Effect of Ayurveda gut therapy protocol in managing dysbiosis of children with autism:study protocol for a randomized controlled trial
2021-01-14DineshKarayilSubramanianAnitaPatelMadathaniyilJosephGeorgeSwapnaChitraSugunanandagopanSanthiKrishnaSujithaVariyattukunnuKeluJayakrishnanKalluvirathArchanaMadhavi
Dinesh Karayil Subramanian,Anita Patel,Madathaniyil Joseph George,Swapna Chitra Sugunanandagopan,Santhi Krishna,Sujitha Variyattukunnu Kelu,Jayakrishnan Kalluvirath,Archana Madhavi
1 Department of Kaumarabhrithya,Vaidyaratnam P S Varier Ayurveda College,Kottakkal,Kerala,India
2 S.C.S.V.M.V University,Tamil Nadu,India
3 Department of Shalyatantra,Vaidyaratnam P S Varier Ayurveda College,Kottakkal,Kerala,India
4 Department of Kayachikitsa,Vaidyaratnam P S Varier Ayurveda College,Kottakkal,Kerala,India
Abstract Background and objectives:Emerging evidences indicate an invariable relationship between gut dysbiosis and neurobehavioral symptoms of autism spectrum disorder.In India,Ayurveda is widely accepted among the complementary and alternative medicine.This study aimed to assess the efficacy of an Ayurveda gut therapy protocol in autism spectrum disorder.Subjects and methods:In this randomized controlled trial,60 children with autism spectrum disorder admitted to Vaidyaratnam P S Varier Ayurveda College,India will be randomly assigned to intervention and control groups.The intervention group will undergo Ayurveda gut therapy protocol for 30 days and interdisciplinary interventions for 2 months,whereas the control group will undergo only interdisciplinary interventions for 2 months.A final assessment will be done on the 60th day.Patient recruitment began in July 2018.The primary and secondary outcome measure will be completed in January 2021 and the study will be completed in September 2022.The study was approved by the Institutional Ethical Committee of Vaidyaratnam P S Varier Ayurveda College,India(Proceedings No:IEC/CI/24/17)on May 4,2017.Protocol version:1.0.Outcome measures:The expected primary outcome is to assess the quality and quantity of the gut microbes through 16s rRNA sequencing.The secondary outcome expected is the changes in the neurobehavioral symptoms assessed through the Childhood Autism Rating Scale and also changes in the gastrointestinal symptoms assessed through Ayurveda Gut Health Assessment Questionnaire.Discussion:The current protocol discusses the relationship between Autism and gut dysbiosis and its management through Ayurveda,and provides evidence for the rationality of using Ayurveda gut therapy as an alternative therapy for autism spectrum disorder in clinical practice.Trial registration:The study was registered with Clinical Trial Registry of India(registration No.CTRI/2018/05/014017,registered on May 21,2018).
Key words:16S rRNA;autism;Ayurveda;gut dysbiosis;gut health;gut microbiome;lifestyle guidelines;traditional medicine
INTRODUCTION
With an incremental surge in the last few decades,the prevalence of autism spectrum disorder(ASD)appears to be a global concern with about one in 160 children being identified with ASD according to estimates from the World Health Organization(Christensen et al.,2019).ASD is a neuro-developmental disorder characterized by impairment in social interaction and communication along with restricted and repetitive behaviors(Hodges et al.,2020).A child with ASD creates turbulence in the family which needs to be addressed dexterously(Ryan,2010).The identified etiologies like genetic,toxic and metabolic factors are unfortunately not addressed in terms of its conventional management.Though these treatments have provided beneficial results,dissatisfaction among the parents has led to the adoption of complementary and alternative medicine in ASD management(Ong,2019).In India,people prefer Ayurveda over other alternative systems of medicine,especially in chronic and debilitating conditions(Kumar et al.,2016;Rudra et al.,2017).This alternative medical science provides a holistic,nonlinear,complex and dynamic approach in the management of diseases(Rioux,2012).
