Development of Mg based biomaterial with improved mechanical and degradation properties using powder metallurgy
2020-12-18AjitKumarPulakPandey
Ajit Kumar,Pulak M.Pandey
Department of Mechanical Engineering,Indian Institute of Technology,Delhi,110016,India
Received 8 October 2019;received in revised form 10 February 2020;accepted 14 February 2020 Available online 29 May 2020
Abstract In the present work,biocompatible materials such as niobium(Nb),zinc(Zn)and calcium(Ca)have been blended with magnesium(Mg)to develop a novel biomaterial(BM)with improved mechanical and corrosion resistant properties.Powder metallurgy(PM)technique was used to fabricate Mg based BM.The powder of all aforementioned materials were mixed homogenously in specifi quantities to create a uniform composite component.In order to analyse the influenc of process parameters on the mechanical properties of the fabricated part,experiments were performed considering central composite design(CCD).The effect of powder metallurgical parameters namely percentage Nb,compaction pressure,heating rate,sintering temperature and soaking time on the ultimate compressive strength(UCS)and sintered density was studied in the present study.It was found that the UCS and sintered density increased with increase in compaction pressure,heating rate and sintering temperature.The results also revealed that the increase in soaking time and percentage Nb,increased sintered density and UCS to a certain limit.Subsequent increase in these two parameters,sintered density and UCS decreased.Scanning electron microscopy(SEM)images of the fabricated samples showed reduction in porosity with the increase in heating rate.Moreover,X-ray diffraction(XRD)results revealed that no other phase or impurities were found during sintering of Mg based BMs.The optimum process parameters were obtained to develop Mg based BM for maximum UCS and sintered density.Furthermore,the Mg based BM samples fabricated at optimum process parameters were used for corrosion testing in simulated body flui(SBF)solution at a temperature of 37±0.5°C.The Mg based BM yielded improved mechanical properties with reduced corrosion rates as compared to pure Mg.© 2020 Published by Elsevier B.V.on behalf of Chongqing University.This is an open access article under the CC BY-NC-ND license.(http://creativecommons.org/licenses/by-nc-nd/4.0/)Peer review under responsibility of Chongqing University
Keywords:Biomaterials;Powder metallurgy;Magnesium;Corrosion;Metal matrix composite.
1.Introduction
In current era,biodegradable metals are considered as the most prominent material for implant manufacturing.These materials degrade progressively with time after implantation resulting into exclusion of post healing surgery requirements.The basic characteristic of these materials are that they have mechanical as well as biological properties close to human bone.Generally,three types of biodegradable materials are used for biomedical applications,namely ceramic,polymer and metal.Ceramic and polymer based biomaterials have been studied broadly;however they are not considered for implants manufacturing due to their lower mechanical properties as compared to bone[1].On the other side,the appropriate mechanical properties of metals make their candidature in the domain of bone tissue engineering applications.Currently,biodegradable metal implants have gained more interest among the researchers[2-4].Iron(Fe),Zinc(Zn)and Magnesium(Mg)are some of the extensively studied biodegradable metals[5,6].Iron and its alloys have excellent mechanical strength.However,higher density and huge mismatch in elastic modulus of iron and its alloys are the big concern which restricts its fabrication for implants.Further,too slow degradation rate in physiological environment is the major limitations of iron,which hinders its application in short-term implantation.Moreover,in the case of Zn and its alloy,the major obstacles such as poor mechanical properties and higher density restrict its application as biodegradable implant even after retaining an ideal corrosion rate.Among all these metals,magnesium(Mg)and its alloys represent most suitable candidature for biodegradable material rather than other polymer and metallic biomaterials and got the significan attention of researchers.The main intention of authors to show a special interest on Mg and its alloys owing to their natural ability to biodegrade in aqueous solutions[7].Moreover,remarkable biocompatibility of Mg[8]plays a vital role in maintaining metabolism by releasing of Mg ions throughout degradation of Mg implants and,so far,no side effects have been reported for Mg ions.Furthermore,on a comparison with metallic materials such as titanium alloys(˜ 115 GPa),cobalt-based alloys(˜230 GPa)and stainless steel(˜200 GPa),the closer elastic modulus of Mg(40-45 GPa)to the natural bone(3-20 GPa)reduces the stress shielding effect[9,10].Also,remarkably light weight and density ranging from 1.74 to 2.0 g/cm3which is closest to natural bone(1.8-2.1 g/cm3)[11]gives an edge in the biomedical applications.In addition,Mg is an essential element of the body[12]and is the fourth major cations in human body.Moreover,degradation products from Mg are expected to be nontoxic to the neighbouring tissue[13].Additionally,progressive degradation behaviour of Mg with body fluids emphasizing a new peer of biomaterials for cardiovascular as well as orthopaedic applications.Biodegradable magnesium implants could play a vital role in eliminating several disadvantages of permanent implants(such as stainless steel,Ti alloys and Co-Cr alloys)like stress shielding,requirement of second surgery,bone infection and joint stiffness,etc.[1-3].However,the accelerated corrosion behaviour of Mg based BMs originate serious concerns such as H2gas accumulation,alkalization of body flui and mechanical failure prior to accomplishing the purpose[14].
