Diagnosis and treatment of iron-deficiency anemia in gastrointestinal bleeding: A systematic review
2021-01-15JosCotterCilniaBaldaiaManuelaFerreiraGuilhermeMacedoIsabelPedroto
José Cotter, Cilénia Baldaia, Manuela Ferreira, Guilherme Macedo, Isabel Pedroto
Abstract
Key Words: Anemia iron-deficiency; Erythrocyte transfusion; Ferric carboxymaltose; Gastrointestinal hemorrhage; Iron; Practice guidelines as topic
INTRODUCTION
Anemia is a public health issue affecting approximately 25% of the world’s population[1]. Several anemia etiologies exist, and of these, iron deficiency is the most widespread and is estimated to cause up to 50% of all anemia cases[1]. Iron-deficiency anemia (IDA) often originates from blood loss from lesions in the gastrointestinal tract in men and postmenopausal women[2,3]. The prevalence of IDA among patients with gastrointestinal bleeding has been estimated to be 61%[4]. However, studies have shown that IDA is often underdiagnosed, underrecognized, and undertreated in hospitalized patients with gastrointestinal bleeding[5-7]. Moreover, evidence suggests that therapeutic approaches for iron-deficiency correction have been poorly implemented[5-7], and clinical practice guidelines are not being followed[6].
Treatment options for correcting IDA in patients with gastrointestinal bleeding include the administration of oral or intravenous iron therapy and transfusion. Oral iron is often considered a first-line treatment because it is safe, inexpensive, and convenient[8]. However, many patients with gastrointestinal bleeding have a poor response to oral iron therapeutics because of gastrointestinal side effects, malabsorption, or requirements of higher supplemental iron doses to correct iron deficiency that consequently aggravate side effects[9]. In these situations, intravenous iron formulations may be a more effective and better tolerated therapeutic alternative than oral formulations[10-13]. Proper treatment of IDA alleviates symptoms of iron deficiency, such as fatigue[14], and improves quality of life[14-16].
Clinical practice recommendations and guidelines on the management of IDA in gastrointestinal bleeding patients are still scarce[12,13,17], and there is no standardization on the management of these patients[13], in which different strategies have been used in daily clinical practice. Therefore, it is urgent to develop evidence-based recommendations to better diagnose and treat IDA in patients with gastrointestinal bleeding. The main purpose of this systematic review, developed by the Digestive Bleeding and Anemia Workgroup, is to provide recommendations for simple and uniform diagnostic and therapeutic approaches for IDA in patients with gastrointestinal bleeding.
MATERIALS AND METHODS
Consensus meetings
The Digestive Bleeding and Anemia Workgroup was formed in 2016 by five key opinion leaders in gastroenterology in Portugal. The Workgroup members have significant experience in the management of gastroenterology departments and clinical practice in gastroenterology emergencies. Two meetings were held in Coimbra (Portugal) in March 2017, with the sole purpose of reaching a consensus among the five experts regarding the diagnosis and treatment of IDA in patients with gastrointestinal bleeding. A consensus was reached through discussions during the meetings and was further supported by a systematic literature search.
Search strategy and details
We followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement to report the results of this review[18]. Key research questions were established and approved a priori by the authors.
One reviewer performed a literature search in MEDLINE (PubMed) for studies published from January 1, 2003, to the present. The last search was conducted on April 2, 2019. Search strategies combined MeSH terms and the free text terms “gastrointestinal bleeding” crossed with “iron-deficiency anemia” and “diagnosis” or “treatment” or “management” or “prognosis” or “prevalence” or “safety” or “iron” or “transfusion” or “quality of life” or other terms. Complete search strategies are available in supplemental material SM1. The electronic database search was supplemented by searching for clinical practice guidelines on the websites of worldwide professional associations and reviewing the reference lists of the guidelines for relevant articles. Additional references were included after the peer review process.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) Studies that included adults ≥ 18 years of age; (2) Studies that included patients with gastrointestinal bleeding (all etiologies); (3) Studies that included patients with IDA; (4) Systematic reviews with or without metaanalyses, clinical trials, registry-based studies, cohort studies, population-based studies, and clinical practice guidelines; (5) Studies that were written in English; and (6) Studies that were published after January 1, 2003.
The exclusion criteria were as follows: (1) Studies including children < 18 years of age; (2) Studies including animals; (3) Studies in which abstracts or full-text articles were not available; (4) Studies that included patients under critical care (emergency); (5) Studies that included patients who refuse transfusion treatment; and (6) Review articles, surveys, case reports, case series, case-control studies, comments, letters, conference abstracts or posters, or economic evaluations.
Study selection
One reviewer screened all titles and abstracts retrieved from the electronic searches to identify potentially eligible articles. Full texts of the potentially eligible articles were retrieved, and the same reviewer classified the articles as eligible, potentially eligible, unclear, or not eligible as well as the reason for exclusion. A second reviewer screened all full-text articles and reviewed the classifications. The second reviewer also screened potentially eligible or unclear full-text articles, determined whether they were eligible or not eligible and recorded the reason for exclusion. Disagreements were resolved by consensus.
Data collection
One reviewer extracted the relevant data. The extracted data included article or guideline characteristics (author or organization, year of publication, study type, number of patients, gastrointestinal etiology, subject sex, subject age, study period, country or region), incidence of IDA, mortality, rebleeding, rate of screening, rate of diagnosis, rate of treatment, recommended tests and thresholds for hemoglobin and/or serum ferritin for the diagnosis of IDA, target population for treatment, indications for erythrocyte transfusion, indications for intravenous iron treatment, recommendations for ferric carboxymaltose treatment, and treatment targets and timepoints.
