Extracellular histones stimulate collagen expression in vitro and promote liver fibrogenesis in a mouse model via the TLR4-MyD88 signaling pathway
2021-01-15ZhiWangZhenXingChengSimonAbramsZiQiLinEDYatesQianYuWeiPingYuPingShengChenChengHockTohGuoZhengWang
Zhi Wang, Zhen-Xing Cheng, Simon T Abrams, Zi-Qi Lin, ED Yates, Qian Yu, Wei-Ping Yu, Ping-Sheng Chen,Cheng-Hock Toh, Guo Zheng Wang
Abstract
Key Words: Liver fibrosis; Extracellular histones; Non-anticoagulant heparin; TLR4; MyD88; CCl4
INTRODUCTION
Liver fibrosis progression to liver cirrhosis and hepatocellular carcinoma is common, in China due to high incidence of hepatitis B and in western countries due to alcohol consumption and hepatitis C[1-3]. In 2017, liver cirrhosis caused more than 1.3 million deaths globally, and constituted 2.4% of total deaths[4]. However, elucidating the underlying molecular mechanism and the development of specific therapies for fibrosis and cirrhosis have progressed slowly and significant discoveries in this area are urgently needed.
Liver fibrosis is a natural wound healing response to chronic liver injury, which has many common causes, including viral infection, alcohol abuse, autoimmune diseases, drug toxicity, schistosomiasis, and metabolic diseases[5]. This process can be easily reconstituted in animals by administering chemicals toxic to hepatic cells, including CCl4, thioacetamide, and dimethyl or diethyl nitrosamine[6-8]. Bile duct ligation (BDL) and animal models mimicking specific chronic liver diseases are also used[6]. CCl4is most commonly used to study the underlying molecular mechanisms and evaluate therapeutic reagents for inhibiting liver fibrosis in animals[6]. CCl4directly damages hepatocytes by ligation of CCl3to plasma, lysosomal and mitochondrial membranes, as well as to highly reactive free radical metabolites[9]. A single dose of CCl4causes centrizonal necrosis and steatosis, with recurrent administration leading to liver fibrosis[10].
The pathological feature of liver fibrosis is the extensive deposition of extracellular collagen produced by myofibroblasts[1]. In the healthy liver, no myofibroblasts are present but they accumulate in the damaged liver and play a pivotal role in fibrogenesis[11]. Myofibroblasts are derived from different types of cells. Hepatic stellate cells (HSCs) in the perisinusoidal Disse space are recognized as the most important source[12]. Bone marrow also contributes to both macrophages and to HSCs within the injured liver[13]. Recurrent injury, chronic inflammation and other chronic stimuli can cause HSCs to evolve into a myofibroblast-like phenotype and produce an extracellular matrix[14]. The myofibroblast-like phenotype also develops and expresses α-smooth muscle actin (α-SMA) in vitro when quiescent HSCs were isolated and cultured over a period of 5-10 d[15].
HSCs activated both in vivo and in vitro express TLR4, CD14 and MD2, therefore, lipopolysaccharide (LPS) is able to assemble a complex to activate TLR4 on HSCs[16]. TLR4 signals via MyD88-dependent and TRIF-dependent pathways that can activate fibroblasts, to promote fibrogenesis in numerous organs, including the liver, lungs, kidneys, heart and skin[17-20]. Since circulating LPS is produced primarily in Gramnegative bacterial infections[21], ligands that activate TLR4 in chronic liver injury without concomitant bacterial infection are yet to be identified. Increased intestinal permeability allows LPS to enter the circulation in the later stages of liver cirrhosis, but this leakage has not been demonstrated in the early stages of fibrogenesis[22].
Liver cell injury and death also release damage-associated molecular patterns (DAMPs) that may initiate the wound-repair process[23]. Extracellular histones, the most abundant DAMPs, have gained much attention in recent years, as they play key roles in many pathological processes and human diseases[24-35]. Histones are wellconserved proteins that are essential for DNA packaging and gene regulation[36]. During tissue damage and cell death, nuclear chromatin is cleaved into nucleosomes that are released extracellularly[37]and further degraded into individual histones[38]. Although histones are rapidly cleared by the liver[39]and rarely detected in blood[25,26,31,32], liver cell death is likely to release histones locally[40-43], which stimulate adjacent cells, including HSCs. Histones can activate TLR2, TLR4 and TLR9[24,40,43-46]in the early stage of chronic liver injury and fibrogenesis. Therefore, TLR4 activation is more likely to be maintained by extracellular histones rather than by LPS.
