Is right lobe liver graft without main right hepatic vein suitable for living donor liver transplantation?
2021-01-14KhaledDemyatiSamiAkbulutEgemenCicekAbuzerDiricanCemalettinKocSezaiYilmaz
Khaled Demyati, Sami Akbulut, Egemen Cicek, Abuzer Dirican, Cemalettin Koc, Sezai Yilmaz
Khaled Demyati, Sami Akbulut, Egemen Cicek, Abuzer Dirican, Cemalettin Koc, Sezai Yilmaz, Department of Surgery and Liver Transplant Institute, Inonu University Faculty of Medicine, Malatya 44280, Turkey
Khaled Demyati, An-Najah National University Hospital, An-Najah National University, Nablus 11941, Palestine
Abstract
Key words: Living donor liver transplantation; Congenital-absence of right hepatic vein; Common large opening drainage model; Case report
INTRODUCTION
Since the first successful liver transplantation (LT) performed in 1967, LT has become the gold standard treatment for many liver diseases in adult and pediatric patients[1].In socioculturally developed western countries, most of the liver graft requirements are provided from the cadaveric organ pool, while in Asian and Middle Eastern countries, a significant portion of the organ requirements are provided from the living donor pool[1,2].In deceased donor liver transplantation, whole size liver graft is harvested with the inferior vena cava (IVC) and then venous anastomosis can be performed easily between the IVC of the liver graft and IVC of the recipient using conventional, piggyback, or modified piggyback techniques[1,2].In contrast, variations in the vascular structure of the liver graft obtained from a living liver donor (LLD) cause difficulties during vascular reconstruction in LDLT, especially hepatic venous reconstruction.Venous drainage of the right lobe (RL) is more complex compared to the left lobe of the liver.To both benefit from liver graft optimally and avoid congestion-related complications, all large venous structures including inferior right hepatic vein (IRHV), segment 5 vein (V5) and segment 8 vein (V8) should be integrated into the venous drainage system[3].In other words, meticulous assessment of the vascular structures of the LLD candidates by preoperative radiological instruments and thus identification of variations is critical for both LLDs safety and planning of graft implantation techniques.
To evaluate the hepatic vascular structures of LLD candidates, Doppler ultrasonography (US), multidetector computed tomography (MDCT) and, if necessary, conventional hepatic angiography are the most commonly used techniques[2].Variations detected in the liver vascular anatomy of the potential LLD candidates either result in rejecting the candidate or the surgical team considers alternative surgical techniques such as various venous drainage models.
Congenital absence of the right hepatic vein (RHV) is one of the rarest hepatic vascular anomalies.This anomaly is usually associated with multiple large IRHVs or wider middle hepatic vein (MHV) tributaries.To our knowledge, no clinical studies or case reports related to this RHV anomaly have been published in the English literature except autopsy studies.To our knowledge, a successful LDLT using the RL liver graft without the RHV was performed by our clinic for the first time in the world[2].After that, Ray and colleagues reported that they performed successful LDLT using a RL liver graft without a RHV orifice.Herein, we present hepatic venous drainage reconstruction models of RL liver grafts obtained from two LLDs with congenital RHV anomalies.
CASE PRESENTATION
Chief complaint and history of present illness
Case 1:A 25-year-old healthy male (BMI: 20.2 kg/m2, total liver volume: 1136 cc, RL: 786 cc, remnant liver: 34%) was admitted to our liver transplant institute to give a part of his liver to his 26-year-old sister with Budd Chiari Syndrome.He had no chronic disease.
Case 2:A 31-year-old healthy male (BMI: 23.7 kg/m2, total liver volume: 1428 cc, RL: 1000 cc, remnant liver: 30%) was admitted to our liver transplant institute to give a part of his liver to his 56-year-old uncle with alcoholic liver cirrhosis.
Physical examination
Case 1 and Case 2:Physical examination revealed that vital signs were within normal limits.The LLD candidates were examined according to the donor evaluation algorithm applied in our liver transplant institute.
Laboratory and imaging examinations
Case 1:Biochemical blood tests and viral markers were within normal limits.Contrastenhanced MDCT showed that the RHV was rudimentary and that the RL was drained by three IRHVs, one of them was located in the hepatocaval ligament.As our institute is experienced in RL drainage models, cadaveric organ donation was insufficient, and the recipient could not provide another potential donor candidate; thus, we decided to accept the LLD candidate.
Case 2:The potential LLD was examined according to the donor evaluation algorithm applied in our institute.Contrast-enhanced MDCT showed congenital absence of the RHV and that the RL was drained by two large IRHVs.
FINAL DIAGNOSIS
Case 1
The healthy individual who had a rudimentary RHV was accepted as a suitable LLD candidate.