The prevalence of gastrointestinal symptoms like bloating,gastroesophageal reflux,constipation and vomiting ranges from 23% to 70% in ASD(Chaidez et al.,2014)and a strong association has been postulated with neurobehavioral symptoms among ASD patients(Babinska et al.,2020).Recent studies have discussed the involvement of the gut microbiome in the pathogenesis of ASD and administration of probiotics is the most promising treatment for neurobehavioral symptoms and bowel dysfunction,but clinical trials are still limited and heterogeneous(Fattorusso et al.,2019;Oh and Cheon,2020;Roussin et al.,2020).Ayurveda advocates maintenance of a healthy gut as the primary mode of management in all ailments(Steer,2019).Thus,the current trial is a novel approach to assess the efficacy of an Ayurveda gut therapy protocol involving poly-herbal Ayurveda medications,lifestyle and diet modifications,parental guidelines and other interdisciplinary interventions in the management of ASD.The anticipated outcome of the trial is to introduce an effective treatment for gut dysbiosis in ASD and its associated neurobehavioral symptoms.Our study aimed to study the effect of an Ayurveda gut therapy protocol in gut dysbiosis by assessing the quality and quantity of microbial gut flora in ASD children through 16S rRNA sequencing.
METHODS/DESIGN
Trial design
The current study i s an open-label randomized controlled clinical trial conducted at the Out-patient and In-patient units of Vaidyaratnam P S Varier Ayurveda College,India.A total of 60 children with ASD are planned to be included in this study with random allocation 1:1.
The total period of intervention will be for 30 days,wherein the assessment will be done in three phases namely baseline(0thday),interim(30thday)and final phase(60thday).The details of the trial schedule are outlined in Table 1.
Eligible criteria
Inclusion criteria
The children of either gender,aged between 3 and 12 years,diagnosed with ASD(Diagnostic and Statistical Manual of Mental Disorders V criteria)(Guha,2014)will be assessed for food intake,food-conversion and fecal output through an“Ayurveda gut health assessment questionnaire”(Trikamji,2008;Additional file 1).Selected children will also be verified for their ethnicity,making sure that they follow similar food habits and inhabit in similar environment.
Exclusion criteria
The children on anti-epileptic medications,probiotics,long term or recent(within 3 months)antibiotics,or ASD medications from any disciplines including Ayurveda will be excluded.Children with co-morbid conditions like cerebral palsy,mental retardation or any other chronic neurological or metabolic disorders and those who are suffering from conditions like coma,paralysis will also be excluded from the trial.
Intervention
The participants in the trial will receive a multi-modular intervention comprising of poly-herbal Ayurveda formulations,parental guidelines,lifestyle guidelines and interdisciplinary intervention according to the group allocation(Sabnis,2012;Additional file 2).Patient adherence to the trial setting will be ensured through pill count,diary logging and interim phone calls.Medicines will be collected from a GMP certified government approved reputed company.
The interventions will be carried out in two groups,each comprising of 30 participants.The intervention group will receive Ayurveda poly-herbal formulations(Rajanyadi Churna,Vilwadi Guilka)as per Ayurveda posology principles and food and lifestyle guidelines for 30 days.Both test and control will receive multidisciplinary interventions(speech therapy,occupational therapy and behavioral therapy)in common for 2 months(Table 2).
Primary and secondary outcome measures
Baseline(0thday),interim(30thday)and follow up(60th)data will be collected from the parents of the participants during each visit.The expected primary outcome is to assess the quality and quantity of the gut microbes measured in terms of relative abundance,Alpha and Beta diversity of gut microbiome which will be assessed through 16S rRNA sequencing with Illumina HiSeq2500/Miseq to generate 0.5 M reads,2 × 250 bp for each faecal sample of the participants.16S rRNA illumina sequencing is done by DNA isolation and purification using QiAmp mini stool kit followed by quality check and sequence processing by Quantitative Insight Into Microbiological Ecology(QIIME)wherein nucleic acid sequence data are analyzed and interpreted from fungal,viral,bacterial and archeal communities.It covers a broad range of topics like sequence processing,alpha-diversity,beta-diversity and taxonomic composition(Alcon-Giner et al.,2017).The secondary outcome expected is the changes in the neurobehavioral symptoms,such as hyperactivity,irritability,crankiness and stereotypic behavior,which will be assessed through the Childhood Autism Rating Scale.Total scores can range from a low of 15 to a high of 60;scores below 30 indicate that the individual is in the non-autistic range;scores between 30 and 36.5 indicate mild to moderate autism;and scores from 37 to 60 indicate severe autism(Chlebowski et al.,2010).The reduction of gastrointestinal symptoms like constipation,Gastro Esophageal Reflux Disease,abdominal cramps and undigested food in stool will be assessed through an Ayurveda gut health assessment questionnaire.The abdominal discomfort of the non-verbal subjects will be elicited by vocal and behavioral expressions they give on the appearance of discomfort(Prosperi et al.,2019).The questionnaire was formulated based on the principles of Ayurveda in Charaka Samhita(Trikamji,2008;Additional file 1),which is dichotomous and gives digestive and metabolic homeostasis assessment considering food intake,food-conversion and fecal output.
