Subclinical proximal tubulopathy in hepatitis B: The roles of nucleot(s)ide analogue treatment and the hepatitis B virus
2021-01-13AnaBrayetteMarieEssigPaulCarrierMarilyneDebetteGratienAnaLabrunieSophieAlainMarianneMaynardNathalieGanneCarriEricNguyenKhacPaulinePinetVictorDeLedinghenChristopheRenouPhilippeMathurinClaireVanlemmensVincentDiMartinoAnne
Anaïs Brayette, Marie Essig, Paul Carrier, Marilyne Debette-Gratien, Anaïs Labrunie, Sophie Alain, Marianne Maynard, Nathalie Ganne-Carrié, Eric Nguyen-Khac, Pauline Pinet, Victor De Ledinghen, Christophe Renou,Philippe Mathurin, Claire Vanlemmens, Vincent Di Martino, Anne Gervais, Juliette Foucher, Fouchard-Hubert Isabelle, Julien Vergniol, Isabelle Hourmand-Ollivier, Daniel Cohen, Xavier Duval, Thierry Poynard, Marc Bardou, Armand Abergel, Manh-Thong Dao, Thierry Thévenot, Jean-Baptiste Hiriart, Valérie Canva,Guillaume Lassailly, Christine Aurières, Nathalie Boyer, Dominique Thabut, Pierre-Henri Bernard, Matthieu Schnee, Dominique Larrey, Bertrand Hanslik, Séverine Hommel, Jérémie Jacques, Véronique Loustaud-Ratti
Anaïs Brayette, Paul Carrier, Marilyne Debette-Gratien, Jérémie Jacques, Véronique Loustaud-Ratti, U1248 INSERM, Department of Hepatology and Gastroenterology, Univ.Limoges, CHU Limoges, Limoges F-87000, France
Marie Essig, U1248 INSERM, Department of Nephrology and Transplantation, CHU Limoges, Limoges F-87000, France
Anaïs Labrunie, Department of Center of Epidemiology, Biostatistics and Research Methodology, CHU Limoges, Limoges F-87000, France
Sophie Alain, U1092 INSERM, Department of Virology, CHU Limoges, Limoges F-87000, France
Marianne Maynard, Department of Hepatology, Croix-Rousse University Hospital of Lyon, Lyon 69004, France
Nathalie Ganne-Carrié, Department of Hepatology, Jean Verdier University Hospital of Bondy, Bondy 93140, France
Eric Nguyen-Khac, Department of Hepato-Gastroenterology, Amiens University Hospital, Amiens 80054, France
Pauline Pinet, Department of Infectious Diseases, CHU Limoges, Limoges F-87000, France
Victor De Ledinghen, Juliette Foucher, Julien Vergniol, Jean-Baptiste Hiriart, Department of Hepatology, Haut Leveque Hospital, Bordeaux University Hospital, Pessac 33604, France
Christophe Renou, Department of Gastroenterology, Hyeres Hospital, Hyeres 83407, France
Philippe Mathurin, Valérie Canva, Guillaume Lassailly, Department of Hepato-Gastroenterology, Claude Huriez University Hospital, Lille 59037, France
Claire Vanlemmens, Vincent Di Martino, Thierry Thévenot, Department of Hepatology, Jean Minjoz University Hospital, Besançon 25030, France
Anne Gervais, Xavier Duval, Department of Infectious Diseases, Bichat University Hospital, Paris 75018, France
Fouchard-Hubert Isabelle, Department of Hepatology, University Hospital of Angers, Angers 49933, France
Isabelle Hourmand-Ollivier, Manh-Thong Dao, Department of Hepato-Gastroenterology and Nutrition, University Hospital of Caen, Caen 14033, France
Daniel Cohen, Department of General Medecine, University Hospital of Caen, Caen 14000, France
Thierry Poynard, Dominique Thabut, Department of Hepatology, La Pitié-Salpêtrière University Hospital, Paris 75651, France
Marc Bardou, Department of Hepatology and Gastroenterology, Dijon University Hospital, Dijon 21079, France
Armand Abergel, Department of Hepatology and Gastroenterology, Estaing University Hospital, Clermont Ferrand 63003, France
Christine Aurières, Nathalie Boyer, Department of Hepatology, Beaujon University Hospital, Clichy 92110, France
Pierre-Henri Bernard, Department of Hepatology, Saint-André University Hospital, Bordeaux 33000, France
Matthieu Schnee, Department of Hepatology and Gastroenterology, La Roche-Sur-Yon Hospital Center, La Roche-Sur-Yon 85000, France
Dominique Larrey, Department of Hepatology and Gastroenterology, University Hospital of Montpellier, Montpellier 34295, France
Bertrand Hanslik, Department of Addictology, Hospital of Montpellier, Montpellier 34295, France
Séverine Hommel, Department of Hepatology and Gastroenterology, Hospital Center of Aix en Provence, Aix-en-Provence 13100, France
Abstract
Key Words: Hepatitis B virus; Proximal tubulopathy; Biomarkers; Renal insufficiency; Nucleoside analogues
INTRODUCTION
Chronic hepatitis B virus (HBV) infection is associated with significant morbidity and mortality due to cirrhosis and hepatocellular carcinoma[1,2].Current second-generation antiviral agents are efficient, as they have a high barrier to resistance.They include nucleosidic [e.g., entecavir (ETV)] and nucleotidic [e.g., tenofovir disoproxil (TDF) and tenofovir alafenamide (TAF)] analogues.Nevertheless, the persistence of HBV within hepatocytes in the form of covalently closed circular deoxyribonucleic acid and the low probability of hepatitis B surface antigen (HBsAg) clearance necessitates long-term or even life-long treatment.Currently available antiviral agents are eliminated in an active formviaglomerular filtration and active tubular secretion.Their dosages must be adjusted when the estimated glomerular filtration rate (eGFR) falls under 50 mL/min/1.73 m².Therefore, long-term renal tolerance to antivirals is an important issue.
