Research Progress on the Invasion Mechanism of Erigeron annuus
2021-01-03YunyunZHOUMinGUOXiuLIUGuofuFUDiZOUChenzhongJIN
Yunyun ZHOU Min GUO Xiu LIU Guofu FU Di ZOU Chenzhong JIN

Abstract Erigeron annuus (L.) Pers., an alien species, is widely distributed in most areas of China. It was included in the third batch of invasive alien species list by the Ministry of Ecology and Environment of China, and it is a first-level malignant invasive plant. Due to its great reproducibility with a mass of seeds, power adaptability to the external environment and strong allelopathy, it has highly invasive and adaptability, and has affected the development of the agriculture and forestry, the diversity of local species and the ecosystem. In this paper, we summarized the current invasion situation, invasion and adaptation mechanism of E. annuus , and prospected further research on E. annuus invasion mechanism, hoping to provide a reference for realizing its effective management.
Key words Erigeron annuus (L.) Pers.; Biological invasion; Adaptive mechanism
Received: July 15, 2021 Accepted: September 18, 2021
Supported by Outstanding Youth Project of Hunan Provincial Education Department (18B461); Hunan Science and Technology Innovation Program (2019NK4170).
Yunyun ZHOU (1986-), female, P. R. China, PhD, devoted to research about ecology and utilization of wild plant resources.
*Corresponding author.
Biological invasion is one of the important factors leading to the loss of biodiversity. Mastering the invasion mechanism can improve the ability of prevention, control and management of invasive alien species[1-2]. The successful invasion of an alien plant means that when a plant enters a new area from its natural distribution range, it colonizes, spreads, and grows explosively, which affects the local environment, species, community dynamics, and the stability of ecosystem structure and function and poses a serious threat to local life and ecology[3].
Erigeron annuus (L.) Pers. is native to North America. It is a one-year or two-year-old plant belonging to Erigeron of Compositae. It originally entered China as an ornamental plant and was included in the third batch of invasive alien species list by the Ministry of Ecology and Environment of China. It is a first-level malignant invasive plant and was included in China’s agricultural pest system in 2014[4-5]. In this paper, we summarized the current invasion situation, invasion and adaptation mechanism of E. annuus , and prospected further research on E. annuus invasion mechanism, hoping to provide a reference for realizing its effective management.
Current Situation and Prediction of the Invasion of E. annuus
According to records, E. annuus was collected for the first time in 1886 in Shanghai[5]. E. annuus easily spreads over long distances with media such as vehicles, goods, humans and animals. Human activities provide a bridge for E. annuus to travel across rivers and seas to leave the original environment and spread between regions. With the rapid development of international exchanges and world trade, more and more organisms have been intentionally or unintentionally transferred to vast areas of the world. The distribution range of E. annuus has spread from the original eastern China to central, southwest, northwest, and north China. Nowadays, E. annuus is almost all over the temperate and subtropical regions of China, and it grows widely on the roadside, in the wilderness or on the hillside[6].
E. annuus has not yet reached its saturation period in China, and it will continue to spread rapidly. The GARP niche model was used to predict its distribution in China, and it was found that its distribution range is still smaller than the predicted suitable area, and the areas except Guangdong and southwestern Guangxi, the Qinghai-Tibet Plateau, central and southern Xinjiang, most areas of Inner Mongolia, and the central and northern parts of Heilongjiang, are all suitable areas for the growth of E. annuus [7].
Biological Characteristics and Invasion Mechanism of E. annuus
Propagation and seed dispersal ability of E. annuus
E. annuus is mainly propagated by seeds, and the seed yield is large. Each plant produces more than 10 000 mature seeds in the growing season. The seeds mature quickly, and they have pappi, and are small and light. They can spread with the wind, and the spread area is large. They have an absolute advantage in the early growth of the following year. Its flowering period is long, from early June to mid-to-late August, lasting for more than two months. During this period, the seeds will mature and fall off, which is conducive to its reproduction and spread[5,8-9].