In this study, we investigated the roles and mechanisms of extracellular histones in liver fibrogenesis. Circulating histone levels were measured in a CCl4-induced mouse liver fibrosis model. Extracellular histones were used to stimulate human HSC cell lines to demonstrate their direct effect on collagen I production. In addition, we used non-anticoagulant heparin (NAHP) to neutralize circulating histones in both the cell culture system and mouse liver fibrosis model to explore the role of anti-histone therapy in reducing collagen I production and fibrosis. To clarify the potential molecular mechanisms, we used a TLR4-neutralizing antibody to block histoneenhanced collagen production by an HSC cell line and compared the fibrogenesis in TLR4 and MyD88 knockout mice with their wild-type (WT) parental mice in response to CCl4treatment.
MATERIALS AND METHODS
Cell culture
LX2 cells, a human HSC cell line, were purchased from Shanghai Meixuan Biological Sciences and Technology Ltd. and routinely cultured.
Histone treatment
After optimization of the doses and times, 0-20 μg/mL calf thymus histones (Merck, Armstadt, Germany) were added to cell culture medium to treat the LX2 cells. Medium with different concentrations of histones was changed every 48 h. On day 6, cell supernatant and lysates were collected for western blotting.
TLR4-neutralizing antibody and NAHP treatment
The neutralizing antibody, PAb-hTLR4, was purchased from Invitrogen (Carlsbad, CA, United States). LX2 cells were treated with 5 μg/mL histones with and without 5 μg/mL antibody. NAHP was synthesized and characterized by Dr. Yates at the University of Liverpool. NAHP (25 and 50 μg/mL) was used to treat LX2 cells together with 5 μg/mL histones. The cell culture medium was changed every 48 h. The cell lysates of the treated LX2 cells were collected on day 6.
Western blotting
One milliliter of cell supernatant was collected and proteins were precipitated using ice-cold acetone and suspended in 50 μL clear lysis buffer [1% (w/v) sodium dodecyl sulfate (SDS), 10% (v/v) glycerol, 120 mmol/L Tris–HCl, 25 mmol/L EDTA, pH 6.8]. Cells were washed with phosphate-buffered saline (PBS) three times and lysed using clear lysis buffer. Protein concentrations were determined using Bio-Rad protein assay kit II (Bio-Rad, Watford, United Kingdom). Both supernatant and cell lysates (20 μg protein) were subjected to SDS-PAGE and electrically transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Watford, United Kingdom). After blocking with 5% (w/v) dried milk in TBST buffer [50 mmol/L Tris-HCl, pH 7.6; 150 mmol/L NaCl, 0.1% (v/v) Tween-20], the membranes were incubated with the sheep antihuman collagen I α1 antibody (1:2000, R&D Systems, Abingdon, United Kingdom) overnight at 4 °C. After extensive washings with TBST, the membranes were incubated with rabbit anti-sheep IgG-horseradish peroxidase (HRP, 1:10000, Santa Cruz Biotechnology, Dallas, TX, United States) for 1 h at room temperature. The protein bands were visualized using enhanced chemiluminescence (ECL, Millipore). For βactin, primary antibody was purchased from Abcam (Abcam, Cambridge, United Kingdom) and anti-rabbit IgG-HRP was purchased from Santa Cruz Biotechnology. The band density was analyzed using Image J software and then the ratios of collagen I/β-actin were calculated.
Immunofluorescent staining
LX2 cells were seeded in eight-well chamber slides and treated with or without 5 μg/mL histones for 6 d. The cells were fixed with 4% (w/v) paraformaldehyde for 10 min and permeabilized with PBS + 1% (v/v) Triton X-100 for 10 min. After blocking with 5% (w/v) dried milk + 2% (w/v) bovine serum albumin in PBS, the cells were incubated with sheep anti-human collagen I α1 antibody (1:200) or rabbit-anti-α-SMA antibody (1:200, Proteintech, Rosemont, IL, United States) at 4 °C overnight. After extensive washing, the cells were incubated with anti-sheep-fluorescein isothiocyanate (FITC, Abcam, 1:2000) or anti-rabbit-FITC (Abcam, 1:2000) at room temperature for 45 min. Following extensive washing, the slides were mounted on coverslips using mounting medium and antifade (Thermo Fisher Scientific, Renfrew, United Kingdom). The fluorescent images were visualized using an LSM-10 confocal microscope (excitation: 488 nm and emission: 512 nm).
Animals
Mice were housed and used in sterile conditions at the Research Centre of Genetically Modified Mice, Southeast University, Nanjing, China. The mice were kept in a temperature and humidity controlled laminar flow room with an artificial 10-14 h light/dark cycle. All mice had free access to water. Sample sizes were calculated using power calculation based on our previous data and experience. All procedures were performed according to State laws and monitored by local inspectors, and approved by the Animal Research Ethics Committee at the Medical School of the Southeast University. Wang Z and Cheng ZX hold a full animal license for use of mice (Jiangsu Province, No. 2151981 and No. 2131272).