Although tubular toxicity is well-described in human immunodeficiency virus (HIV) patients treated with TDF[3-5], less data exist for hepatitis B monoinfection.Registration trials report good tolerance profiles, but real-life studies recount cases of lactic acidosis with ETV treatment, and impaired renal function and rare cases of Fanconi syndrome are reported with TDF[6-8].Indeed, these two compounds weakly inhibit host mitochondrial polymerase and may induce tubulopathy[9].TDF toxicity may also result from tubular secretion of its active form (tenofovir) and its potential interaction with the metabolism of tubular cells[3,4,9].Furthermore, transport proteins may interact with TDF, increasing its intracellular concentration and consequently, its toxicity[10-12].Long-term consequences of tubular dysfunction include hypophosphatemia (secondary to hyperphosphaturia), osteomalacia, osteoporosis, and renal failure.
Most studies on nucleot(s)ide analogue (NA) renal toxicity are based on assessments of eGFR and phosphatemia, which are late markers of proximal tubulopathy.Various early markers are available (e.g., non-diabetic glycosuria, hyperaminoaciduria, β2-microglobulinuria, and cystinuria), but no consensus exists on their use[3,4,13,14].
In this study, two early, easy-to-perform, and inexpensive markers were selected: Maximal tubular reabsorption of phosphate per unit volume of eGFR (TmPi/eGFR) and fractional excretion rate of uric acid (FEUA).The objective was to detect and monitor the evolution of subclinical proximal tubulopathy (SPT) over a 2-year period in three populations of HBV-monoinfected patients.The three populations included those who were treatment naïve or those starting treatment with either ETV or TDF.
MATERIALS AND METHODS
Patient selection
A prospective, non-randomized phase IV study involving 20 French centers was conducted.Adult patients with HBV monoinfection and an eGFR above 50 mL/min/1.73 m2were included.They were separated into three populations: Naïve, ETV treatment, or TDF treatment, depending on the investigator’s choice.The following exclusion criteria were employed in this study: Patients already receiving the planned treatment; those who have hepatocellular carcinoma; those coinfected with the hepatitis C virus, hepatitis D virus, or HIV; those with serum phosphate levels < 0.48 mmoL/L; and pregnant or breast-feeding women.
Data collection
On day 0 (D0), data on the following characteristics were collected: Age, gender, ethnicity, body mass index (BMI), potentially nephrotoxic treatments (e.g., diuretics, non-steroidal anti-inflammatory drugs), prior anti-HBV treatment, viral load, and fibrosis stage.On D0 and then every 3 mo thereafter until month 24 (M24), the eGFR, phosphatemia, 25-hydroxyvitamin D3 [25(OH)D3] vitamin levels, and dipstick test levels were measured.The TmPi/eGFR and FEUA were calculated.Patients with serum 25(OH)D3 vitamin < 30 ng/mL were supplemented systemically.
The monitoring visits were planned according to the patients’ usual follow-up appointments.Treatment choices and any modifications made during the study complied with the recommendations made by the European Association for the Study of the Liver in 2012[15].
TmPi/eGFR and FEUA calculations
The main objective of this study was to determine the prevalence of SPT at M24 in the three groups.SPT was defined as a TmPi/eGFR below 0.8 mmoL/L and/or FEUA above 10%.
TmPi/eGFR was estimated according to Bijvoet’s diagram and included serum and urine phosphate and creatinine measured from fasting morning blood and urine samples.The eGFR was estimated with a simplified Modification of Diet in Renal Disease formula.FEUA was calculated as follows: [(urine uric acid × serum creatinine)/(serum uric acid x urine creatinine)] × 100%.If data at inclusion (M0) and M24 were missing, M3 and M21 data were used, respectively.
Prevalence and incidence data
The prevalence of SPT resulting from anti-HBV treatment prior to inclusion, if any, was retrospectively described.
At M24, the prevalence of eGFR < 50 mL/min/1.73 m2or serum phosphate < 0.48 mmoL/L and the cumulative incidence of SPT were calculated.High urine calcium defined by a urine calcium/blood calcium ratio above 0.5 mmoL/mmoL was used as a marker of bone involvement at M24.
Ethical considerations
The study was conducted in full compliance with the European and French guidelines of good clinical practices.It was approved by the French Institutional Review Board and the Independent Ethics Committee of Limoges.The study is registered with ClinicalTrials.gov under the number NCT01500265.Eligible patients were given information describing the study in readily understandable language detailing the investigational nature of the study.All patients gave written informed consent for study participation and blood sample conservation.
Statistical methods
Statistical analyses were performed by the Methodological, Epidemiological, and Biostatistical Research Center of the University Hospital of Limoges, using SAS V9.3®software (SAS Institute; Cary, NC, United States).The packagesurvivalin R v3.2.2 software was used for survival analyses.
Quantitative variables were described using means and standard deviations, medians, and interquartile ranges.Analyses of variance or Kruskal-Wallis tests were used to compare treatment-naïve patients to ETV- and TDF-treated patients.
Qualitative variables were described using the numbers and percentages associated with their 95% confidence intervals (CIs).They were compared using chi-squared or Fisher’s exact tests.These tests were also performed to compare the prevalence of SPT at inclusion between previously-treated patients and patients who had not received any antiviral treatment before inclusion, as well as the M24 prevalence of renal insufficiency, hypophosphatemia, or hypercalciura, depending on the occurrence of SPT during follow-up.
Pvalues less than 0.05 were considered to denote significance except for the main objective and the differences between the naïve group and each treatment group at inclusion, which were deemed significant atP< 0.025.
The SPT-free survival curves of the different groups over the 24 mo were plotted using the Kaplan-Meier method.The log-rank test was used to compare survival curves between the groups.
Analyses were adjusted to account for potential confounders.For the main analysis (i.e.prevalence of SPT at M24) a multivariate binary logistic regression model was used, whereas a Cox model was used for the cumulative incidence of SPT.The models included variables associated withPvalues of less than 0.2 in the univariate analysis; variables were strained using the step-by-step method.
RESULTS
Study population
Data were obtained from 214 patients between December 2011 and December 2013; 18 were excluded from the analysis (Figure 1).The final dataset was compiled from 196 patients: 116 in the naïve group, 38 in the ETV group, and 42 in the TDF group.
Prevalence of SPT at baseline with or without previous HBV treatment
Of the 196 patients analyzed, 22 (11.2%) had received previous HBV therapy: Adefovir (36%), lamivudine (27.3%), or both (36.7%).At baseline, 40 patients (22.5%) presented with SPT.SPT prevalence did not differ significantly between previously treated and untreated patients (21.5%vs30%, respectively;P= 0.40).