RESULTS
Study and guideline characteristics
The results of the screening and selection process are shown in the flowchart in Figure 1. Our initial search yielded 494 literature citations, of which 51 were duplicates and were removed. The remaining 443 studies were screened by title and abstract. After excluding irrelevant studies, 69 studies were further assessed by reading the full texts. Of these, 38 were excluded; the remaining 31 studies were included in our analysis and comprised 17 original articles, 1 meta-analysis (Table 1), and 13 clinical practice guidelines (Table 2). Ten additional references regarding the differential diagnosis of IDA and anemia caused by inflammation, the potential role of ascorbic acid in increasing iron absorption, and the characteristics and advantages and disadvantages of oral and intravenous iron preparations were included after the peerreview process [Northrop-Clewes[19], World Health Organization (WHO)[20], Shinet al[21], Infusinoet al[22], Shubhamet al[23], Koulaouzidiset al[24], Muñozet al[25], Drozdet al[26], Jimenezet al[27], McDonaghet al[28]].
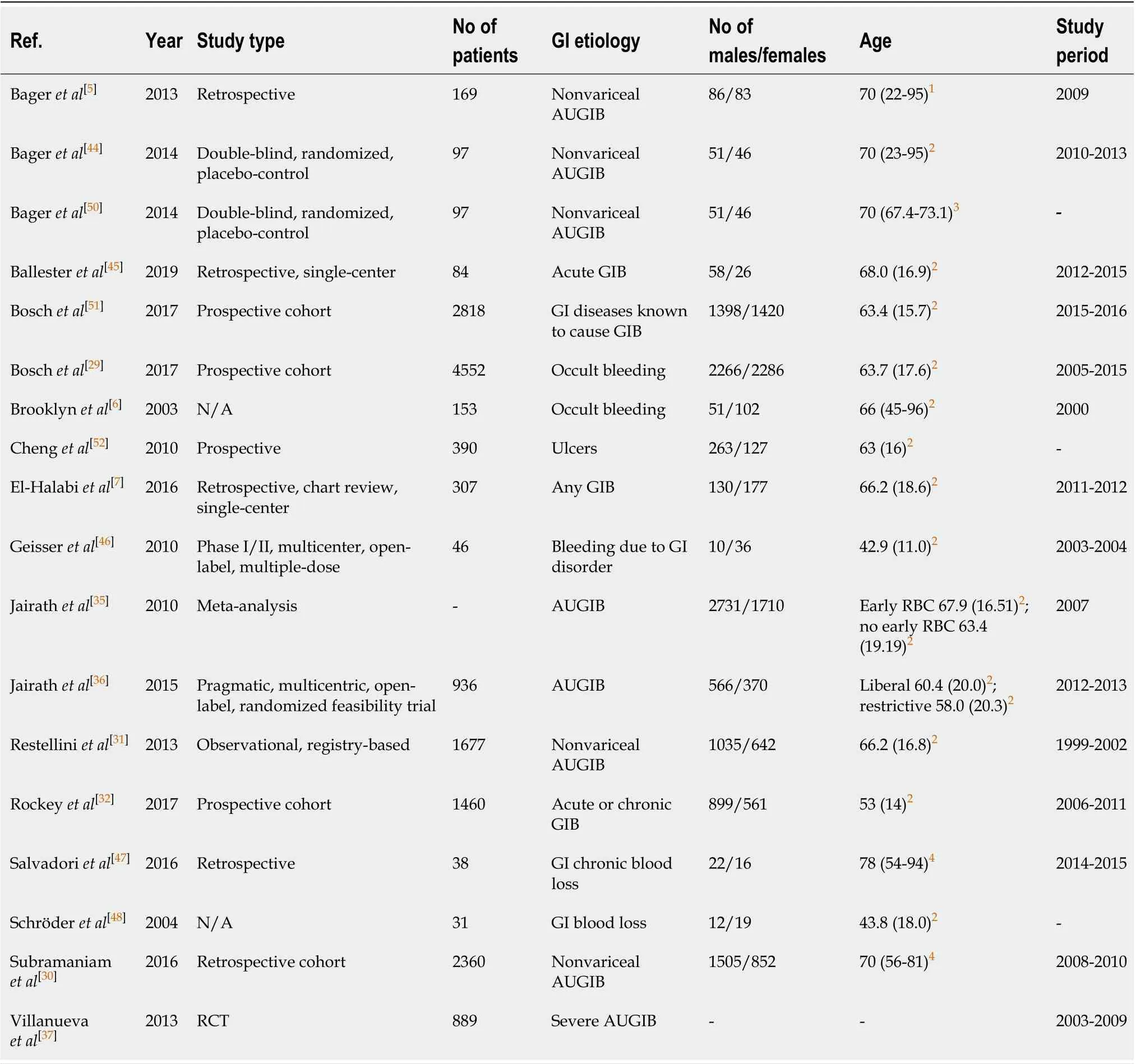
Table 1 Characteristics of included original articles and meta-analyses
Recommendations for the management of IDA in patients with gastrointestinal bleeding
Diagnosis:Hemoglobin and iron status should be routinely evaluated in all patients with gastrointestinal bleeding.
Although IDA is common in patients with gastrointestinal bleeding, the rate of IDA screening is generally low[6,7]. Moreover, patients with IDA are less likely to be investigated than patients with iron deficiency according to published guidelines[6].
The recommendation that all patients with gastrointestinal bleeding should be assessed for hemoglobin and iron status is based on data from studies reporting a high prevalence of anemia and IDA and a high incidence of mortality among patients with gastrointestinal bleeding. Two retrospective studies assessed the prevalence of IDA ingastrointestinal bleeding. The first study from Bageret al[5]included 169 patients with nonvariceal acute upper gastrointestinal bleeding (AUGIB) and found that 82% of the patients had anemia at hospital discharge. The second study was a single-center study by El-Halabiet al[7],which included 307 patients with any gastrointestinal bleeding and reported that 47.4% of the patients had IDA.