CCl4 model in ICR mice
Healthy male, 6-week-old ICR mice were purchased from Yangzhou University Animal Experiment Centre and divided randomly into three groups after blood sampling: (1) 5 μL/g body weight of 25% (w/v) CCl4in olive oil injected intraperitoneally (i.p.), twice per week; (2) CCl4+ NAHP: On the day of CCl4injection, 8 μL/g body weight NAHP (4 mg/mL in saline, filtered for sterility) were injected subcutaneously (s.c.) every 8 h. On the remaining days, 8 μL/g body weight NAHP was injected s.c. every 12 h. Both groups of mice were monitored every day and euthanized by neck dislocation at 4, 8 and 24 h (3 mice per group at each time point) after the first dose of CCl4each week to collect the blood and organs for further analysis (Supplementary Figure 1); and (3) Saline was injected i.p. and NAHP injected s.c. as a control; nine mice in total were euthanized by the end of 4 wk. No mice died before euthanization.
CCl4 model using genetically modified mice
C57BL/6JGpt (WT), TLR-4-KO (B6/JGpt-Tlr4em1Cd/Gpt) and MyD88-KO(B6/JGpt-Myd88em1Cd/Gpt) mice were purchased from Gempharmatech (Nanjing, China). Each type of mouse was divided into three groups as above after blood sampling. Eight mice in each group were treated with 5 μL/g body weight of 25% (w/v) CCl4in olive oil i.p., twice per week for 4 wk. All mice were euthanized by neck dislocation and blood and organs were collected for further analysis (Supplementary Figure 2). No mice died before euthanization.
Tissue processing and staining
In our pathology laboratory, organ tissues were routinely processed and 4-μm sections were prepared. Staining with hematoxylin and eosin (H&E) and Sirius red was routinely performed and α-SMA was detected by immunohistochemical staining using anti-α-SMA (Proteintech), as described previously[47]. Liver histological grading was as previously reported[48]and as performed by two investigators in a blinded manner. The staining areas of α-SMA and fibrosis were quantified using Image J software.
Measurement of blood histones and alanine aminotransferase
Venous blood was collected with citrate anticoagulant and immediately centrifuged at 1500 × g for 25 min at room temperature. Plasma was collected and alanine aminotransferase (ALT) levels were measured by an automatic blood biochemical analyzer in the clinical laboratory and histone levels were measured by western blotting using anti-histone H3 (Abcam), as described previously[32]. Plasma and histone H3 protein (New England Biolabs, Herts, United Kingdom) as standard were subjected to SDS-PAGE and electrically transferred onto PVDF membranes (Millipore). After blocking, the blot was probed by anti-histone H3 (Abcam, 1:2000) at 4 °C overnight. After extensive washings, HRP-conjugated secondary antibody (Santa Cruz Biotechnology, 1:10000) was incubated at room temperature for 1 h. After extensive washing in TBST, ECL (Millipore) was used to visualize the protein bands, which were quantified against histone H3 protein standard to obtain the concentration of histone H3 in plasma using Image J software. The total histones were estimated based on the molar ratio of H3 in the cell nuclei.
Measurement of hydroxyproline in liver tissues
Part of the liver from mice was washed with saline and frozen at -80 °C. Two hundred and fifty milligrams of frozen liver tissues were ground using a low temperature Tissuelyser (Shanghai Jingxin Industrial Development Co. Ltd.) and hydroxyproline was measured using a colorimetric kit from Beijing Solarbio Science & Technology Co. Ltd.
Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD) or standard error (SE). Differences in means between any two groups were compared using unpaired Student’s t test. Differences in means between more than two groups were compared using analysis of variance followed by Student-Newman-Keuls test. P < 0.05 was considered significant. Statistical analysis was performed using SPSS version 25.