SPT prevalence at M24
Forty patients met the criteria of SPT at D0.Eighteen patients with incomplete biological reports, including at D0, were further excluded.The final number of patients with no SPT at D0 was 138: 84 in the naïve group, 28 in the ETV group, and 26 in the TDF group (Figure 1).Clinical and para-clinical characteristics of these 138 patients at inclusion are summarized in Tables 1 and 2.

1Chi2 test.2Mann-Whitney test.3Fisher’s exact test.4Evaluated by liver biopsy or FibroScan.5METAVIR classification.BMI: Body mass index; ALAT: Alanine aminotransferase; ETV: Entecavir; HbeAG: Hepatitis B e-antigen; HBV: Hepatitis B virus; Max: Maximum; Min: Minimum; Q1: First quartile; Q3: Third quartile; TDF: Tenofovir disoproxil.
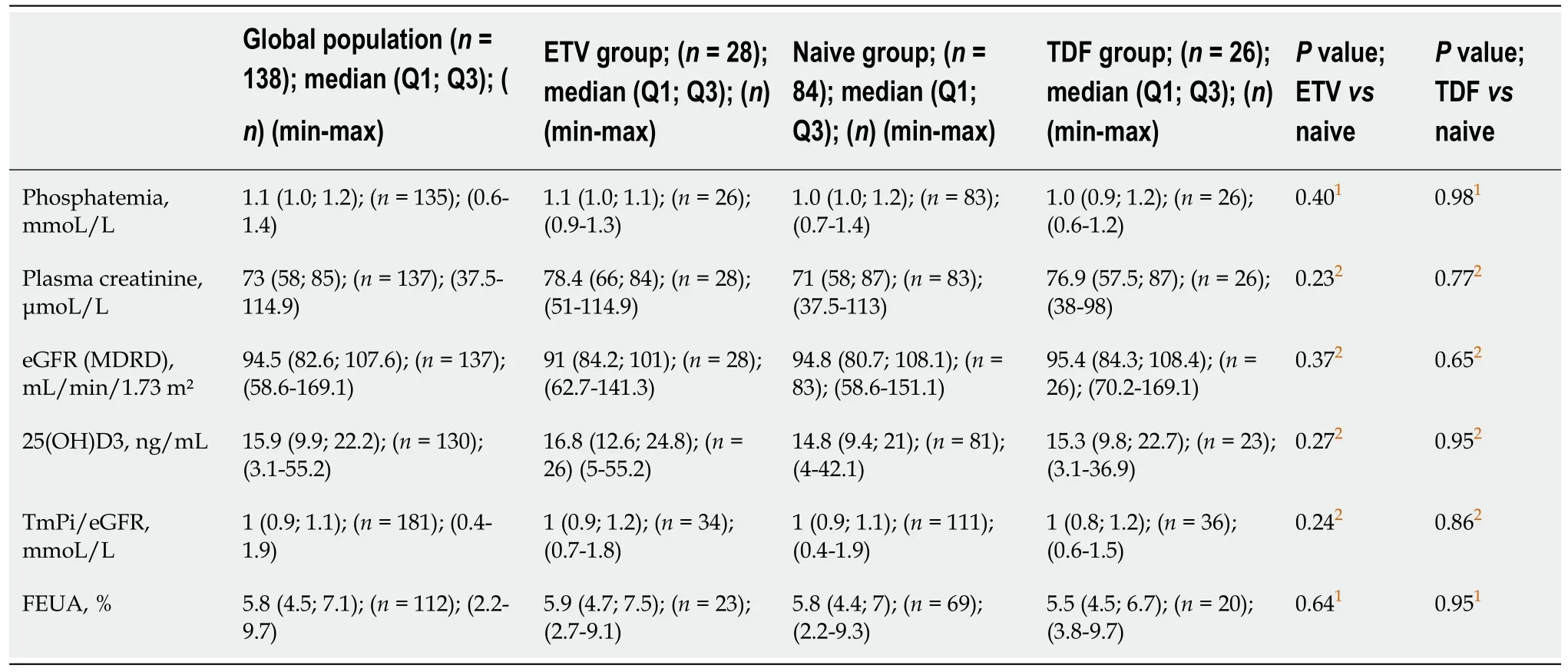
Table 2 Clinical characteristics of the patients with no subclinical proximal tubulopathy at on day 0
Statistically significant differences in chronic hepatitis (vsinfection), HBsAg-status, viremia levels, alanine aminotransferase (ALT) levels, and fibrosis stage were found between the treated groups (ETV or TDF)vsthe naïve group.Some unexpected differences were also observed.Compared to the naïve group, the ETV group contained more Asian patients, and patients in the TDF group had lower BMIs.These differences were accounted for in the adjusted analyses.
Of the 138 patients without SPT at baseline, 45 had missing data at M24 and had to be excluded from the analysis of SPT prevalence at that timepoint.Therefore, the main analysis included data from 93 patients, with 62 in the naïve group, 19 in the ETV group, and 12 in the TDF group.Accordingly, the overall prevalence of SPT at M24 was 31.2% (n= 29/93; 95%CI: 22.0–41.6).Among the three treatment groups, the prevalence was 30.7% (n= 19/62; 95%CI: 19.6–43.7) in the naïve group, 21.1% (n= 4/19; 95%CI: 6.1–45.6) in the ETV group, and 50% (n= 6/12; 95%CI: 21.1–78.9) in the TDF group.No statistically significant differences were observed between the naïve group and the ETV (P= 0.42) or the TDF group (P= 0.42) (Table 3).
Adjusted analyses of SPT prevalence at 24 mo
Potential confounding factors among the different groups were assayed at baseline: Age, gender, ethnicity, virological status, diabetes, hypertension, potential nephrotoxic drugs, ALT and viremia levels, fibrosis stage, and previous HBV therapy (Table 1).Ethnicity was not included in the model because no Asian patient had SPT at M24.Table 4 contains the results of the univariate models given as raw odds ratios (ORs).Variables associated withPvalues of less than 0.20 (gender and age) were tested in a multivariate model comparing ETV and naïve groups.The effect of group on the presence or absence of SPT at M24 was not affected by any adjustment variables (OR = 0.60; 95%CI: 0.17–2.06;P= 0.42).No multivariate model could be built to compare TDF and naïve groups (no variable had aPvalue less than 0.20).