Case 2
The healthy individual who had no RHV was accepted as a suitable LLD candidate.
TREATMENT
Case 1
RL hepatectomy was performed as previously described in our institute.Three IRHVs of 5-6 mm diameter, which drained the RL into the IVC, were preserved until the parenchymal transection was completed.Parenchymal transection was performed using the CUSA (Cavtron Ultrasonic Surgical Aspirator, Integra, United States) without Pringles maneuver.During transection, two V5 and one V8 were marked and preserved to be integrated into the venous drainage model.Bloodless RL graft volume and graft-recipient weight ratio were measured as 765 g and 1.03%, respectively.
Case 2
RL hepatectomy was performed as previously described in our institute, all three IRHVs were preserved until the parenchymal transection was completed and transection was performed using the CUSA without Pringles maneuver (Figure 1).During transection, two V5 and two V8 were marked and preserved to be integrated into the venous drainage model.Bloodless RL graft volume and graft-recipient weight ratio were measured as 1000 g and 1.02%, respectively.The drainage model was found to be successful by postoperative MDCT.Finally, the recipient was discharged without postoperative complications.
综上所述,结核合并感染的CNS对比非结核感染的CNS,特别是MRCNS,PCR法与MIC法相互印证其准确性,而结核合并感染的MRCNS引发的多重耐药特性比非结核合并感染的MRCNS更强。耐药基因mecA是MRS的金标准,它不但可在同种细菌内传播,更可通过不同种类细菌间传播,必须警惕其严重的耐药性。
OUTCOME AND FOLLOW-UP
Case 1
The LLD had an uneventful postoperative clinical course.The drainage model was found to be successful by postoperative MDCT.Finally, the recipient was discharged on postoperative day 21 without complications.

Figure 1 Dissection plan between the right lobe of the liver and inferior vena cava.
Case 2
The LLD had an uneventful postoperative clinical course.The drainage model was found to be successful by postoperative MDCT.Finally, the recipient was discharged with minimal biliary complications.
DISCUSSION
Definition of back-table reconstruction techniques for both patients
The perfusion and washing of the liver grafts with preservation solutions on the backtable stage were performed as described previously[2].Using the cryopreserved vascular graft materials, a common large opening drainage model was created to include three IRHVs and V8.The rudimentary RHV was also integrated into the drainage model.For this common large opening drainage model, an aortic vascular graft was used as a quilt, while a saphenous vein graft was used to both create a circumferential fence and extend V8 to the main drainage model.Both V5 orifices on the cut surface were first created as a single orifice and then anastomosed directly to the recipient's left hepatic vein stump using an expanded polytetrafluoroethylene vascular graft (Figures 2 and 3).The liver implantation techniques used in both cases were not different from other LT recipients with normal RHV.Postoperative US and MDCT were performed to determine whether the venous drainage model was successful in both recipients (Figure 4).
LDLT has been expanded to overcome the graft shortage and disparity between supply and demand in patients on the LT waiting list.However, unlike a whole size deceased donor liver graft, most of the living liver grafts require reconstruction of the venous structures including RHV, IRHVs and MHV tributaries to restore venous drainage of the corresponding segments to prevent any postoperative congestion.Hepatic venous structures may be delineated using modern imaging techniques: Doppler US, MDCT, and conventional angiography are particularly useful for observing the venous structures.Variations or congenital anomalies in hepatic venous structure in LLD candidates can disqualify the candidate or alter surgical choice.One such hepatic venous anomaly is congenital absence of the RHV or a rudimentary RHV.As vascular anomalies and variations in LLD candidates may cause unexpected complications and difficulties, these vascular anomalies and variations must be evaluated and documented clearly by imaging techniques before surgery.In our cases, the rudimentary or congenitally absent RHV and presence of the IRHVs were identified easily on preoperative MDCT, which allowed us to plan the surgery.
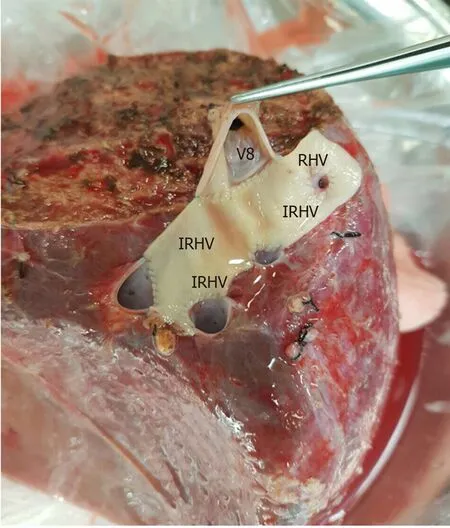
Figure 2 The cryopreserved aortic vascular graft patch was placed between the four orifices as a quilt.