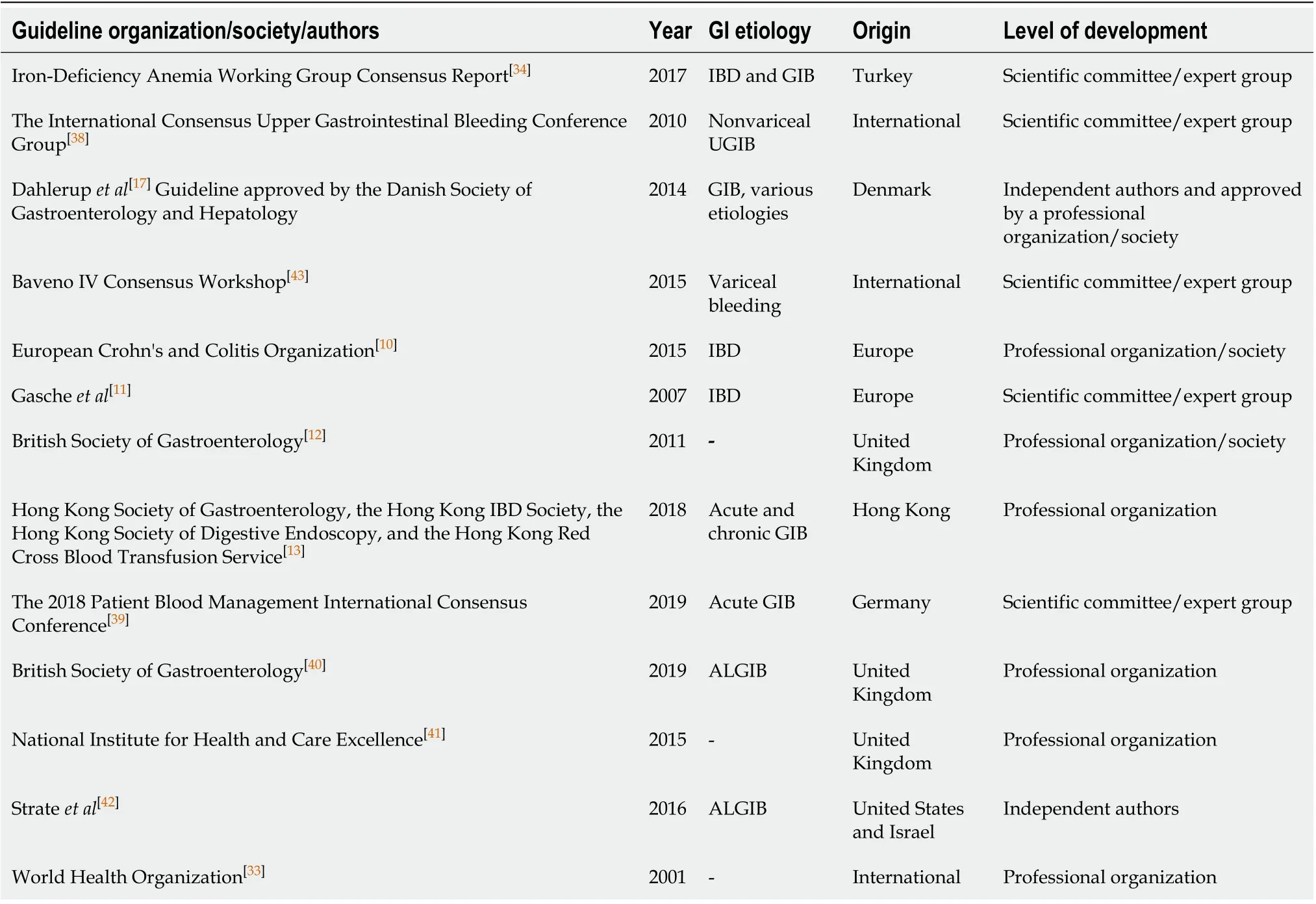
Table 2 Characteristics of the included guidelines
Moreover, mortality among patients with gastrointestinal bleeding was reported in two observational studies[29,30]. Among patients with nonvariceal upper gastrointestinal bleeding (UGIB), 30 d mortality ranged from 4.9% to 5.4%[30,31], 1-year mortality was 13.9%, and 2-year mortality was 19.6%[30]. For patients with occult gastrointestinal bleeding, 10-year mortality was 13%[29]. In addition, a study by Rockeyet al[32]found that 30 d mortality was higher among patients with acute bleeding (7%) than among patients with chronic bleeding (0%).