Finally, group membership had no significant effect on the presence or absence of SPT at M24 (OR = 2.26; 95%CI: 0.65-7.93;P= 0.20).
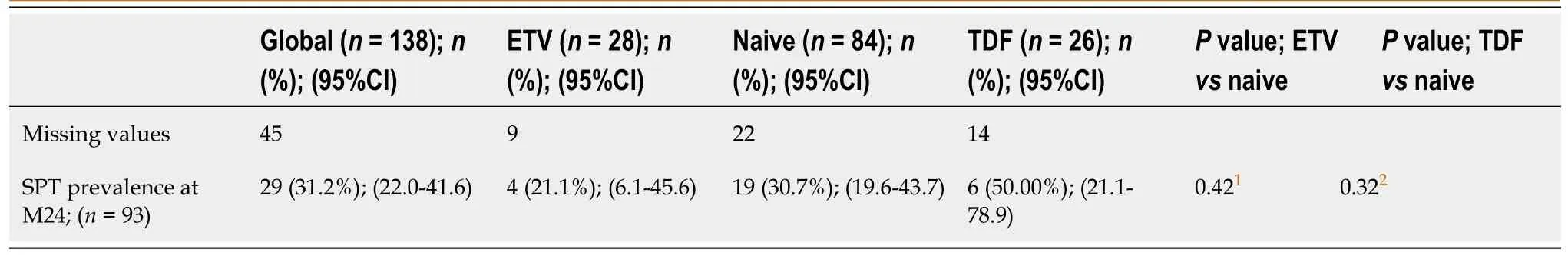
Table 3 Subclinical proximal tubulopathy prevalence at month 24 in the entecavir, naive and tenofovir disoproxil groups
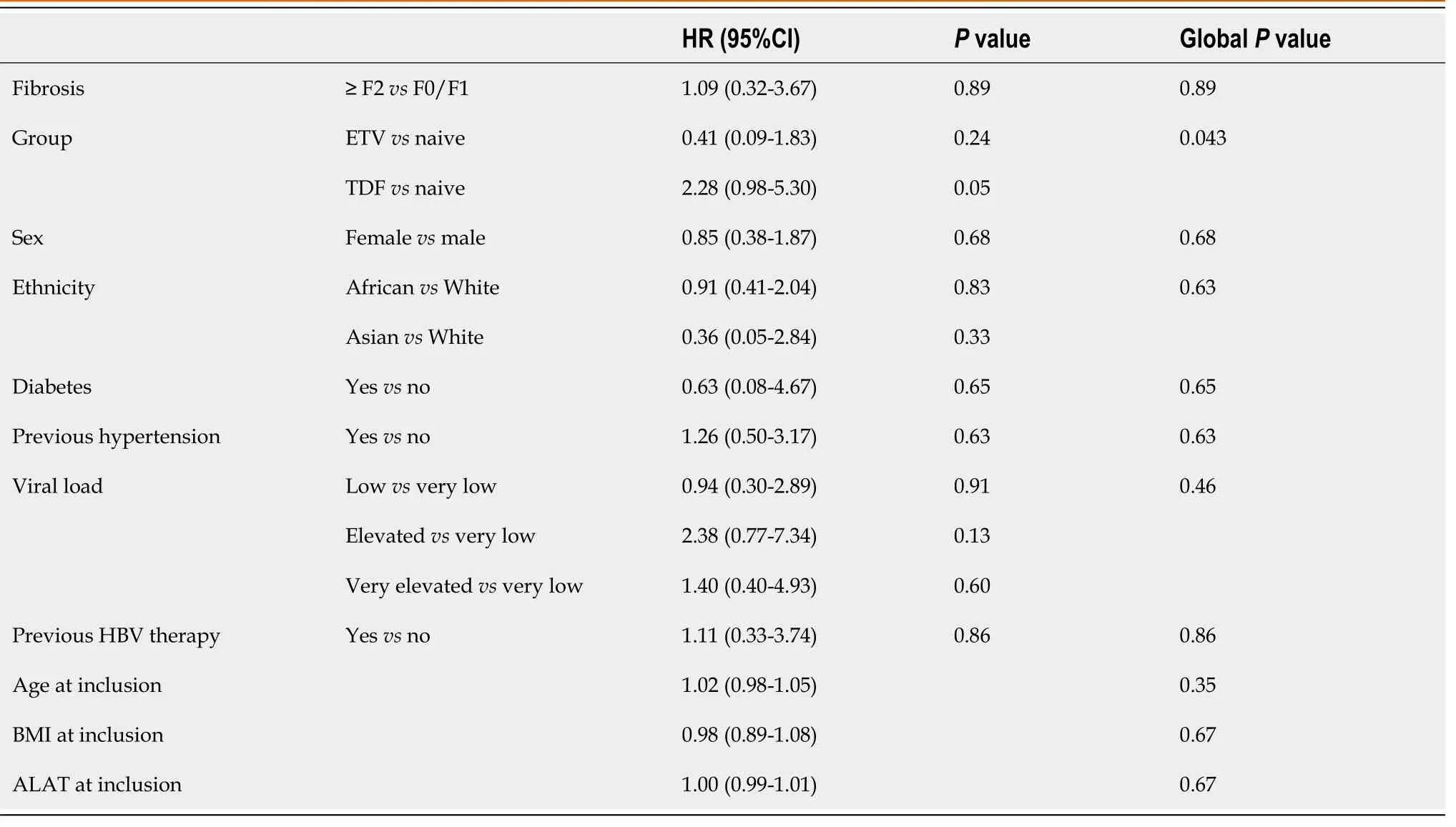
Table 4 Potential confounding factors at baseline susceptible to influence the prevalence of subclinical proximal tubulopathy at month 24 between the different groups in univariate analysis
Cumulative incidence of SPT over 24 mo
The overall survival rate of SPT-free patients at M24 was 52.2% (95%CI: 38.3-71.2).Among the three groups, the survival rates were 57.6% (95%CI: 47.1-79.6) in the naïve group, 68.8% (95%CI: 38.1-100) in the ETV group, and 23.5% (95%CI: 5.3-100) in the TDF group.
The median survival time, corresponding to the time during which more than 50% of the patients remained SPT-free, was analyzable only in the TDF group.The median survival time in this group was 5.9 mo.The occurrence of SPT in the TDF group differed significantly from that in the other two groups (log-rank test;P= 0.0283; Figure 2).