RESULTS
High levels of circulating histones are detected in mice treated with CCl4
CCl4is a hepatotoxin that directly damages hepatocytes. CCl4administration in mice is the most commonly used animal model to generate liver fibrosis, but it is not clear whether high levels of histones actually exist under these conditions in the circulation. Circulating histone H3 was detected by western blotting and the total circulating histone levels were calculated based on these levels of H3, as described previously[32]. Histone H3 was detectable at 4 h after the first administration of CCl4alone or CCl4+ NAHP (Figure 1A and B) at week 1. At week 4, before or 24 h after the last dose of CCl4, H3 was also detectable (Figure 1C). Total circulating histones reached peak values (around 200 μg/mL) between 8 and 24 h after CCl4was administered and then gradually reduced to low levels (10-60 μg/mL) prior to the subsequent dose of CCl4(Figure 1D). The mean ± SD of total histones during each week from 8 and 24 h after CCl4injection is shown in Figure 1E. In contrast, histone H3 was barely detectable in the control group treated with saline + NAHP (data not shown). To monitor liver injury, blood ALT level was measured using a clinical biochemical analyzer. The mean ± SD from nine mice on day 28 was compared, and NAHP alone did not increase ALT levels, while CCl4caused a significant elevation in circulating ALT (approximately 10-fold), but this elevation was significantly reduced by NAHP (approximately 40% reduction) (Figure 1F). Liver injury scores of H&E-stained liver sections were also significantly reduced by NAHP treatment (Figure 1G). These observations indicated that NAHP treatment reduced liver injury.
Extracellular histones stimulate HSCs to increase production of collagen I and α-SMA
Extracellular histones are TLR ligands, including TLR2 and TLR4[40,45]; both of which signal via the downstream MyD88 pathway. During liver fibrogenesis, HSCs express TLR4 and its signaling enhances activation of fibroblasts[17]. We directly treated LX2 cells with calf thymus histones and found that histone concentrations between 2 and 10 μg/mL significantly increased collagen I production. Histones (5 μg/mL) incubated for 6 d were the optimal condition. This increased collagen I released into the culture media by nearly 2.5-fold and increased collagen I expression by 2.7-fold in cell lysates (Figure 2A and B). Concentrations of ≥ 20 μg/mL histones caused cell death (data not shown). Using immunofluorescent staining, we found that LX2 cells treated with 5 μg/mL histones were positively stained with anti-collagen I and anti-α-SMA compared to LX2 cells treated without histones (Figure 2C), supporting that LX2 cells were activated by histone treatment and induced collagen production.
NAHP reduces histone-enhanced collagen production in LX2 cells and liver fibrosis induced by CCl4
NAHP has been demonstrated to bind histones and block their cytotoxicity[26,32,49]. We added NAHP (25 and 50 μg/mL) to culture medium to neutralize 5 μg/mL calf thymus histones (Histones: NAHP approximately 1:2 and 1:4 molar ratio), and found that NAHP inhibited histone-enhanced collagen I synthesis in LX2 cells (Figure 3A and B). In H&E-stained liver sections from the CCl4-induced mouse liver fibrosis model, CCl4caused extensive hepatocytic damage compared to controls (Figure 3C), including hepatocyte swelling, vacuole formation, necrosis, Kupffer cell proliferation and immune cell infiltration (Figure 3C). NAHP intervention significantly reduced CCl4-induced hepatocyte vacuolization, necrosis and immune cell infiltration (Figure 3C). Immunohistochemical staining with anti-α-SMA showed a larger area of positive staining in CCl4-treated mice compared to controls (Figure 3C). In NAHPtreated mice, the CCl4-induced α-SMA staining was reduced (Figure 3C). Using Sirius red staining to visualize collagen, only the vascular walls were stained positively in controls (Figure 3C). In contrast, large areas of positive staining appeared in CCl4-treated mice (Figure 3C), which were reduced by NAHP intervention (Figure 3C). Using Image J software to quantify the areas of positive staining for α-SMA (Figure 3D) and collagen (Figure 3E), we demonstrated that NAHP significantly reduced both α-SMA and collagen production by 50%-70%. Using a colorimetric assay, hydroxyproline in liver tissues was detected and NAHP was shown to significantly reduce CCl4-induced hydroxyproline elevation by about 40% (Figure 3F).