Laboratory tests used for testing serum ammonia,serum lactate and serum pyruvate are glutamate dehydrogenase technique,ion selective electrode technique and spectrophotometry respectively in any laboratory certified by National Accreditation Board for Testing and Calibration Laboratories.The research staff will monitor the whole process.
Patient recruitment began in July 2018.The primary and secondary outcome measures will be completed in January 2021 and the study will be completed in September 2022.
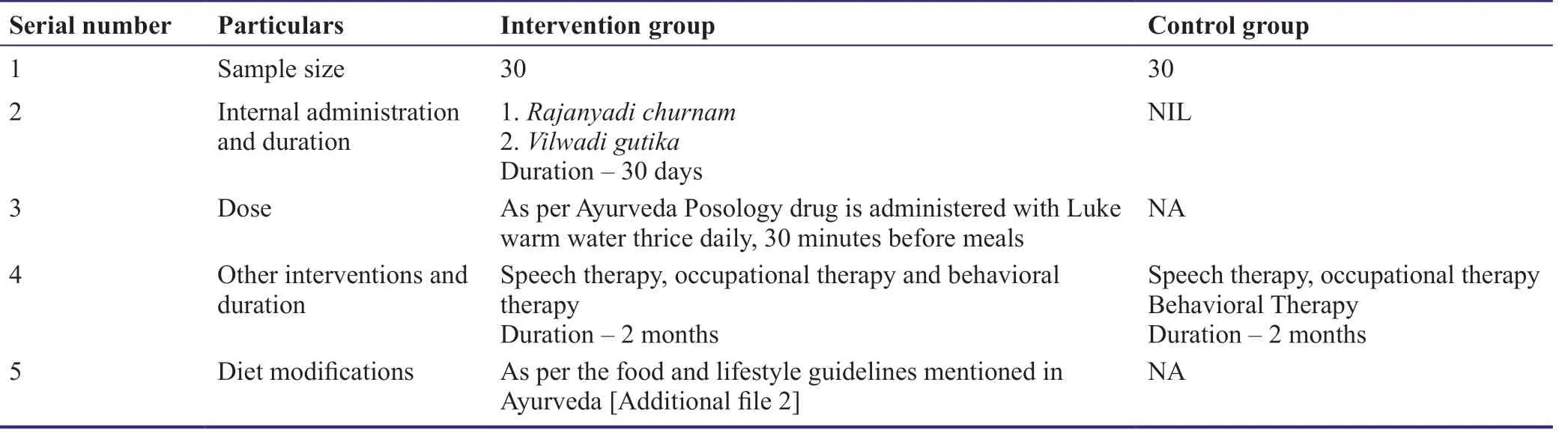
Table 2:Description of the intervention plan
Sample size
The study will follow a completely randomized design with two treatments.The error degrees of freedom for completely randomized design aret(r-1),wheretis number of treatments andris the number of replications.Equating the error degrees of freedom to 12,the minimum required,the number of replications was worked out to 7 with just two treatments.However,considering the novel nature of the trial and lack of any previous information on the error variance,the number of replications is taken as 30 for each treatment.With repeated measurements,the precision of treatment comparison is expected to be still higher.After receiving an informed assent from the parents,they will be allocated randomly through computer generated randomization(1:1)into two groups - an intervention group and a control group each constituting of 30 participants(See Figure 1 for the details of the participant flow).