Laboratory tests should include determining serum hemoglobin and ferritin levels and transferrin saturation percentage
The WHO defines anemia as a hemoglobin level below 13 g/dL in men and below 12 g/dL in nonpregnant women[33]. Hemoglobin together with serum ferritin are commonly recommended by international and national guidelines as markers for IDA, and most guidelines agree with the cutoff value for hemoglobin as defined by the WHO[10,11,17,34]. According to several guidelines, the recommended serum ferritin cutoff value for diagnosing IDA ranges from 12 to 30 µg/L in the absence of inflammation and from 30 to above 100 µg/L in the presence of inflammation[10-12,17,34]. However, as serum ferritin is an acute-phase reactant, additional markers, such as transferrin saturation, may be required to confirm IDA. Three guidelines recommend measuring transferrin saturation, and the suggested cutoff for diagnosing IDA is below 16%[11,34]or below 20% in the presence of inflammation[10]. To the differential diagnosis of IDA and anemia caused by inflammation, WHO recommends to assess both the concentration of serum transferrin receptor and the serum transferrin receptor/log ferritin ratio[19,20]or log (serum transferrin receptor/ferritin) ratio[19]. Shin and colleagues reported that serum transferrin receptor/log ferritin ratio enabled an accurate diagnosis of IDA, as well as the differential diagnosis between IDA and anemia of chronic disease[21]. Infusinoet al[22].performed a meta-analysis and suggested that both serum transferrin receptor and the serum transferrin receptor/log ferritin ratio are useful to distinguish between patients with IDA and anemia of chronic disease, adding that serum transferrin receptor may be more efficient than the latter[22]. Koulaouzidis and colleagues critically reviewed the use of serum transferrin receptor as a marker for the evaluation of iron stores and suggested a cutoff value of 2.5 mg/L (29.5 nmoL/L) for the identification of IDA[24]. Nevertheless, serum transferrin receptor levels should not be used alone to distinguish between patients with or without iron deficiency in the presence of inflammation, because their levels are affected by the rate of erythropoiesis from any cause[20].
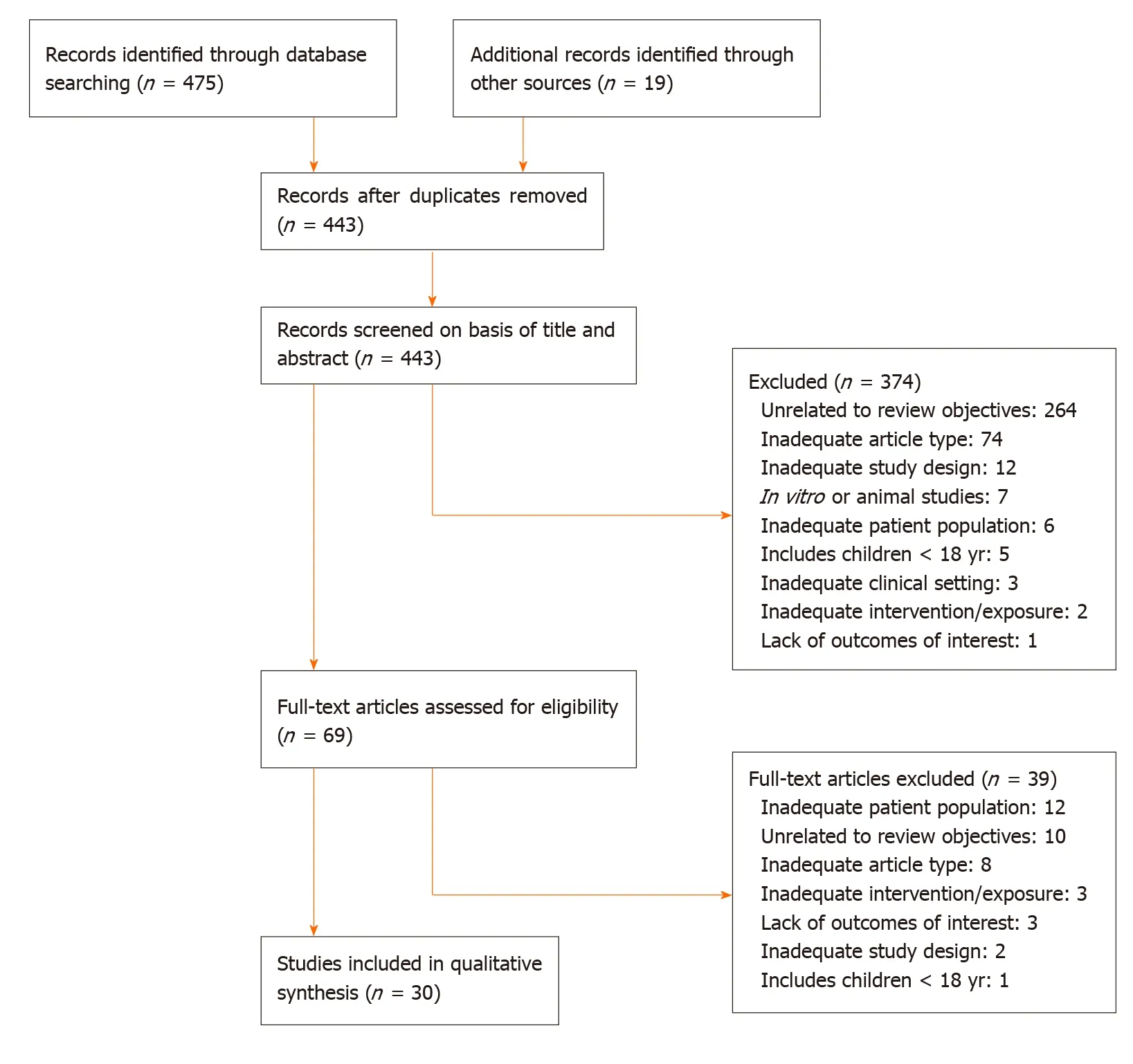
Figure 1 Flowchart of the selected articles. Adapted from Moher et al[18].
Target population for treatment
Treatment for IDA should be considered in patients with one or more of the following conditions:Evidence or clinical suspicion of gastrointestinal bleeding,i.e., the presence of melena or hematochezia or hematemesis or if there was IDA (hemoglobin < 13 g/dL in men, < 12 g/dL in women) or a positive fecal occult blood test; previously diagnosed but untreated anemia; hemoglobin levels > 7 g/dL and ≤ 10 g/dL and no indication for erythrocyte transfusion (see indications for erythrocyte transfusion); hemodynamic stability,i.e., the absence of visible active hemorrhaging, with systolic blood pressure > 100 mmHg and a heart rate < 100 beatsperminute; comorbidities (for instance, heart or kidney disease); concomitant IDA with erythropoiesis-stimulating agents.