Figure 3 A common large opening drainage model was created using the cryopreserved saphenous vein graft.
Difficulties in hepatic venous drainage in LDLT has been addressed by many studies with many technical considerations and modifications investigated[1-12].While controversy exists regarding the ideal criteria and method of incorporating the IRHVs into the graft’s drainage system and the ideal method of draining segments 5 and 8, it is agreed that venous congestion due to inadequate outflow reconstruction impairs regeneration, and is associated with increased complications including graft loss[8,12].
In our patients with absent or rudimentary main RHV and the presence of multiple major IRHVs, a common large opening drainage model allowed for a wider ostium, which achieved faster and easier anastomosis with the IVC reducing the warm ischemia time with an ostium tolerating compression with less risk of compression and obstruction.We reported a similar case from the same center in 2013, but did not find similar reported cases in the English literature[2].One case was reported with a liver transplant in the absence of a RHV ostium with the right hepatic vein present and drained into the IVC through a single ostial opening by the middle and left hepatic veins, in that case a subtotal MHV was to be taken leaving behind the proximal MHV with drainage of the segment 4b and RHV vein into it, as the patient’s RHV joined the MHV intra-hepatically[13].
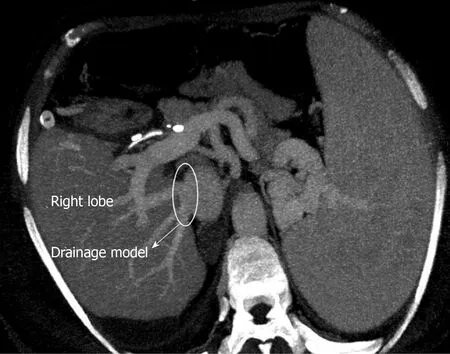
Figure 4 Postoperative contrast axial multidetector computed tomography image shows that the venous drainage model is functional.
The venous outflow reconstruction is technically challenging for RL liver grafts with an undrained anterior sector, along with the presence of multiple IRHVs with vulnerability of congestion if not adequately reconstructed.Adding to the complexity is the presence of a wide variability in the pattern of branching of hepatic veins, difficulty in determining the optimal anastomotic site and direction especially in the presence of major IRHVs to anastomose which requires further time[14,15].Authors recommend that short hepatic veins with a diameter ≥ 4 mm should be integrated into the drainage system, which is our approach[2,3,15].The need for IRHVs to be integrated into the drainage system is even more essential in the absence of adequate drainage through the RHV due to its absence or in cases where it is rudimentary, where in these cases the major IRHVs dominate the venous outflow.
A common large opening reconstruction technique diminishes morbidity as well as potential mortality associated with compromised graft outflow and has been proved to be safe[1,4-6].A single, wide orifice is achieved by various venoplasty techniques during back-table procedures using cryopreserved conduits, or the recipient's saphenous vein, or synthetic vascular grafts[3,5-7].The technique used to perform a back-table venoplasty to form a single, large orifice remains an easy procedure without added risks[5].Also, in the presence of dense adhesions due to previous surgeries, reduced available length of IVC, and multiple collaterals, the outflow reconstruction becomes technically less complex with this technique in addition to reducing the warm ischemia time with one single anastomosis to the IVC.
With regard to the MHV tributaries, which drain the central region of the liver, our approach is to leave the MHV in the donor’s side in cases without a segment 4b vein.In cases with a segment 4b vein, the decision to include the MHV in the graft is made with respect to the remnant liver volume.If the remnant liver volume is ≤ 30%, the MHV should be left in the donor’s side.If the remnant liver volume is > 30%, the decision is made with respect to the diameters of veins draining segment 5 and 8[2,6].
CONCLUSION
In conclusion, rudimentary or congenital absence of RHV is not an absolute contraindication for RL-LDLT in centers with experience in venous outflow reconstruction and various drainage models.However, it is important to meticulously examine the vascular structures of donor candidates using preoperative radiological instruments.
猜你喜欢
杂志排行
World Journal of Hepatology的其它文章
- Diagnosis and management of hepatic artery in-stent restenosis after liver transplantation by optical coherence tomography: A case report
- Effect of zinc treatment on clinical outcomes in patients with liver cirrhosis: A systematic review and meta-analysis
- Non-alcoholic steatohepatitis and the risk of myocardial infarction: A population-based national study
- Anti-inflammatory and anti-oxidant effects of aloe vera in rats with non-alcoholic steatohepatitis
- Ipragliflozin-induced improvement of liver steatosis in obese mice may involve sirtuin signaling
- Oxidative stress in alcohol-related liver disease