Recruitment
The parents of children with ASD will be approached by the research fellows to participate in the trial during their visit to the hospital.A participant information file detailing the purpose of the study,the procedures to be followed and the risk and benefits of participation will be provided to the parents.Thereafter,screening will be done to verify whether the inclusion and exclusion criteria are met.A brief discussion regarding the informed assent will be done by the research fellows.Principal investigator will ensure that the information provided is understood and will clarify any queries related to the study.The participants can withdraw from the study at any stage and the reason for this will be duly recorded.
Data collection methods
After recruitment,the research fellows will be thoroughly trained on the protocol to be followed in the study and its methodology.The study will be carried out in the surveillance of the principal investigator and the progress will be audited by the principal investigator at regular intervals.The research fellows will collect the data from the participants during the screening time,which will be recorded in a paper-based case report form.The data collection and assessment will be repeated during baseline period,interim period(30thday)and final phase(60thday).Care will be taken in the collection and storage of biological samples without breaking the cold chain.The samples will be collected by the parents in sterile conditions as advised by the research fellows and will be strictly stored at a specific temperature of -40°C at the research office.Later,the specimens will be transported maintaining the cold chain to the concerned genetic laboratory,where the isolation and sequencing techniques are performed.The data collected after the assessment will be analyzed and verified by the principal investigator and experts in the concerned field.Since,the infrastructure of the institution offers interdisciplinary intervention and long-term in-patient facilities,participant retention and follow up can be ensured.The implementation strategy for intervention and control groups is given in Table 3.
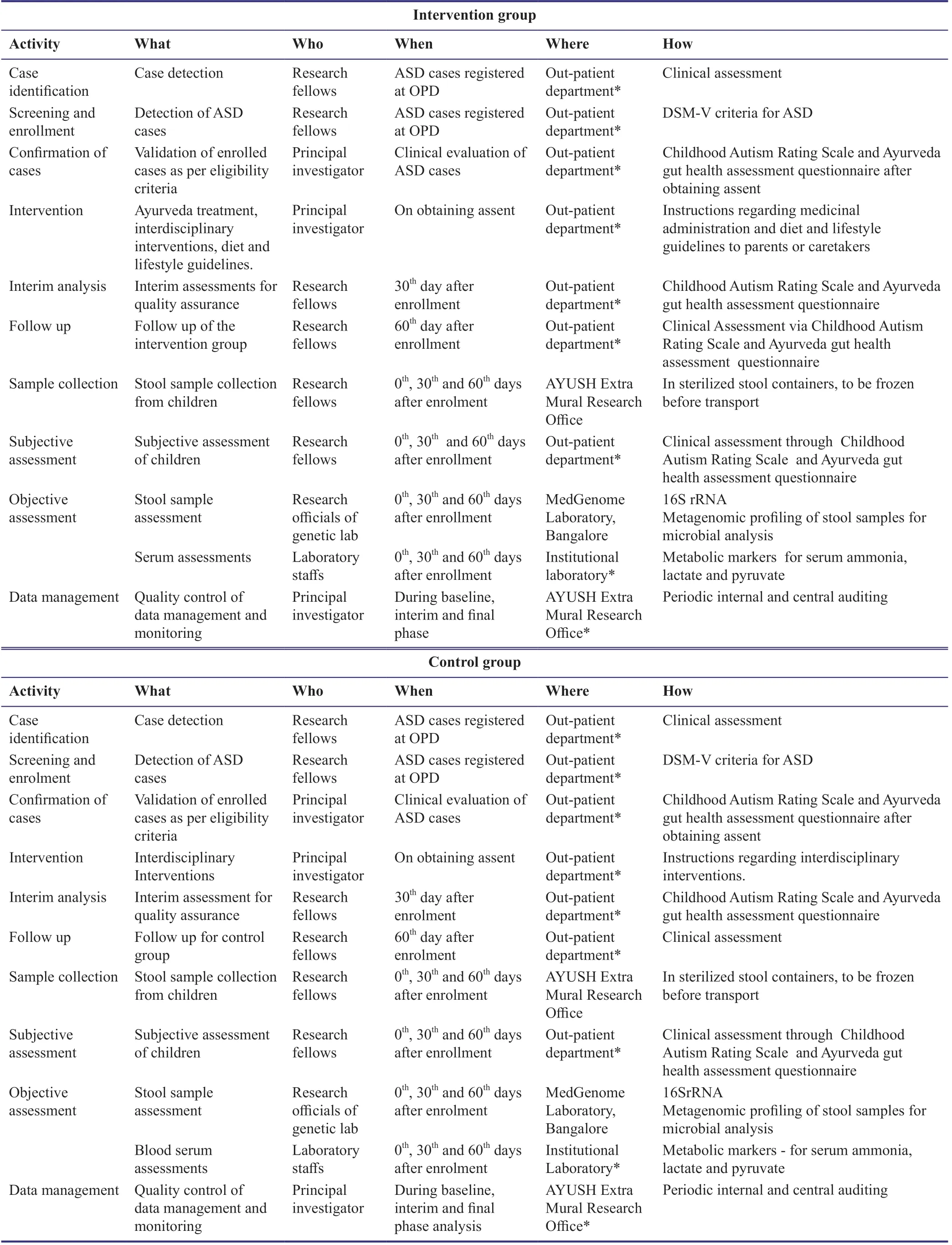
Table 3:Implementation strategy for intervention and control groups

Figure 1:Study flow chart.