TLR4 is involved in histone-enhanced collagen production in LX2 cells and in liver fibrosis induced by CCl4
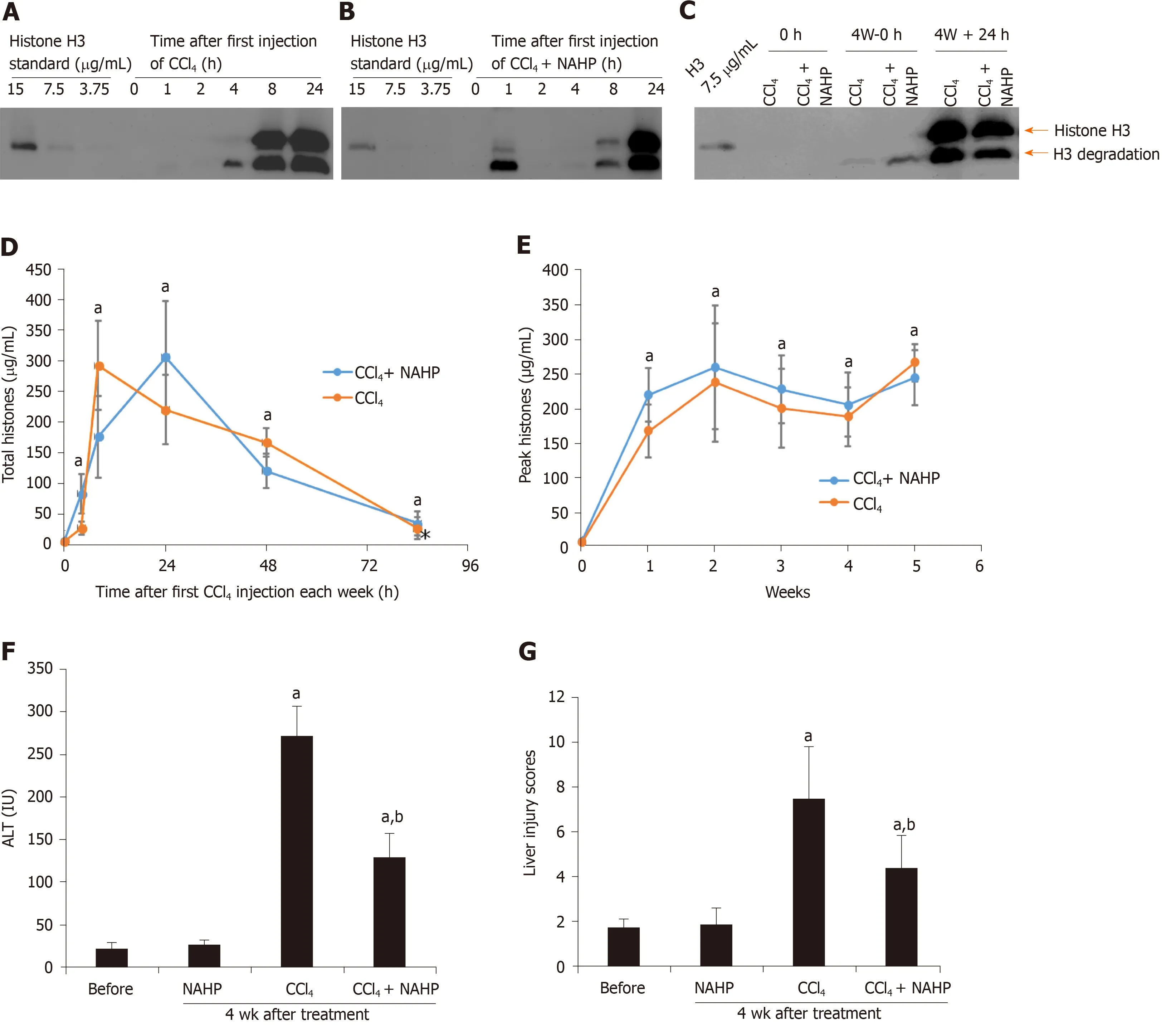
Figure 1 Circulating histones are elevated in ICR mice treated with CCl4. Typical western blots of histone H3 standard and histone H3 in plasma from mice treated with the first dose of CCl4 (A) and CCl4 + non-anticoagulant heparin (NAHP) (B), and a typical H3 blot before and 24 h after the last dose of CCl4 (C); D: The mean ± SD of circulating histones at different time points, including 0 h (before the experiment), and 4, 8, 24, 48 and 84 h after first dose of CCl4 (orange) and CCl4 + NAHP (blue) of each week are presented. ANOVA test, aP < 0.05 compared to time 0; E: The mean ± SD of peak circulating histones (8 and 24 h after first CCl4 injection of each week). ANOVA test, aP < 0.05 compared to time 0 (before first injection). The mean ± SE of blood alanine aminotransferase levels (F) and liver injury scores (G) from nine mice in each group following 4 wk of treatment. ANOVA test, aP < 0.05 compared to mice without treatment (before). bP < 0.05 compared to CCl4 group. ANOVA: Analysis of variance; NAHP: Non-anticoagulant heparin; ALT: Alanine aminotransferase.