Iron deficiency should be corrected by iron treatment, with the goal of restoring hemoglobin levels, serum ferritin levels, and transferrin saturation to normal levels to avoid or reduce the need for erythrocyte transfusion. The decision to initiate iron treatment should be based on the patient’s history and symptoms and should consider comorbidities, hemodynamic stability, hemoglobin level and additional treatments. Gascheet al[11]suggested that the absolute indications for initiating intravenous iron treatment include hemoglobin levels below 10 g/dL, intolerance or inappropriate response to oral iron, severe intestinal disease activity, concomitant use of an erythropoietic agent, and patient preference. In patients who are not considered for iron treatment, other treatment options should be considered.
Indications for erythrocyte transfusion
The decision to transfuse erythrocytes should be individualized and based on multiple factors related to the patient’s clinical status. In general, erythrocyte transfusion should be considered in patients with: Hemoglobin levels below 7 g/dL; hemoglobin levels above 7 g/dL and below 8 g/dL and comorbidities or under postoperative care; hemoglobin levels between 8 g/dL and below 10 g/dL and symptomatic anemia (i.e.,resulting in asthenia and a change in attention capacity), persistent bleeding, or heart disease; in exceptional cases, in patients with hemoglobin levels above 10 g/dL.
Treatment outcomes will depend on etiology, duration, and volume of blood loss. In general, a hemoglobin target of 7 to 9 g/dL should be considered in patients with levels below 7 g/dL and no comorbidities. For patients with comorbid illnesses (cardiovascular disease), hemoglobin target levels of 10 g/dL or above should be considered.
Current clinical practice restricts erythrocyte transfusion to special situations, such as severe anemia or anemia with comorbidities. Treatment with erythrocyte transfusion in patients with UGIB was associated with an increased risk of rebleeding in a meta-analysis of three randomized controlled trials (RCTs)[35]and one observational study[31]. Moreover, erythrocyte transfusion may also be linked to increased mortality, although the evidence is less conclusive than that for rebleeding. The abovementioned meta-analysis found an association between erythrocyte transfusion and increased mortality[35], whereas the observational study did not find an association[31]. Two large RCTs studied the hemoglobin threshold for initiating erythrocyte transfusion in patients with UGIB and its association with treatment complications. The study by Jairathet al[36]did not find any association between a restrictive transfusion strategy (8 g/dL) and rebleeding or mortality. The study by Villanuevaet al[37]showed that a restrictive transfusion strategy (7 g/dL) was associated with better survival, a lower rate of further bleeding, and fewer complications than a more liberal transfusion strategy (9 g/dL). Similarly, an observational study by Subramaniamet al[30]found that for patients with hemoglobin levels above 9 g/dL, there was an association between the number of red blood cell units transfused and increased odds of rebleeding.
Most guidelines recommend a restrictive transfusion strategy for patients without comorbid illnesses, although the indications to initiate transfusion vary[10,13,38-42]. Most guidelines recommend transfusion when hemoglobin levels fall below 7 g/dL[10,38,40,41]and the target hemoglobin level is above 7 g/dL[40-43]. The recommended hemoglobin threshold and target levels for the included guidelines are shown in Table 3.
In addition to the recommendations mentioned above, there are exceptions in which a more liberal transfusion strategy can be adopted, such as in patients with comorbidities such as cardiovascular disease or massive bleeding[13,40-42]. However, the threshold differs between the guidelines, such as hemoglobin concentrations above 8 g/dL in patients with a history of cardiovascular disease[40]or acute coronary syndrome[41], above 9 g/dL in patients with cardiovascular ischemia or massive bleeding[42], or between 9 and 10 g/dL in patients with symptomatic coronary artery disease[13].
The decision to initiate erythrocyte transfusion should be decided on an individual basis after carefully weighing the potential benefits and risks. Patients with heart disease, symptomatic anemia, or persistent bleeding or those receiving postoperative care generally benefit from a less restrictive transfusion strategy. Moreover, the goal of erythrocyte transfusion should be to restore the hemoglobin concentration to a safe level and should generally be followed by iron supplementation to replenish iron stores.
Indications for intravenous iron
Intravenous iron should be considered for patients undergoing oral iron supplementation but not achieving IDA correction, reporting treatment-emergent adverse events, or reporting nonadherence to oral supplementation.
The goal of iron therapy is to normalize hemoglobin levels, serum ferritin levels, and transferrin saturation to avoid the need for erythrocyte transfusion. Two treatment approaches are available for restoring iron levels in patients with gastrointestinal bleeding: Oral and intravenous iron. Oral iron is the conventional treatment for IDAbut has been associated with gastrointestinal side effects, malabsorption, and nonadherence. Intravenous iron, on the other hand, is generally well tolerated and considered safe[44-48]. One of the identified studies directly compared the effects of intravenous iron with oral iron in patients with nonvariceal AUGIB and found that both treatments were equally effective in restoring hemoglobin levels[44]. These findings were supported by clinical trials and retrospective studies showing that intravenous iron (ferric carboxymaltose or iron sucrose) was effective in restoring hemoglobin levels in patients with AUGIB and acute lower gastrointestinal bleeding[45], chronic gastrointestinal bleeding[47], and gastrointestinal disorder[46]. Intravenous iron (ferric carboxymaltose), however, has been shown to be more efficient in restoring ferritin levels and iron stores in patients with nonvariceal AUGIB than oral iron[44]. Although evidence is still scarce, in cases of suspected malabsorption, the co-administration of oral ascorbic acid may be considered to increase oral iron absorption[12,13,23]. Some pharmacological characteristics of worldwide available oral and intravenous iron preparations, as well as their advantages and disadvantages are shown in Table 4.