Data management
The preliminary data and the assessments will be entered in paper-based case report form,while the biochemical and microbiological analysis data will be recorded and stored electronically.The participants will be labeled in numerical order and the data will be stored in a secure file in the research office.The case report form will be reviewed and validated by the Institutional Ethical Committee(IEC).Any modifications in the data will be duly documented and reported in audit trials.The principal investigator of the project will evaluate any changes occurring in the interim results and will make a decision to continue or terminate the trial of any participants.The follow up will be ensured for discontinued participants,even if they are excluded from the trial.The data safety management techniques as per Indian Council of Medical Research guidelines will be followed.
Statistical analysis
The statistical analysis for the primary outcome will be done using analysis of variance and secondary outcome measure analysis will be done using chi-square and correlation statistics.The microbiome taxonomic classification and sequencing is done using operational taxonomic unit table(Principal Component Analysis Plot),Alpha and Beta Diversity through 16S rRNA sequencing.
Data monitoring
A Data Management Committee independent from the sponsor agency will be associated with the research team for data monitoring,and periodic updating will also be done in Clinical Trials Registry of India.The National Pharmacovigilance Unit of the Institute will monitor the trial for any adverse events.The chances of harm or unintended effects are estimated to be minimal in the current trial.Although,triggering factors of any means as a result of intervention will be subjected to the stopping guidelines.The final decision regarding the termination of participants will be done by the principal investigator.Care will be taken to include subjects from same ethnicity and diet practices to avoid confounding factors.The subjects undergoing chemical and medical interventions due to gut disease will be exempted from the study.Appropriate ancillary and post-trial care will be considered for the trial participants,if any.The respective investigatory reports will be provided to the participants and effective results will be published according to the guidelines and data safety management policy.An annual central auditing will be done by the sponsor agency.
Ethics and dissemination
The protocol of this clinical research has undergone an initial peer-review and has been approved by the IEC of Vaidyaratnam P S Varier Ayurveda College,India(Proceedings No:IEC/CI/24/17)on May 4,2017(Additional file 3).The study was registered with Clinical Trial Registry of India(registration No.CTRI/2018/05/014017,registered on May 21,2018).Any revision at any stages of the trial will be submitted before the IEC for approval.
The study protocol and results will be presented at national and international conferences.The results will also be published in a peer reviewed,open access international journal.Authorship on the publishing journal will follow the International Committee of Medical Journal Editors and respective journal guidelines.This protocol was written in accordance with Standard Protocol Items:Recommendations for Interventional Trials(SPIRIT)to improve the quality of reporting(Chan et al.,2013).
Informedconsent
Assent from the parents of concerned participants will be obtained after detailing every provision of the trial protocol.The confidentiality of the participants will be assured throughout the study.However,the Ministry of Ayurveda,Yoga and Naturopathy,Unani,Siddha and Homoeopathy,Government of India will have an access to the final trial dataset.The study will be conducted with the highest respect for the individual participants in accordance with the ethical principles laid down by theDeclaration of Helsinki,the International Conference on Harmonization Good Clinical Practice Guidelines and all applicable laws and regulations,including data safety management,clinical trial disclosure laws and regulations.