Adjusted analysis of cumulative incidence of SPT over 24 mo
No multivariate analysis was conducted as no potential confounding factors hadP< 0.20.The univariate model found no significant effects between ETV and naïve groups [hazard ratio (HR): 0.41; 95%CI: 0.09-1.83;P= 0.24].The HR associated with the TDF groupvsthe naïve group was 2.28 (95%CI: 0.98-5.30;P= 0.0546).Thus, TDF treatment tended to be associated with TDF-induced tubular toxicity.
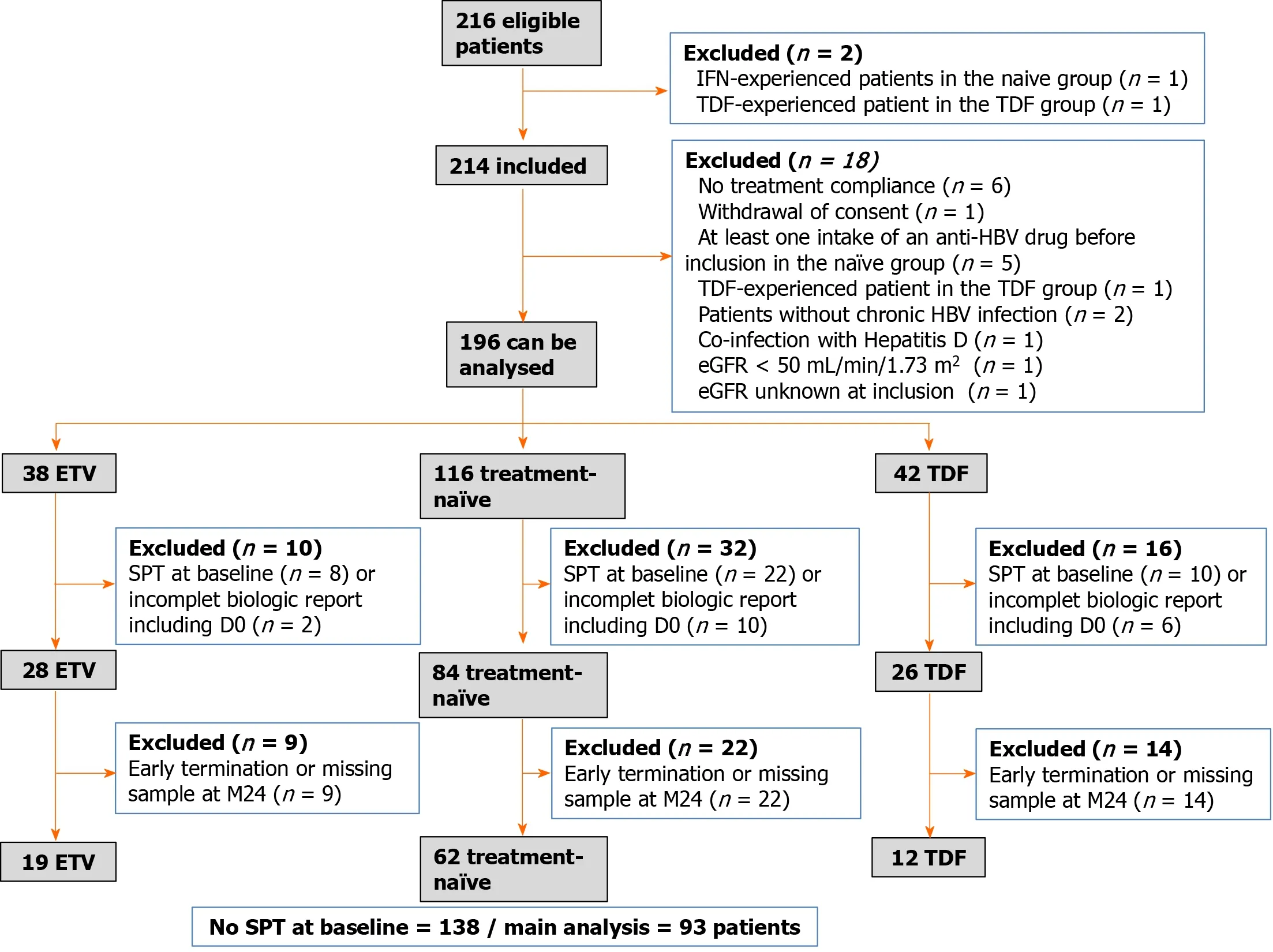
Figure 1 Data were obtained from 214 patients between December 2011 and December 2013; 18 were excluded from the analysis.