E. annuus can increase reproduction capacity by adjusting phenology. Usually, seeds are produced in autumn. After overwintering, the flowers are vegetative flowers, which rely on photosynthesis to accumulate energy, making them more competitive in seed survival rate than plants that germinate in spring[10-11]. Under the condition of increasing temperature, E. annuus increases reproduction by flowering in advance, increasing the amount of flowering, prolonging the duration of flowering, increasing the size and quality of its seeds, and adapts to rising temperatures by optimizing biomass allocation, thereby improving the adaptability and invasiveness of E. annuus [11]. The E. annuus populations distributed in Jiangsu and Zhejiang in eastern China invaded relatively earlier, and they have stronger growth ability and reproduction performance, which is reflected in plant height and the distribution of biomass in flowers[12]. Studies have shown that there are two genotypes in E. annuus , and it is found that there is a high degree of genetic differentiation ( GST of RAPDs=0.58, ISSRs of GST =0.64), indicating that E. annuus can quickly adapt to the new environment during its reproduction process, providing a theoretical basis for the successful invasion of E. annuus [13].
Morphological characteristics of E. annuus and its adaptability to the environment
The stalk of E. annuus is strong, upright, 30-100 cm high, with branches on the upper part; the basal leaves are oblong or broadly ovoid, rarely nearly round, and wither at the flowering stage; the lower leaves are in the same shape as the basal leaves, but the petiole is shorter; the middle and upper leaves are small, oblong-lanceolate or lanceolate; and the uppermost leaves are linear. Several or more capitula are arranged in an umbrella-shaped or conical shape, with tongue-shaped side flowers, a tube-shaped heart flower, and the flowering period is six or nine months; and the fruit is achene, narrowly obovate to oblong, about 1.5 cm in length, and has special pappi and membranous pericarp, and each piece of fruit contains 1 seed[5,14].
The phenotypic plasticity of invasive alien plants broadens ecological breadth through the pre-adaptive mechanism in the process of colonization and population establishment and the post-adaptive mechanism in the process of incubation and diffusion, and acts on the speed of evolution and natural selection, thereby enhancing the invasiveness of invasive alien plants[15-16]. In Zhenjiang, Jiangsu, the differences in functional traits and reproduction distribution between five species of invasive plants such as E. annuus and native plants and the successful invasion of invasive plants were studied. It was found that the plant height, number of branches, number of reproductive branches, ratio of underground biomass/aboveground biomass, and reproductive allocation index of invasive plants such as E. annuus were significantly higher than those of native plants, and they believed that these indexes might play an important role in the process of their successful invasion[17]. Through the analysis of the growth and reproduction characteristics of E. annuus in different regions of China (eastern Zhejiang, central Hubei, western Chongqing), the northern and southern locations, and at different altitudes (300-2 100 m) of Chongqing in the west, it was found that the plant morphology, biomass, and leaf functional traits of E. annuus had significant changes with changes in region, location and altitude. For example, the north-south direction had a significant impact on the plant height and number of basal branches of E. annuus . Region and altitude had a significant impact on the distribution of plant biomass. Below 1 200-1 400 m altitude, plant height and the biomass of flowers and stems increased with altitude, and the biomass of leaves decreased. Above 1 200-1 400 m altitude, the biomass allocation between stems and leaves changed, and as the altitude increased, the stem allocation ratio decreased, while the leaf allocation ratio increased significantly. The phenotypic plasticity of E. annuus maintained a relatively high adaptability to the environment.
Physiological characteristics and adaptability of E. annuus
E. annuus can adapt to a variety of environments, and E. annuus grows easily in places with fertile soil and sufficient sunlight, but can survive in places with poor soil such as cliffs and steep walls, and even in rocky cracks with sparse soil[18]. Photosynthesis is the physiological basis of plant growth and development, and is the carbon skeleton source of all organic matter produced in the plant life cycle[19]. In the study of the physiological and ecological characteristics of E. annuus and its companion species in Jilin Province, it was found that E. annuus showed the strongest average daily net photosynthetic rate among the companion species, and had better photosynthetic capacity, and therefore, it had an ability of growing faster and could form a dominant population easier and quickly form a canopy environment to gain an advantage in the competition with other plants.