To understand how histones are involved in liver fibrosis, we specifically tested if histone-activated TLR/MyD88 signaling played a major role in this process. This hypothesis was based on previous reports that TLR4 and MyD88 were involved in fibrogenesis[17,19]. We used human TLR4-neutralizing antibodies to treat LX2 cells and found that histone-enhanced collagen I production was significantly reduced (Figure 4A). To confirm that the TLR4-MyD88 signaling pathway was involved in CCl4-induced liver fibrosis, TLR4 and MyD88 gene knockout mice were used. The extent of fibrosis was significantly less in both TLR4-/-and MyD88-/-mice than in WT mice treated with CCl4(Figure 4B and C). These results are consistent with a previous study that demonstrated TLR4 and MyD88 deficiency reduced liver fibrosis in a mouse BDL model[50]. These findings strongly suggest that extracellular histones activate the TLR4-MyD88 signaling pathway to enhance collagen I production and liver fibrosis. However, further investigation is required to substantiate this point and the downstream pathways.
DISCUSSION

Figure 2 Extracellular histones induced collagen I and α-smooth muscle actin production in LX2 cells. A: Typical western blots of collagen I in medium (upper panel) and lysates (middle panel) of LX2 cells treated with different concentrations of histones at day 6. Lower panel: β-actin in the cell lysates; B: The mean ± SD of the relative percentages of collagen/β-actin ratios with untreated LX2 cells set at 100% from five independent experiments. Analysis of variance test, aP < 0.05 compared to untreated cells; C: Immunofluorescent staining of LX2 cells with anti-collagen I and anti-α- smooth muscle actin (SMA) antibodies. Control: Cells were treated with culture medium without histones for 6 d. Histones: Cells were treated with culture medium + 5 μg/mL histones. Typical images are shown. White arrows indicate staining for collagen I, yellow arrows indicate staining for α-SMA. Bar = 50 m. α-SMA: α-smooth muscle actin.
We proposed a novel concept that extracellular histones act as pathogenic factors in liver fibrosis. High levels of circulating histones in CCl4-treated mice were detected from 4 h after the first administration of CCl4and remained high during fibrosis induction. In vitro, extracellular histones directly enhanced collagen I production in LX2 (HSC) cells by up to 2.7-fold. When NAHP was used to neutralize histones, it significantly reduced histone-enhanced collagen I production in LX2 cells and significantly reduced fibrosis in CCl4-treated mice. When TLR4 neutralizing antibody was used, histone-enhanced collagen I production in LX2 cells was also reduced. When CCl4was injected into TLR4 or MyD88 knockout mice, they showed significantly less fibrosis compared to WT mice, suggesting that both TLR4 and MyD88 were involved in histone-enhanced fibrosis.
TLRs not only recognize pathogen-associated molecular patterns from various microbial infections, but also respond to DAMPs from host cell damage[51]. TLR4 recognizes LPS from Gram-negative bacteria as well as histones released following cell death. TLR4 can activate nuclear factor-κB via MyD88[40,44,52,53]. The TLR4-MyD88 pathway has been demonstrated to promote liver fibrosis in a mouse BDL model by enhancing transforming growth factor (TGF)-β signaling[50]. LPS as a TLR4 ligand was elevated 3-6-fold in this model likely due to obstruction of bile ducts and subsequent bacterial infection[50]. However, circulating LPS concentration did not increase in the CCl4mouse model[54]. Without infection, LPS from digestive tracts enters the circulation in the late stage of fibrosis or cirrhosis, while the wound-healing process is initiated immediately after injury. Therefore, LPS is unlikely to be an initiating factor in fibrosis. Extracellular histones are newly identified TLR4 ligands and are most likely to mediate the initial stage of fibrogenesis by activating the TLR4-MyD88 signaling pathway[40,44]. However, the downstream signaling pathways requires further experimental clarification. In addition, fibrogenesis not only involves collagen production but also many molecular and cellular mechanisms[55]. The co-operation of the TLR4-MyD88 pathway with other signaling pathways, such as TGF-β signaling and inflammatory response, also requires further investigation to determine their relative contributions to liver fibrosis.