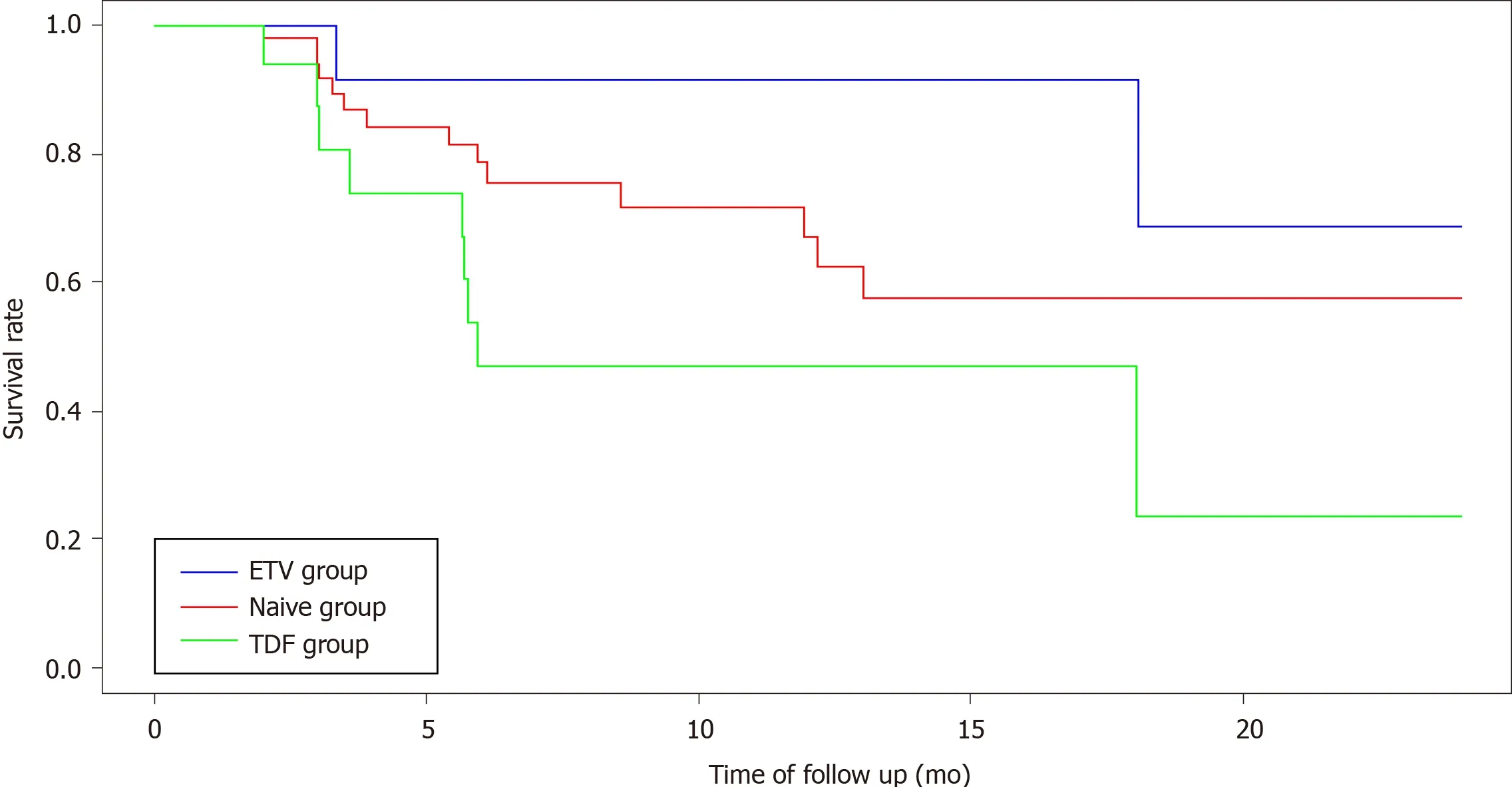
Figure 2 Kaplan Meier curves for free subclinical proximal tubulopathy survival among the different groups (entecavir, naive, tenofovir disoproxil).E
Prevalence of impaired renal function (eGFR < 50 mL/min/1.73 m2), hypophosphatemia (< 0.48 mmoL/L), and hypercalciuria (> 0.5 mmoL/mmoL) at M24
In patients without SPT at baseline, no renal function impairment or hypophosphatemia was observed at M24, regardless of whether they had developed SPT during follow-up.However, four patients (6.5%) experienced hypercalciuria at M24.Three (7.0%) did not develop SPT within 24 mo, whereas one (5.3%) developed SPT after M12 with simultaneous alterations of TmPi/eGFR and FEUA.This latter patient was an HBsAg-negative African female belonging to the naïve group and presented with hypertension and grade I obesity.
DISCUSSION
Most of the studies investigating the renal tolerance of NAs have focused on glomerular markers (serum creatinine and eGFR) instead of tubular markers[16-18].Although data on the tubular toxicity caused by TDF in HIV-positive patients are widely available[3-5], analogous data in HBV-monoinfected populations are sparse[3,4].
This paper reports on the first prospective, multicenter study that evaluated the prevalence and incidence of SPT for an extended duration (24 mo) using early markers in a population of HBV-monoinfected patients starting treatment with ETV or TDF.
A strong point of this study was the comparison of the treated groups with a control naïve group.The latter allowed for an evaluation of the role of HBV on tubular function in the absence of any treatments.Additionally, the effects of confounding factors on the interpretation of SPT prevalence or incidence were limited, as the patient population was homogeneous, relatively young (median age: 37.5 years), and had very few renal comorbidities.
Tubular markers
Optimal markers of proximal tubulopathy are not agreed upon the literature.The most commonly used markers, whether early or late, are increased urinary α1-microglobulin, urinary β2-microglobulin, urinary retinol binding protein (RBP) or mixed proteinuria, fractional phosphate or uric acid excretion, non-diabetic glycosuria, hypophosphatemia, hypouricemia, hypokalemia, aminoaciduria, and renal tubular acidosis[3,4,13].None of these markers have demonstrated superiority in terms of sensitivity and specificity.The more sophisticated markers such as RBP or β2-microglobulin are interesting, but they are expensive to analyze and not widely used.Kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin are markers of acute tubular injury, which is passed the early prevention stage[19].The markers chosen in this study, TmPi/eGFR and FEUA, are easy to use, inexpensive, repeatable over time, and thus ideal for routine follow-up.
TDF and SPT
In this study, the prevalence of SPT at M24 was higher in patients treated with TDF compared to naïve patients (50%vs30%).However, this difference was not statistically significant.Nevertheless, the HR for the cumulative incidence of SPT in the TDF groupvsthe naïve group was 2.28, with a trend towards significance and TDF-induced tubular toxicity.
In the literature, many study designs are heterogeneous.Two main studies used the same population as this one, except they were cross-sectional.In the first, Tienet al[20]compared the prevalence of SPT (defined as decreased TmPi/eGFR) in 146 HBVmonoinfected patients (60 naïve, 44 treated with ETV, and 42 treated with TDF), of whom fewer than 2% had an eGFR < 60 mL/min/1.73 m²[20].SPT prevalence was 30%, 23%, and 43% in naïve, ETV, and TDF patients, respectively.Differences among these groups were not statistically different.Nonetheless, in a subgroup of patients treated with ETV and TDF for more than 18 mo, the prevalence of SPT was significantly higher in the TDF-treated group than in the ETV-treated group (48.5%vs12.5%;P= 0.005).The second study was the multicenter "MENTE" study consisting of 280 HBVmonoinfected patients (122 naïve, 89 ETV, and 69 TDF), which reported an association between the TDF group and the presence of SPT[21].Here, the urinary RBP/ creatininuria ratio was used as an SPT marker[21].
In brief, no study has rigorously demonstrated a causal link between TDF and SPT or directly compared patients treated with TDF and ETV.Moreover, no other study has prospectively evaluated SPT incidence according to treatment type (i.e.naïve, ETV, and TDF).