After the formation of inflorescences, the photosynthesis of E. annuus reaches a peak. This period is the heyday of the vegetative growth of E. annuus , during which the leaf ratio is closest to the stem ratio, indicating that the ability of E. annuus to intercept light energy has reached a strong level, and the efficiency is also higher. The transpiration rate of E. annuus is much higher than those of other companion species, and the increase of the transpiration rate accelerates the absorption and transportation of water and minerals in E. annuus and lowers leaf temperature, which is conducive to the fixation of CO2, thereby promoting photosynthesis. The stomatal conductance value of E. annuus is much higher than those of other companion species, the intercellular CO2 concentration is more stable than other companion species, and the whole-day average value is higher than those of other species, which is conducive to the process of photosynthesis and can better adapt to the environment. However, because its transpiration capacity is much higher than those of other companion species, and its water use efficiency is low, E. annuus is not suitable for spreading in arid areas[19].
Allelopathic effect of E. annuus
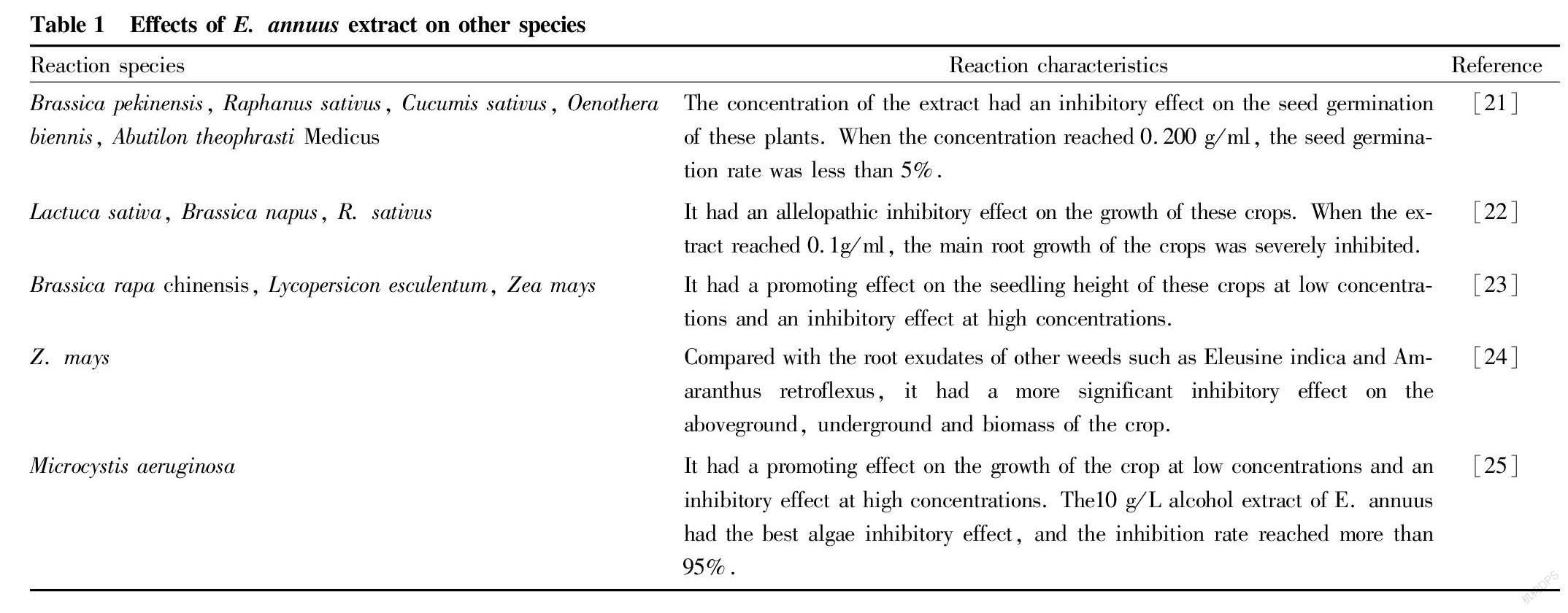
Allelopathy refers to the direct or indirect harmful effects of plants or microorganisms on the growth and development of other plants or microorganisms by releasing certain chemical substances into the environment. It is one of the important mechanisms for the successful invasion of alien plants and is considered to be an important means for invasive plants to improve their competitive advantage[20]. Studies have shown that the extract of E. annuus has an inhibitory effect on the germination and growth of crops, which is more significant than the root exudates of other weeds such as Eleusine indica and Amaranthus retroflexus , as shown in Table 1. Therefore, it is inferred that the successful invasion of E. annuus is inseparable from its allelopathic effects and the allelopathic substances produced enable it to gain an advantage in the competition for limited resources.