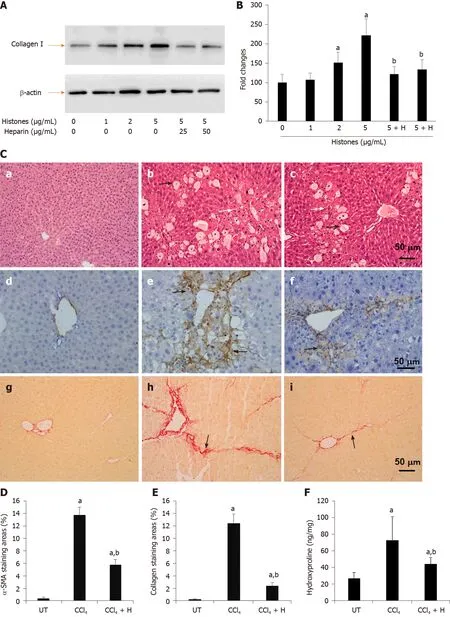
Figure 3 Effect of anti-histone reagent, non-anticoagulant heparin, on histone-enhanced collagen I production in LX2 cells and CCl4-induced fibrosis in mice. A: Typical western blots of collagen I in LX2 cells treated with histones or histones + non-anticoagulant heparin (NAHP). Beta-actin is shown as a loading reference; B: The mean ± SD of relative percentage of collagen/actin ratios in untreated LX-2 cells set at 100% from three independent experiments. ANOVA test, aP < 0.05 compared to untreated cells. bP < 0.05 compared to cells treated with 5 μg/mL histones; C: Typical images of stained liver sections (hematoxylin and eosin staining: a-c; immunohistochemical staining with anti-α-SMA: d-f; and Sirius red staining: g-i) from normal mice (a, d, g); mice treated with CCl4 (b, e, h) and mice treated with CCl4 + NAHP (CCl4 + H) (c, f, i). Arrows in b and c: Black indicate hepatocyte swelling and white indicate necrosis and immune cell infiltration. Arrows in e and f: Indicate staining for smooth muscle actin. Arrows in h and i: Indicate collagen deposition. The mean ± SD of percentage of areas of staining for α-SMA (D) and Sirius red staining for collagen (E) (nine mice per group, and six randomly selected sections per mouse). ANOVA test, aP < 0.05 compared to controls. bP < 0.05 compared to CCl4 alone; F: The mean ± SD of hydroxyproline levels in liver tissues from nine mice per group. ANOVA test, aP < 0.05 compared to controls. bP < 0.05 compared to CCl4 alone. α-SMA: α-smooth muscle actin; ANOVA: Analysis of variance; NAHP: Non-anticoagulant heparin.
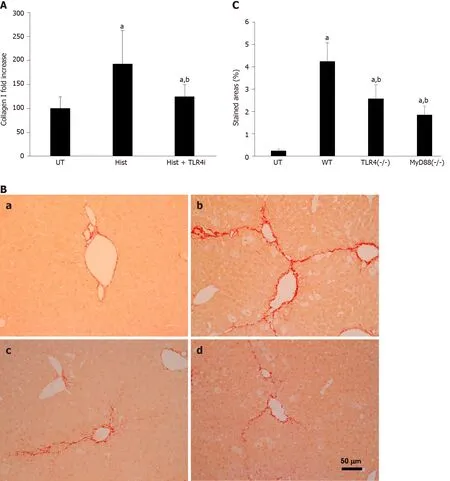
Figure 4 TLR4 is involved in histone-enhanced collagen I production and CCl4-induced mouse liver fibrosis. A: LX2 cells were treated with 5 μg/mL histones (Hist) in the presence or absence of TLR4 neutralizing antibody (TLR4i). The mean ± SD of the relative percentage of collagen Ι/β-actin ratios are presented with control (UT) set at 100% from three independent experiments. ANOVA test, aP < 0.05 compared to UT. bP < 0.05 compared to histone alone; B: Typical images of Sirius red staining of liver section from untreated wt C57BL/j mice (a), CCl4-treated wt mice (b), and TLR4 and MyD88 knockout mice; C: The mean ± SD of stained areas of liver sections from untreated wt mice (UT), CCl4-treated wt mice (WT), CCl4-treated TLR4-/- and MyD88-/- mice. Eight mice were in each group and six sections from each mouse were analyses. ANOVA test, aP < 0.05 compared to untreated wt mice (UT). bP < 0.05 compared to CCl4-treated wt mice. ANOVA: Analysis of variance; wt: Wild type; UT: Control.
The level of extracellular histones required for stimulating LX2 (HSC) cells is 2-10 μg/mL, which is lower than that detected in the circulation of CCl4-treated mice. All groups, including ICR mice treated with CCl4and CCl4+ NAHP, and C57BL/6, TLR4-/-and MyD88-/-mice treated with CCl4, had comparable levels of peak circulating histones (Supplementary Figure 3). This is due to the severe and extensive damage to hepatocytes caused by the radical CCl3, a metabolite of CCl4. The highest levels of histones detected after CCl4administration are comparable to those we have detected in critical illnesses[26,31,32]and are most likely able to cause further liver injury[56]. However, plasma can neutralize histones at a concentration of up to 50 μg/mL in vivo or ex vivo, making cells more resistant than those in culture medium[32]. Additionally, in our mouse model, the interval between two doses of CCl4was 84 h; therefore, more than half of this period had histone levels around 50 μg/mL, which may be sufficient in maintaining TLR4 activation. Elevation of circulating histones was also found in many types of liver disease and the high levels of circulating histones could cause secondary liver injury[26,40,57]. Some human chronic liver diseases, such as chronic hepatitis B and C, and alcoholic and autoimmune liver diseases may have low but constant levels of circulating histones to serve as alarmins, which signal tissue injury and initiate repair processes. However, this needs further clinical investigation.