Table 3 Erythrocyte transfusion: Guidelines for hemoglobin thresholds and targets
The decision to treat patients with intravenous iron should be based on the patient’s clinical history and preference. Moreover, international guidelines recommend intravenous iron as the first-line therapy for patients with inflammatory bowel disease[10,11,13,17,34], intolerance to oral iron[10-13,17], or poor response to oral iron[13,17], or according to patient preference[11].
Recommendations for intravenous ferric carboxymaltose treatment
The recommended maximum cumulative dose of ferric carboxymaltose is 1000 mg of iron (20 mL of ferric carboxymaltose)perweek. Ferric carboxymaltose treatment can be administered one or two times, with an interval of at least one week (in cases in which patient iron dose requirements are > 1000 mg).
Patients should be monitored during the infusion and for 30 min after each administration of intravenous ferric carboxymaltose.
Ferric carboxymaltose treatment should not be considered in patients with one or more of the following conditions: (1) active bleeding; (2) first-trimester pregnancy; (3) active bacterial infection; (4) hemochromatosis or hemosiderosis; (5) Evidence of iron overload (ferritin levels > 800 µg/L and transferrin saturation > 50%); (6) hypersensitivity to ferric carboxymaltose preparations or any of its excipients; and (7) known severe hypersensitivity to other intravenous iron formulations.
Concentrations of ferric carboxymaltose up to 1000 mg have been administered in clinical trials without serious adverse events[44-47]. The recommendations regarding the administration of ferric carboxymaltose are based on the Digestive Bleeding andAnemia Workgroup’s clinical experience and the Summary of Product Characteristics[49]. In our opinion, the first step before administering ferric carboxymaltose is to evaluate the patients’ iron requirements. Calculations according to patient body weight and hemoglobin level are shown in Table 5. The second step is to calculate the required dosage using the criteria in Table 5 while considering the following conditions: A maximum single dose of ferric carboxymaltose containing 1000 mg of iron (without exceeding 20 mg/kg of body weight) with an infusion duration of 15 min.

Table 4 Pharmacological characteristics, advantages and disadvantages of worldwide available oral and intravenous iron preparations
The recommendation to monitor patients for 30 min after administration reflects common clinical practice and the findings from a clinical trial showing that ferric carboxymaltose may induce adverse events during drug infusion[47].
Treatment targets and monitoring of patients
After administration of intravenous iron and in the absence of persistent bleeding, the goal of IDA treatment is to increase the hemoglobin level by 1 to 2 g/dL within 2 to 4 wk and maintain a serum ferritin level ≥ 50 ng/mL (in the absence of inflammatory conditions), increase the number of reticulocytes within 3 to 5 d, and maintain transferrin saturation ≥ 30% for 4 to 6 mo after normalization of hemoglobin levels and once the etiological cause of anemia has been corrected.
Clinical trials have shown that increasing hemoglobin levels by 1 to 2 g/dL within 4 wk can be achieved with the administration of intravenous iron (ferric carboxymaltose)[44,46]. Similarly, a retrospective study of 38 patients with chronic gastrointestinal bleeding reported a median hemoglobin increase of 2.4 g/dL at 5 wk after ferric carboxymaltose treatment[47]. Moreover, an 2 g/dL increase in hemoglobin levels within 4 wk is also considered an acceptable speed of response according to several guidelines[10,11,34].
Two guidelines recommend serum ferritin target levels. Gascheet al[11]recommend maintaining serum ferritin above 100 µg/L, whereas the European Crohn’s and Colitis Organization guidelines recommend restoring serum ferritin to normal[10].
Reticulocytes are a relatively new biomarker for assessing response to iron treatment. Evidence supporting the monitoring of reticulocytes and their relevant target levels was therefore limited to one small clinical trial and recommendations inone guideline. Geisseret al[46]measured reticulocyte levels at baseline and 14 d after ferric carboxymaltose supplementation and found that the levels increased from 60 × 109/L to 89 × 109/L. Moreover, one guideline recommends measuring reticulocytes at one week after iron treatment to confirm an increase compared to the level before treatment[17]. Generally, reticulocyte levels increase within one week in response to intravenous treatment. We recommend measuring the number of reticulocytes after 3-5 d to assess whether a proper response to initial intravenous iron has been achieved to evaluate further treatment.
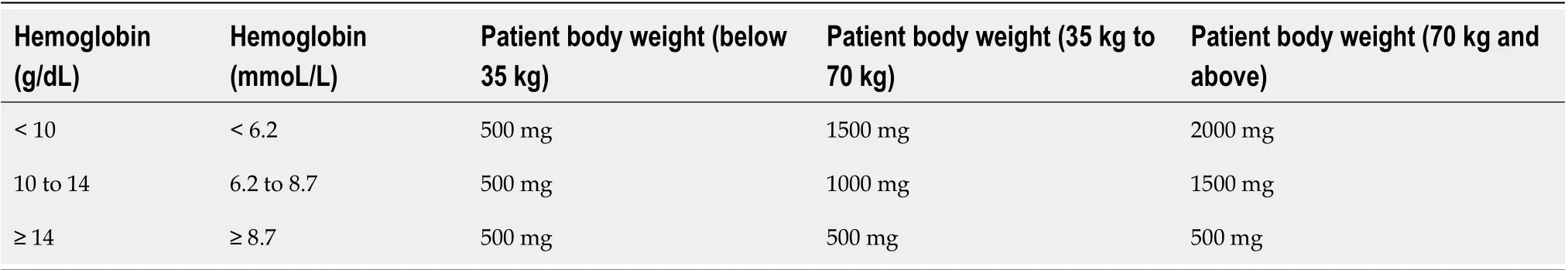
Table 5 Calculation of iron requirement according to patient body weight and hemoglobin level
Only one guideline recommends measuring transferrin saturation to assess therapeutic response. This guideline recommends target levels of transferrin saturation between 16% and 50% after anemia treatment[11]. Transferrin saturation may temporarily increase in response to intravenous iron treatment and should therefore not be used for monitoring initial treatment response.