ETV and SPT
The prevalence and cumulative incidence of SPT in the ETV-treated group compared to those in the naïve group were not significantly different.This negative result reinforces the good renal safety profile of ETV in humans and mouse models[22-24].Accordingly, Viganòet al[25]argued that SPT in TDF-treated patients improved after switching to ETV[25].
HBV and SPT
As previously highlightedin vitro, HBV-specific tubular toxicity may result from HBV replication and transcription activity in proximal tubular cells.In tubular cell cultures, the serum of infected patients had potential apoptotic effects[20,21,26,27].Detection of SPT in our HBV-monoinfected naïve patients supports this hypothesisin vivo.
A limitation of this study is the absence of a matched control population not infected with HBV.However, the tubular markers chosen here had been in use for many decades and validated in populations of healthy subjects.For instance, the adult 95% reference range for TmPi/eGFR is 0.80–1.35 mmoL/L.Independent of age, normal values are above 0.8 mmoL/L in healthy subjects[28].The normal value of FEUA is approximately 8%; values above 10% are considered to reflect a reabsorption defect[29].Consequently, using as a reference the normal values as defined in healthy populations, the observation that nearly 30% of the naïve HBV-monoinfected population met the definition of SPT implies a link between SPT and HBV infection.
Renal insufficiency, hypophosphatemia, and hypercalciuria
In this study, SPT screened at baseline or during follow-up in the low renal risk population did not impact eGFR, phosphatemia, and urinary calcium at M24 of NAtherapy.Data from the literature are highly variable due to the heterogeneity of the populations in terms of renal risk factors, age, pre-existing renal insufficiency, concomitant nephrotoxic drugs, and/or HIV co-infection[6,16,18].
In contrast to the results reported here, Tienet al[20]found that the eGFR was lower in the ETV- and TDF-treated groups compared to the naïve group (P= 0.002) but not significantly different between the ETV- and TDF-treated groups[20].However, the decline in eGFR correlated with age and not with antiviral treatment.Further, their study design did not allow for any conclusions regarding an association between the observed reduction in eGFR and changes in tubular function (TmPi/eGFR).
In a prospective, single-center study, Viganòet al[30]evaluated the prevalence and incidence of hypophosphatemia and hyperphosphaturia within a median duration of 27 mo in 156 NA-naïve patients receiving TDF[30].During the follow-up, hyperphosphaturia appearedde novoin 26% of the patients, of whom only 4% developed mild hypophosphatemia (≤ 2.5 mg/dL)[30].None of the hypophosphatemia patients developed a severe, diffuse stage of tubulopathy that is characteristic of Fanconi syndrome.
The occurrence of hypophosphatemia following a correction of 25(OH)D3 deficiency reflects major perturbation in proximal tubular function in which compensatory mechanisms are exceeded.Cases of Fanconi syndrome are exceptional in HBV-monoinfected patients and have been described only with nucleotide analogues (e.g., adefovir and exceptionally, TDF)[31-33].Regarding bone toxicity, the "MENTE" study failed to find a clear association between SPT and abnormal markers of bone remodeling[21].
In summary, the few studies focusing on SPT following NA treatment are mainly cross-sectional and consequently do not allow for the long-term evaluation of their effects on renal and bone health.This prospective study suggests that, in low renal risk patients, SPT does not have clinical impacts on renal or bone health at M24.
25(OH)D3 insufficiency
In this study, the prevalence of 25(OH)D3 insufficiency and severe deficiency was 66.9% and 25.4% at baseline and 84.7% and 7.1% at M24, respectively, despite iterative supplementation.These results are very similar to those reported in the literature.In the Maggi study, which evaluated renal and bone toxicity in chronic hepatitis B patients treated with lamivudine and adefovir, the prevalence of vitamin D insufficiency and severe deficiency was 72.2% and 20.4%, respectively[34].Vitamin D insufficiency is common in chronic liver disease irrespective of etiology[35].Additionally, 25(OH)D3 has been suggested to increase tubular reabsorption of phosphate, in particular by directly modifying the lipid structure of the cell membrane of proximal tubular cells[36].In line with this hypothesis, the patients in this study had their 25(OH)D3 levels measured and supplemented to limit renal phosphate loss and misinterpretation of TmPi/eGFR levels.
Limitations of the study
The main limitation of this study was the small number of patients who completed SPT markers follow-up.Also, some missing SPT markers were substituted with values from the nearest available date (< 3 mo).Moreover, the choice of the primary endpoint (TmPi/eGFR < 0.8 mmoL/L and/or FEUA > 10%) favored sensitivity over specificity.When the two markers, TmPi/eGFR < 0.8 mmoL/L and FEUA > 10%, were combined, the prevalence of SPT was 2.6%, 0%, and 9.5% in the naïve, ETV, and TDF groups, respectively, with no significant differences among the groups.
The absence of randomization could have generated a selection bias as baseline parameters potentially influencing renal function might not have been well-balanced in the treatment assignments, which were selected by the investigator.However, these potential confounders were limited in the overall population, which was characterized by a young age (median, 37.5 years) and very few renal comorbidities.
The dose-dependence of tubular toxicity caused by NAs could have been explored, especially with TDF.Unfortunately, TDF dosages were not readily available and were not recommended at the time of this study.Gene polymorphisms in the transporter proteins involved in TDF elimination (ABCC2 or ABCC4 genes) have been linked to renal tubular damage, implying that overexposure to TDF could cause kidney tubular cell damage.In HIV-infected patients, Rodríguez-Nóvoaet al[37]reported that median TDF plasma trough concentration was higher in patients with SPT as defined by the same early markers used in this study.However, even if this result implies cumulative toxicity, whether elevated TDF plasma concentration causes the development of SPT could not be determined due to their cross-sectional analysis.
The overexposure of tenofovir has so far been suggested but not proven in terms of the mechanism of toxicity.Indeed, the mechanism underlying tubular toxicity is probably not singular and could involve a cumulative dose effect; a recent paper proposed progressive mitochondrial dysfunction as a mechanism of TDF tubular toxicity[38].
TAF: An opportunity
TAF represents real progress in terms of renal tolerance, but it is not available in all countries for HBV-monoinfected patients, including France.It is similar to TDF, in that it is a tenofovir prodrug but has better renal and bone tolerance profiles, most likely due to its higher intracellular and much lower plasma concentrations.