NAHP can bind to histones, but does not lower the levels of circulating histones. In the first week, NAHP seems to increase the levels of circulating histones in the CCl4-treated mice and this result could be explained by its ability to reduce histone clearance, as we have observed previously[26]. NAHP binding to extracellular histones prevents their binding to the cell plasma membrane and other targets, such as prothrombin[32-34]and thereby detoxifies histones. In this study, we found that ALT and liver injury scores were significantly reduced by NAHP in CCl4-treated mice, strongly indicating that high levels of circulating histones are toxic to liver cells, which can be neutralized by NAHP. This protective effect may also contribute to the reduction of liver fibrosis.
Heparin was reported to reduce collagen fiber formation and liver fibrosis two decades ago[58-60]. Enzymatically depolymerized low-molecular-weight heparins were shown to inhibit CCl4-induced liver fibrosis by reducing tumor necrosis factor (TNF) α and interleukin (IL)-1β[61]. Although extracellular histones could enhance cytokine release, including IL-6, IL-1β, and TNFα[33,40], we suggest that the major mechanism for heparin-inhibited liver fibrosis is most likely due to its blocking the ability of extracellular histones to activate the TLR4-MyD88 signaling pathway. Heparin has been used as an anticoagulant for many decades and overdoses may cause bleeding, which can be avoided by using NAHP[26]. Additionally, NAHP alone appears to have no significant liver toxicity, but it significantly reduced histone-enhanced collagen I expression in LX2 cells and reduced CCl4-induced liver fibrosis. Therefore, this novel finding may help to advance translating this old discovery into clinical application.
CONCLUSION
This study has demonstrated that high levels of circulating histones exist in mice treated with CCl4during induction of liver fibrosis. Treatment of LX2 cells with extracellular histones enhanced production of collagen I and α-SMA. Neutralizing histones using NAHP significantly reduced the production of collagen I and the extent of liver fibrosis. These data demonstrate that extracellular histones released after liver injury promote liver fibrosis. Blocking TLR4 receptor on LX2 cells reduced histoneenhanced collagen I production and CCl4induced less fibrosis in TLR4 and MyD88 knockout mice than in WT parental mice, strongly suggesting that histone-enhanced collagen production is via the TLR4-MyD88 signaling pathway. However, there were a few limitations to this study. Firstly, in our in vivo study, no direct link was established between histones and their binding to TLR4. The relative contribution of the histone-TLR4-MyD88 axis and downstream signaling pathways is still not clear. Secondly, only one type of animal model for liver fibrosis was used and it may not truly represent the fibrosis caused by different diseases. However, the results are sufficient to demonstrate the novel concept that extracellular histones are involved in liver fibrosis. Thirdly, liver fibrosis is a complicated process involving many types of cells and signaling pathways. Besides collagen overproduction, many other biological processes are also involved in liver fibrosis. High levels of extracellular histones may also affect many other types of cells besides HSCs and collagen production. The detailed roles of extracellular histones in the complex processes of fibrogenesis require extensive investigation. Similarly, NAHP may have other effects besides blocking histones to activate TLR4 and to injury hepatocytes. Therefore, further in vivo mechanistic studies and development of better reagents to target histones and potential signaling pathways may offer a better understanding of fibrogenesis and more effective therapies in the future.
ARTICLE HIGHLIGHTS

ACKNOWLEDGEMENTS
Thanks to all the staff in the animal house for their support and to the technicians in the pathology laboratory for technical support and clinical biochemistry for ALT measurement in Zhongda Hospital, Nanjing, China. Thanks to Professor Rudland P in the University of Liverpool for his critical reading and correction of the manuscript. Thanks to Dr. Lane S in The Department of Statistics, University of Liverpool for his assistance with statistical analysis.
杂志排行
World Journal of Gastroenterology的其它文章
- Artificial intelligence-aided colonoscopy: Recent developments and future perspectives
- Invasive fungal infection before and after liver transplantation
- Molecular overview of progressive familial intrahepatic cholestasis
- Evaluation of an educational telephone intervention strategy to improve non-screening colonoscopy attendance: A randomized controlled trial
- Towards an evaluation of alcoholic liver cirrhosis and nonalcoholic fatty liver disease patients with hematological scales
- Prevalence and associated factors of obesity in inflammatory bowel disease: A case-control study