DISCUSSION
Summary algorithm for investigation and treatment
Figure 2 presents the algorithm for the diagnosis and treatment of IDA in patients with acute or chronic gastrointestinal bleeding. The algorithm is based on the literature and the experience of the members of the Digestive Bleeding and Anemia Workgroup. This algorithm should be implemented during the hospital stay and at follow-up visits after patient discharge and is not applicable for emergencies or critical care.
Limitations
This consensus recommendation had several limitations. First, the quality of the identified studies was generally low, and we found few RCTs on IDA and gastrointestinal bleeding. Therefore, the recommendations are mainly based on observational studies, guidelines, and clinical experience of the members of the Digestive Bleeding and Anemia Workgroup. Second, studies on lower gastrointestinal bleeding are scarce, and our recommendations are mainly based on studies on patients with UGIB. Third, the search strategy included only studies indexed in MEDLINE (PubMed), published from January 2003 until April 2019, and written in English.
CONCLUSION
Diagnosing and treating patients with IDA is challenging in clinical practice. IDA in patients with gastrointestinal bleeding should be diagnosed and treated promptly to improve the quality of life and reduce morbidity and, eventually, mortality. This consensus recommendation provides a starting point for diagnosing and treating IDA in patients with gastrointestinal bleeding by gastroenterologists and other physicians in daily clinical practice and should serve to optimize the decision-making process for the management of these patients. We believe that this guideline may facilitate improvements in the management of IDA in patients with gastrointestinal bleeding, which ultimately translates to improved health outcomes for these patients.
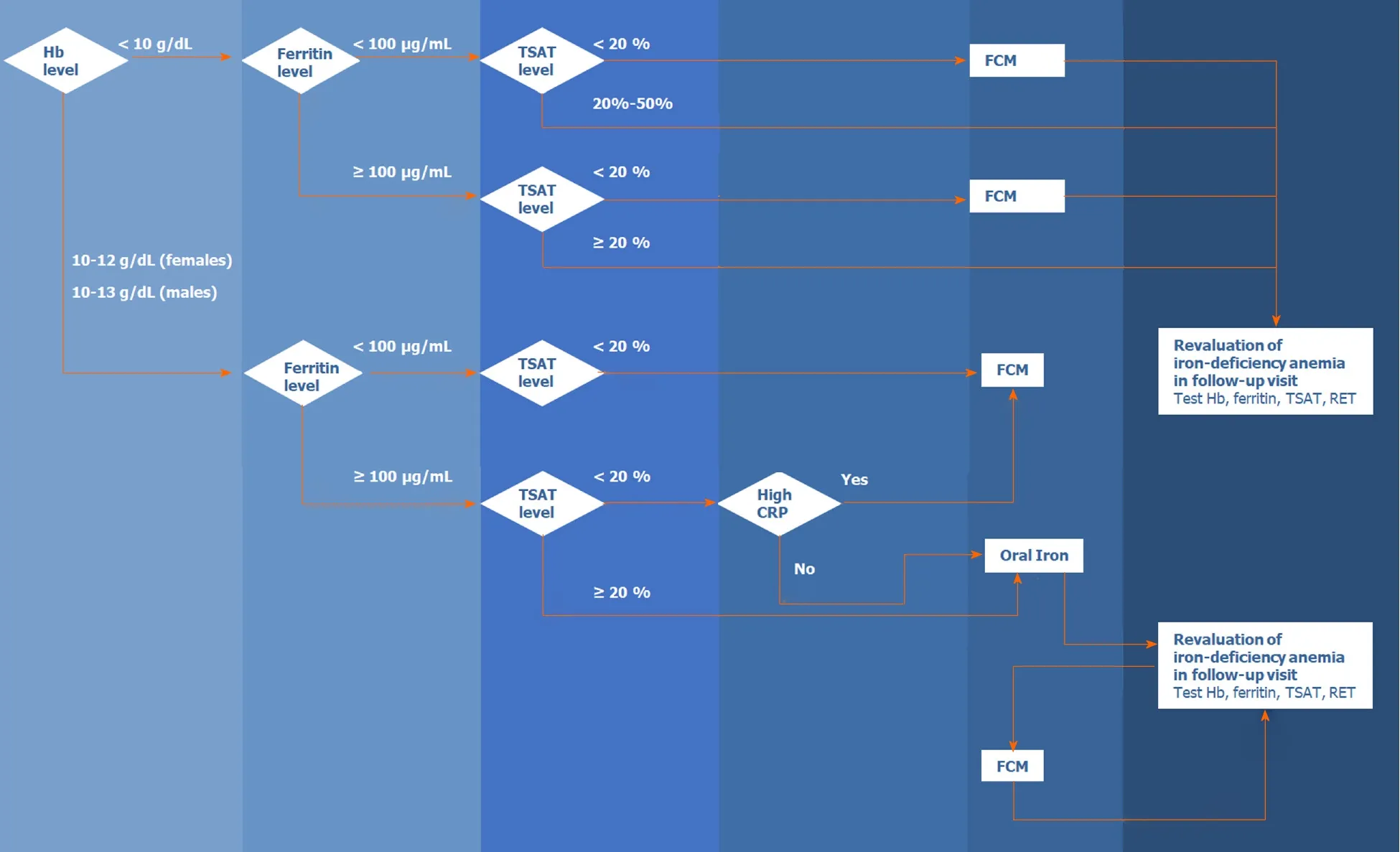
Figure 2 Algorithm for the diagnosis and treatment of iron-deficiency anemia in patients with acute or chronic gastrointestinal bleeding. Hb: Hemoglobin; TSAT: Transferrin saturation; CRP: C-reactive protein; FCM: Ferric carboxy maltose; RET: Reticulocyte.