Two recent randomized, double-blind phase 3 studies evaluated the utility of renal biomarkers in HBV-monoinfected patients treated with TAF or TDF.At 48 wk, glomerular and tubular proteinuria (RBP/creatininuria and β2-microglobulinuria/ creatininuria) was lower in the TAF group (percent change from baseline: 0.3%vs25.1%;P< 0.001 and -3.5%vs37.9%;P< 0.001, respectively)[39].The reversibility of SPT after TDF/TAF switching, as assayed with early tubular markers, remains unknown.
CONCLUSION
This prospective study did not find significant differences in SPT prevalence and incidence at M24 between low renal risk HBV-monoinfected patients treated with ETV or TDF and treatment-naïve patients.Nonetheless, the prevalence and incidence of SPT tended to be higher in the TDF group, which had a low survival time (5.9 mo) without SPT.The data presented here confirm that after 24 mo of NA therapy, patients exhibited a good renal safety profile irrespective of whether SPT was detected at baseline or during follow-up.However, these data should be treated with caution, as additional prospective studies involving large cohorts over several years are still warranted.
Current recommendations include monitoring phosphatemia, serum creatinine, and eGFR to screen renal toxicity, but these are late markers of tubular pathology.In clinical practice, proximal tubular damage would ideally be screened at an early stage using simple and inexpensive tools, especially in populations with renal risk (e.g., patients with hypertension or diabetes or who underwent kidney transplantation).Indeed, the detection here of SPT markers in some HBV-monoinfected patients prior to any antiviral treatment confirms the hypothesis that HBV exerts specific toxicity on proximal tubular cells.
It has been suggested that at 1 year after stopping treatment, SPT could be reversible in approximately 80% of cases[13].Finally, TAF is a promising agent and should be used preferentially, at least in patients at risk of renal toxicity.
ARTICLE HIGHLIGHTS
Research background
Proximal tubular renal toxicity is a main concern in prolonged nucleot(s)ide analogue therapy in hepatitis B virus (HBV)-infected patients.Currently available data for HBVmonoinfected patients are either retrospective or cross-sectional.The recommended screening tools for renal toxicity, estimated glomerular filtration rate (eGFR) and phosphatemia, are late markers for subclinical proximal tubular (SPT) damage.Thus,early SPT detection with tools that are simple, inexpensive, and repeatable over time are needed.Moreover, preclinical studies have reported that HBV exhibits potential toxicity in proximal tubular cells before any antiviral treatment.
Research motivation
Early detection of tubulopathy could allow clinicians to choose less toxic therapeutic alternatives such as tenofovir alafenamide (TAF), particularly in patients with renal comorbidities.TAF is not available in all countries for HBV-monoinfected patients, but its use may be transitionally authorized.Clinical evidence in favor of HBV-induced renal toxicity may assist in improving interpretations of SPT markers over time, as well as explain why these markers improve under antiviral use.
Research objectives
The main objective was to determine the prevalence of SPT at month 24 (M24) in three populations: Treatment-naïve patients and patients starting entecavir (ETV) or tenofovir disoproxil (TDF) at M0.The secondary objectives were to evaluate the cumulative incidence of SPT over 24 mo in the three groups as well as the prevalence of SPT in the naïve population at baseline.
Research methods
This first real-life, prospective, multicenter, French study of patients with low renal risk aimed to determine SPT in three groups of HBV-monoinfected patients:Treatment-naïve and those starting ETV or TDF.Markers for SPT, the eGFR and phosphatemia, were assessed quarterly.SPT was defined using early and low-cost simple markers: TmPi/eGFR below 0.8 mmoL/L and/or fractional excretion rate of uric acid above 10%.Confounding factors potentially impacting kidney function across the groups were assayed.
Research results
At M24, the prevalence of SPT was 30.7% in the naïve group, 21.1% in the ETV-treated group, and 50.0% in the TDF-treated group.However, differences in SPT prevalence between the naïve group and each treatment group (ETV and TDF groups) were not significantly different.In the multivariate analysis, no post-adjustment variables were identified.The incidence of SPT over 24 mo (25.5%, 13.3%, and 52.9% in the naïve,ETV-treated, and TDF-treated groups, respectively) tended to be higher in the TDF group compared to the naïve group (hazard ratio: 2.283; P = 0.05).The median survival time without SPT was 5.9 mo in the TDF group.In patients without SPT at baseline, no renal insufficiency or hypophosphatemia was observed at M24.
Research conclusions
This prospective, multicenter study is the first to evaluate the prevalence and incidence of SPT in low renal risk HBV-monoinfected patients using early markers.Patients were divided into treatment-naïve, ETV-treated, or TDF-treated groups.The prevalence of SPT at M24 was high (21%–50%), but it had no clinical impacts in terms of renal insufficiency or hypophosphatemia.The incidence of SPT tended to be higher in the TDF group.Moreover, the detection of SPT in HBV-monoinfected naïve patients supports the hypothesis of HBV-specific tubular toxicity.
Research perspectives
To better evaluate the clinical impacts of nucleot(s)ide analogue-induced SPT on renal function, future prospective studies tracking both simple and sophisticated SPT markers over a longer period of time are warranted.Furthermore and paradoxically,these early markers may be also used to evaluate treatment reversibility of HBVinduced SPT.
ACKNOWLEDGEMENTS
We would like to thank Céline Rigaud for her assistance with manuscript revision and Sarah Demay for proofreading the manuscript.
杂志排行
World Journal of Hepatology的其它文章
- Autophagy related protein 9A increase in hepatitis B virusassociated hepatocellular carcinoma and the role in apoptosis
- Hepatitis E virus re-infection accelerates hepatocellular carcinoma development and relapse in a patient with liver cirrhosis: A case report and review of literature
- Successful hepatic resection for recurrent hepatocellular carcinoma after lenvatinib treatment: A case report
- Safety and efficacy of sofosbuvir/velpatasvir/voxilaprevir in postliver transplant patients with previous direct-acting antiviral failure:Six case reports
- HIPPOCRATES® project: A proof of concept of a collaborative program for hepatitis C virus microelimination in a prison setting
- Effect of non-alcoholic beer, diet and exercise on endothelial function, nutrition and quality of life in patients with cirrhosis
