipients on hydrolyzing enzymes(human carboxylesterases)were studied[110].Out of the excipients tested,surfactants significantly inhibited carboxylesterase(CES)activity.It was suggested that such inhibition should be taken into consideration during drug administration[110].In addition,the impacts of 19 excipients on human pregnane X receptor(PXR)activation,and CYP3A4 mRNA expression in immortalized human liver cells(HepG2 and Fa2N4),human primary hepatocytes,and the intestinal LS174T cell models were determined.Pregelatinized starch,polysorbate 80,and hydroxypropyl methylcellulose decreased mRNA and protein expression across these models[111].Additionally,the effects of 22 pharmaceutical excipients on CYP3A4 were studied using midazolam as a probe substrate.The results showed that 15 of 22(68.2%)tested excipients inhibited the activity of CYP3A4 by more than 50%,particularly the surfactants and polymers[112].A summary of excipients causing drug interactions is represented in Table 2.
Overall,most of the promising excipients have been reported to impact the drug disposition.As the usage of these excipients is inevitable,researchers should perform prior risk versus benefit analysis.However,while dealing with highly hydrophilic compounds,aqueous formulations could be a better choice[37].Alternatively,pH adjustment might be employed as one of the strategies to solubilize highly lipophilic compounds.Both these approaches can be adopted keeping in view of the high solubility requirements in late stages of drug discovery[37].Wherever possible,it is advisable to deploy more efforts in early stages of drug discovery to find out the right formulation vehicle.These early efforts in optimization can help to keep the formulation vehicle uniform throughout the course of project and support to generate unbiased results.Otherwise,when abrupt/intermittent changes are made in the formulation,it becomes difficult to correlate the results.
6.Conclusion
Pharmaceutical/formulation excipients are essential for formulating the new chemical entities/drug candidates under preclinical investigation.Firstly,even though there exist many excipient recipes in pharmaceutical formulations,not all are used in preclinical drug discovery.In this review,we have emphasized more on these orphan excipients.Higher LD50values of these excipients suggest their safety in preclinical species.Additionally,orphan excipients are capable of solubilizing highly lipophilic compounds.Secondly,we have discussed excipients-mediated bioanalytical matrix effects,the underlying mechanisms and overall impacts on the study outcomes.If not addressed,matrix effects will alter the bioanalytical results,which eventually leads to incorrect pharmacokinetic attributes of the compounds.Various strategies to mitigate matrix effects including sample preparation,sample dilution,chromatographic conditions and change of ionization source were proposed.Finally,formulation excipients-mediated drug interactions impacting the drug metabolizing enzymes/drug transporters were discussed using numerous case studies.As usage of excipients is inevitable,researchers should always be cognizant of these interactions and outweigh risk versus benefit.It is also advised not to make abrupt changes in formulation vehicle at different stages of drug discovery.Rather,more efforts should be levied at early stages of the program to optimize suitable formulation vehicles(keeping in view of highly lipophilic compounds).
Declaration of competing interest
The authors declare that there are no conflicts of interest.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Challenges for cysteamine stabilization,quantification,and biological effects improvement
- Offline two-dimensional liquid chromatography coupled with ion mobility-quadrupole time-of-flight mass spectrometry enabling fourdimensional separation and characterization of the multicomponents from white ginseng and red ginseng
- Single-run reversed-phase HPLC method for determining sertraline content,enantiomeric purity,and related substances in drug substance and finished product
- Development of a UHPLC-MS/MS method for the quantification of ilaprazole enantiomers in rat plasma and its pharmacokinetic application
- Use of subcutaneous tocilizumab to prepare intravenous solutions for COVID-19 emergency shortage:Comparative analytical study of physicochemical quality attributes
- Discovery of human coronaviruses pan-papain-like protease inhibitors using computational approaches