ARTICLE HIGHLIGHTS
Research background
Anemia is a public health issue affecting approximately 25% of the world’s population, being often caused by iron deficiency. Iron-deficiency anemia (IDA) often originates from blood loss from lesions in the gastrointestinal tract in men and postmenopausal women, and its prevalence among patients with gastrointestinal bleeding has been estimated to be 61%. However, studies have shown that IDA is often underdiagnosed, underrecognized, and undertreated in hospitalized patients with gastrointestinal bleeding, and that therapeutic approaches for iron-deficiency correction have been poorly implemented, and clinical practice guidelines are not being followed. Furthermore, clinical practice recommendations and guidelines on the management of IDA in gastrointestinal bleeding patients are still scarce and there is no standardization on the management of these patients. Therefore, standardized recommendations on the management of IDA in gastrointestinal bleeding patients, based on a systematic review of the current evidence, are needed.
Research motivation
Given the scarcity of clinical practice recommendations and guidelines on the management of IDA in gastrointestinal bleeding patients, and the need of standardization regarding the management of these patients, it is urgent to develop evidence-based standardized diagnostic and therapeutic approaches on the management of patients with IDA due to gastrointestinal bleeding.
Research objectives
With this study, we aimed to review the current evidence and guidelines concerning IDA management in gastrointestinal bleeding patients to develop recommendations for its diagnosis and therapy.
Research methods
Five gastroenterology experts formed the Digestive Bleeding and Anemia Workgroup and conducted a systematic literature search in PubMed and professional association websites. MEDLINE (viaPubMed) searches combined MeSH terms and the keywords “gastrointestinal bleeding” with “iron-deficiency anemia” and “diagnosis” or “treatment” or “management” or “prognosis” or “prevalence” or “safety” or “iron” or “transfusion” or “quality of life”, or other terms to identify relevant articles reporting the management of IDA in patients over the age of 18 years with gastrointestinal bleeding; retrieved studies were published in English between January 2003 and April 2019. Worldwide professional association websites were searched for clinical practice guidelines. Reference lists from guidelines were reviewed to identify additional relevant articles. The recommendations were developed by consensus during two meetings and were supported by the published literature identified during the systematic search.
Research results
From 494 Literature citations found during the initial literature search, 17 original articles, one meta-analysis, and 13 clinical practice guidelines were analyzed. Ten additional references were included after the peer review process. Based on the published evidence and clinical experience, the workgroup developed the following ten recommendations for the management of IDA in patients with gastrointestinal bleeding: (1) evaluation of hemoglobin and iron status; (2) laboratory testing; (3) target treatment population identification; (4) indications for erythrocyte transfusion; (5) treatment targets for erythrocyte transfusion; (6) indications for intravenous iron; (7) dosages, (8) monitoring; (9) indications for intravenous ferric carboxymaltose treatment; and (10) treatment targets and monitoring of patients. The workgroup also proposed a summary algorithm for the diagnosis and treatment of IDA in patients with acute or chronic gastrointestinal bleeding, which should be implemented during the hospital stay and follow-up visits after patient discharge.
Research conclusions
Ten evidence-based recommendations were developed for screening, treatment indications, appropriate therapies, and treatment goals of IDA in patients with acute or chronic gastrointestinal bleeding. An algorithm for the diagnosis and treatment of these patients was also developed, based on the literature and on the experience of the members of the Digestive Bleeding and Anemia Workgroup. Therefore, this work serves as a starting point for diagnosing and treating IDA in patients with gastrointestinal bleeding by gastroenterologists and other physicians in daily clinical practice and should serve to optimize the decision-making process for the management of these patients. This guideline may facilitate improvements in the management of IDA in patients with gastrointestinal bleeding, which ultimately may improve health outcomes in these patients.
Research perspectives
This consensus recommendation provides a starting point for clinicians to better diagnose and treat IDA in patients with gastrointestinal bleeding. Nevertheless, more studies, specially RCTs on IDA and gastrointestinal bleeding are needed to further improve the management of these patients.
ACKNOWLEDGEMENTS
The authors would like to thank Scientific Toolbox Consulting (Lisbon, Portugal) for providing medical writing support.
杂志排行
World Journal of Gastroenterology的其它文章
- Discovery of unique African Helicobacter pylori CagAmultimerization motif in the Dominican Republic
- Association between ADAMTS13 activity–VWF antigen imbalance and the therapeutic effect of HAIC in patients with hepatocellular carcinoma
- Liver fibrosis index-based nomograms for identifying esophageal varices in patients with chronic hepatitis B related cirrhosis
- High mortality associated with gram-negative bacterial bloodstream infection in liver transplant recipients undergoing immunosuppression reduction
- Nimbolide inhibits tumor growth by restoring hepatic tight junction protein expression and reduced inflammation in an experimental hepatocarcinogenesis
- Role of pancreatography in the endoscopic management of encapsulated pancreatic collections – review and new proposed classification