Relationship between E. annuus and biological factors
Insects pollinate flowers, flowers provide food for pollinators, so flowers and insects become important carriers of alien plant invasion[5,26]. Through a two-year field investigation, the diversity of native flower-visiting insects and the factors influencing flower-visiting behaviors for alien plant E. annuus were studied, and the hurdle model was used to analyze the influences of environmental factors on insects’ flower visitation selectivity. It was found that there were 145 species of insects that visited the flowers in one year, belonging to 54 families of 9 Orders. The dominant orders were Hymenoptera, Lepidoptera and Coleoptera, each of which accounted for 20.75% of the total number of families, followed by Diptera (18.87%) and Hemiptera (13.21%); and the density of E. annuus affected the acceptance probability of Hemiptera and Hymenoptera, while the flower density affected the acceptance probability and frequency of visits of Hemiptera and Diptera insects, indicating that the plant density and flower density cause E. annuus to become the target of native insects, which has also accelerated the invasion of E. annuus .
The growth of E. annuus severely inhibits the growth of other plants and poses a great harm to the local ecosystem and biodiversity, and the species diversity decreases with the increase of the important value of E. annuus [6]. After studying the impact of E. annuus on the biodiversity of Wuling Mountain, it was found that E. annuus had an adverse effect on the species diversity of Wuling Mountain[27]. It was found that the functional similarity of the alien species and the native coexisting species during the invasion of E. annuus mainly depended on habitat filtration, and antagonism occurred when it invaded together with Solidago canadensis L.
E. annuus will affect soil microbial community structure. Through comparative studies with native plants, it was found that the physicochemical properties of the soil were affected after the invasion of E. annuus , such as rhizosphere soil water content, electrical conductivity, total porosity, soil bulk density and pH; the number of bacteria in the rhizosphere soil increased significantly, and the quantities of fungi and actinomycetes were significantly inhibited; and the activity of enzymes (cellulase, invertase, urease and acid phosphatase) in the rhizosphere soil was significantly affected, which might be because the root system released different chemical substances which had different effects on the quantity and activity of soil microorganisms[28-29]. Invasive plants significantly increased the total number of microorganisms, and correlation analysis showed that except for soil bulk density, electrical conductivity, and other physical and chemical properties of rhizosphere soil, there was a strong correlation with soil enzyme activity and the number of microorganisms, indicating the internal factors of the soil system are in dynamic change and balance, and they show unity and synchronization as a whole influencing each other[30]. Soil microorganisms (especially soil functional microorganisms) play an important role in the successful invasion of foreign plants, and plant rhizosphere provides a diverse and heterogeneous habitat for microorganisms. They may participate in the metabolic cycle of different nutrients in plants, or affect foreign plants or native plants through their metabolites, or act as signals of soil metabolism. They play an important role in the successful invasion of alien plants[31-32].
Conclusions
Invasive alien species can change their own structure and function to adapt to different environments through the process of coordinating with their living environment. It is a complex biological process involving multiple trophic levels and the result of the combined effects of multiple mechanisms[33]. This review showed that E. annuus plays an important role in the invasion process due to its large seed production, strong dispersal ability, strong phenotypic plasticity, allelopathy influence on local plants, and strong adaptability to external environments. With the development of various modern biotechnologies, it is necessary to conduct systematic and comprehensive analysis and research on the internal links between various invasion mechanisms of E. annuus , laying a foundation for the prevention, control and utilization of E. annuus , such as whether the adaptability of E. annuus to the environment a result of heredity or phenotypic plasticity, the research on relation of phenotypic differences and adaptability differences in different regions with adaptive evolution, and related regulatory genes, the relationship and coordination mechanism between E. annuus and other organisms, related allelochemicals and their physiological transport pathways, changes in substances in different growth periods and environments, and their relationships with native plants, rhizosphere microorganisms and other organisms (studies have shown that E. annuus has a certain allelopathy to other plants), and the relationship and mechanism between E. annuus and soil microbial community.