DISCUSSION
A wide range of review and metanalysis on the relation of autism and gut microbiome has shown a potential research link of autistic features and gut dysbiosis(Srikantha and Mohajeri,2019).The digestive health of an individual is the reflection of the health and equilibrium of his/her gut microbiome.Though literature reviews explaining the potential influence of food,cogitation and environment on the gut microbial flora are available,only a few relates the influence of Ayurveda poly-herbal formulations as its intervention.The current protocol gives emphasis on the existing claims of relationship between Autism and gut dysbiosis and its management through Ayurveda.The trial also aims to effectively manage and improve associated hyperactivity,sensory integration problems and intense world feeling of Autistic children(Markram and Markram,2010;Conlon and Bird,2014).The guidelines incorporated in the research will help to develop result-oriented home-based practices to the beneficiaries by increasing the parental awareness.Assessing the potential serum biomarkers like serum pyruvate,lactate and ammonia in all participants could have an add-on benefit in the management of ASD.In addition to these objective parameters,the inclusion of assessment of behavioral feelings,changes in the autistic traits and subjective assessment of the gut will impart a bench to bed side implementation of treatment through a holistic approach.
If the study reveals significant outcomes in terms of research,it will refine the existing Autism management to a cost-effective,parent-oriented,multi-axial Ayurveda approach rather than the existing probiotic management for dysbiosis.This in due course can be developed to a composite protocol-Ayurveda drugs,Gut therapy,Ayurveda Standards of living,Training of parents and Yogic Assistance(AGASTYA)beneficial to doctors,parents and public via various training programs in ASD.As trial is conducted without blinding the expected degree of bias cannot be estimated.Since the study involves multi-modular interventions,effect of the individual interventions in the improvement in gut dysbiosis cannot be determined.
TRIAL STATUS
Recruiting is ongoing at the time of submission.
Additional files
Additional file 1:Ayurveda Gut Health Assessment Questionnaire.
Additional file 2:Lifestyle guidelines and diet modifications.
Additional file 3:Ethical approval documentation.
Author contributions
DKS conceived and designed the study.SCS and SK acquired data;DKS,SCS and SVK drafted the manuscript.JK and AM made substantial contributions and revisions to the manuscript.AP and MJG reviewed and approved the final version to be published.
Conflicts of interest
None declared.
Financial support
The financial support as grant-in-aid was provided by The Ministry of Ayurveda,Yoga and Naturopathy,Unani,Siddha and Homoeopathy,Government of India(grant No.Z28015/22/2018-HPC(EMR)-AYUSH-A).
Institutional review board statement
The study was approved by the IEC of Vaidyaratnam P S Varier Ayurveda College,India(Proceedings No:IEC/CI/24/17)on May 4,2017.
Declaration of patient consent
The authors certify that they will obtain all appropriate patient consent forms.In the forms the patients will give their consent for their images and other clinical information to be reported in the journal.The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity,but anonymity cannot be guaranteed.
Reporting statement
This study followed the Standard Protocol Items:Recommendations for Interventional Trials(SPIRIT)guidance for protocol reporting.
Biostatistics statement
The statistical methods of this study were reviewed by the biostatistician of Ayurdata,Penta Gardens,Thrissur,Kerala,India.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
For data sharing,data analysis plan,informed consent,clinical study report will be made available from beginning 9 months and 36 months following article publication to investigations whose proposed use of the data has been approved by an independent review committee.The proposal should be directed to drayurksd@gmail.com.All these are subjected data sharing policies of Kerala Ayurveda Studies and Research Society and Department of Ayurveda,Yoga and Naturopathy,Unani,Siddha and Homoeopathy,Government of India.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal,and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
Asia Pacific Journal of Clinical Trials:Nervous System Diseases的其它文章
- Key enzymes of glutamate metabolisms in the brain of neonatal and adult rats exposed to monosodium glutamate
- Comparison of factor structure and psychometric properties of original and abbreviated version of the Penn State Worry Questionnaire in a nonclinical sample:a cross-sectional psychometric study