References
[1] Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis: Synthesis. Washington, DC: Island Press.
[2] XIONG YQ, ZHAO CY. Phenotypic plasticity and the successful invasion of alien plants[J]. Chinese Journal of Ecology,2020,39(11):3853-3864. (in Chinese)
[3] RANDALL JM. Weed control for the preservation of biological diversity[J]. Weed Technology, 1996(10): 370-383.
[4] YAN XL, LIU QR, SHOU HY, et al. The categorization and analysis on the geographic distribution patterns of Chinese alien invasive plants[J]. Biodiversity Science, 2014, 22(5): 667-676. (in Chinese)
[5] FAN JJ, YI YM, ZHU XZ. Advances on the invasive weed, Erigeron annuus [J]. Weed Science, 2020, 38(2): 1-8. (in Chinese)
[6] LIU TT, ZHANG HJ, WANG XL. Influence of alien invasive specie Erigeron annuus on biodiversity in region of Wuling Mountain[J]. Acta Scientiarum Naturalium Universitatis Pekinensis, 2010, 46(3): 365-370. (in Chinese)
[7] WANG R, WANG YZ, WAN FH. Spatiotemporal expansion pattern and potential spread of invasive alien plant Erigeron annuus (Asteraceae)in China[J]. Chinese Journal of Ecology, 2010, 29(6):1068-1074. (in Chinese)
[8] TRTIKOVA M. Effects of competition and mowing on growth and reproduction of the invasive plant Erigeron annuus at two contrasting altitudes[J]. Botan Helv, 2009, 119(1):1-6.
[9] PACANOSKI Z. Current situation with invasive Erigeron annuus (L.) Pers. (daisy fleabane) in the Republic of Macedonia[J]. EPPO Bull, 2017(47): 118-124.
[10] TUNAITIENE V, PATAMSYTE J, NAUGZEMYS D, et al. Genetic and allelopathic differences between populations of daisy fleabane Erigeron annuus (L.) Pers. (Asteraceae) from disturbed and stable habitats[J]. Biochem Systecol, 2017(70): 294-303.
[11] ZHANG SS, XIAO YA, DENG HP, et al. Effects of short-term warming on flowering phenology and reproductive allocation of Erigeron annuus [J]. Journal of Southwest University: Natural Science, 2016, 38(1): 53-59. (in Chinese)
[12] ZHONG CF. Construction of steady-state chlorophyll fluorescence dynamics theory and study on photosynthetic physiology and ecology of evergreen broad-leaved plants during overwintering [D]. Beijing: Beijing Forestry University, 2008. (in Chinese)
[13] PATAMYSYTE J, RANCELIS V, CESNIENE T, et al. Clonal structure and reduced diversity of the invasive alien plant Erigeron annuus in Lithuania[J]. Cent Eur J Bio, 2013, 8(9): 898 -911.
[14] HE YF, JI LW, ZHANG LZ. Research progress on Erigeron annuus [J]. Heilongjiang Medicine Journal, 2010, 23(5): 736-737. (in Chinese)
[15] GON WN, WAN FH, XIE BY, et al. Phenotypic plasticity and adaptability of the invasive alien species[J]. Plant Protection, 2009, 35(4): 1-7. (in Chinese)
[16] WANG CY, ZHOU JW, LIU J, et al. Differences in functional traits and reproductive allocations between native and invasive plants[J].Journal of Central South University, 2018, 25(3): 516-525.
[17] LI Z. Adaptation in traits of growth and reproduction of Erigeron annuus in different regions of China[D]. Wuhan: Huazhong Agricultural University, 2014. (in Chinese)
[18] JIN P, YANG LM, HAN M. Isolation and bioassay of active allelochemicals from Erigeron annuus [J]. Journal of Jilin Agricultural University, 2011, 33(1): 36-41, 46.
[19] GAO K. Studies on the comparison of biological characteristics and eco-physiology between the alien species of Conyza canadensis and Erigeron annus and its accompanying species[D]. Changchun: Jilin Agricultural University, 2007. (in Chinese)
[20] BAIS HP, VEPACHEDU R, GILROY S, et al. Allelopathy and exotic plant invasion: From molecules and genes to species interactions[J]. Science, 2003, 301(5638): 1377-80.
[21] JIN P, YANG LM, HAN M. Preliminary studies of Erigeron annuus water extracts on allelopathic effects of five kinds of plants[J]. Journal of Jilin Agricultural University, 2010, 32(4): 419-424. (in Chinese)
[22] LI JH, TIAN SN, DU WW. Preliminary study on the allelopathy of alien Erigeron annuus [J]. Anhui Agricultural Science Bulletin, 2007(17): 23-26.
[23] FANG F, MAO W, GUO SL. Study on allelopathic effects of the invasive plant annual fleabane[J]. Bulletin of Botanical Research, 2005(4): 449-452. (in Chinese)
[24] ZHANG Y. Allelopathic effects of alien invasive plants on crops and their risk assessment in Guangzhong region[D]. Yangling: Northwest Agriculture & Forestry University, 2008.
[25] LIU YT. Study on allelopathy of extracts of compositae plants on Microcystis aeruginosa [D]. Nanjing: Nanjing Normal University, 2011. (in Chinese)
[26] SONG HT, LI BP, MENG L. Flower-visiting insect diversity of the alien plant Erigeron annuus (Asteraceae) in Nanjing, southeastern China and an analysis of factors influencing their foraging preference[J]. Acta Entomologica Sinica, 2013, 56(3): 293-298. (in Chinese)
[27] WANG C, WEI M, WANG S, et al. Erigeron annuus (L.) Pers. and Solidago canadensis L antagonistically affect community stability and community invasibility under the co-invasion condition[J]. The Science of the Total Environment, 2020, 716(May10): 137128.1-137128.10.
[28] WANG CY, XIANG JG, DU DL. The ecological effects of two invasive plants on soil microorganism community in rhizosphere[J]. Ecology and Environment, 2012, 21(7): 1247-1251. (in Chinese)
[29] WEI M, WANG S, XIAO HG, et al. Co-invasion of daisy fleabane and Canada goldenrod pose synergistic impacts on soil bacterial richness[J]. Journal of Central South University, 2020, 27(6): 1790-1801.
[30] ZHANG HX. Effects of different invasive plants on rhizosphere soil enzyme activities and microbe quantities of native plant[J]. Guangdong Agricultural Sciences, 2014, 41(21): 61-66. (in Chinese)
[31] NI GY, PENG SL. Research advances in the interactions between allelopathy and soil in exotic plant invasions[J]. Ecology and Environment, 2007(2): 644-648. (in Chinese)
[32] CHEN BM, WEI HJ, CHEN WB, et al. Effects of plant invasion on soil nitrogen transformation processes and it’s associated microbial[J]. Chinese Journal of Plant Ecology, 2018, 42(11): 1071-1081. (in Chinese)
[33] LI XM, YU M, LI J. Research progress of plant invasion mechanism[J]. Bulletin of Biology, 2020, 55(3): 5-9. (in Chinese)
杂志排行
农业生物技术(英文版)的其它文章
- Rice Blast Resistance-associated Genes Based on Different RNA-seq Resources
- Research Progresses on QTLs for Main Grain Shape Genes in Rice
- Effects of Raising Chickens Under Moringa oleifera
- Preliminary Research on Radiation Breeding of Pteroceltis tatarinowii Maxim
- Comparison of Spring Radish Varieties with Entire Leaves
- Occurrence and Chemical Control Techniques of Rice Black-streaked Dwarf Disease in Rongshui County