So far,improvisation of the corrosion performance and mechanical integrity of metallic Mg is being done either by alloying/reinforcement strategies or by manufacturing technique.Numerous alloying element have been used where many of the essential Mg alloys show certain cytotoxicity[15,16]and inadequate corrosion resistance which decreases rapidly in biological fluid and leads to reduction of mechanical properties[16,17].In spite of the aforementioned problems,still extensive investigations are under progress in the domain of composition design.Moreover,till date very few manufacturing techniques like casting,forming,layered manufacturing and sintering have been used to fabricate pure magnesium or its alloys.All these manufacturing techniques have some prime limitations in fabrication of Mg.The limitations of casting could appear as segregations,precipitation shrinkage,micro and macro porosity,inhomogeneous grain size and grain size distribution during solidification These limitations of casting are difficul to eliminate even by heat treatment techniques[18].Forming has comparatively limited cold workability owing to hexagonal lattice structure of magnesium alloys.This behaviour leads to critical stress concentrations at the grain boundaries under tension and compression which possesses the danger of an early material failure[19].The major obstacle of layered manufacturing includes optimization of the shielding box to minimize the effect of oxidation,the heat affected zone,optimize parameters in the development of multi-layer parts by means of process parameters[20].Apart from this,initial investment and high energy consumption are also a big concern to use this technique for Mg fabrication[20].Considering the most economical and less energy-consuming technique,powder metallurgy processing is one of the most suitable technique to fabricate high melting point materials like tungsten,carbon etc.[21].
Extensive research[22-27]has been carried out in the fiel of Mg based BMs which involved the development of Mg based BMs,production of various Mg for excellent cell integration,surface modificatio via coatings,microstructural alteration through mechanical processing.Many researchers[28-32]attempted to control the corrosion rate of Mg by alloying with elements such as zinc(Zn),calcium(Ca),manganese(Mn)etc.But,the Mg based BMs still lack the desired improvement in corrosion resistance properties.The higher corrosion rate of Mg based implants results in deterioration of mechanical properties which leads to implants failure prior to accomplish their purpose[14,33,34].So,this necessitates the development of Mg based BMs which could act as inhibitors in the way of deterioration of mechanical and corrosion properties.
Moreover,a significan quantity of research has been done on Mg-Zn-Ca alloys which exhibit a better mechanical as well as corrosion behaviour than pure Mg[29-32].However,Mg-Zn-Ca alloys need further improvisation to become a biodegradable implant material.The incorporation of reinforcements to Mg matrix is an encouraging alternative to enhance the mechanical properties,corrosion performance and wear resistance[35].Recently,Magnesium-Niobium(Mg-Nb)biomaterial systems have fascinated an attention for in-vivo applications as Nb is nontoxic as well as physiologically inert material which neither forms intermetallic phase with Mg matrix nor dissolves in it.Nb is considered as a valve metal,which easily creates protective surface oxides and hence,exhibits excellent stability against corrosion in most of the aqueous medium[36].A bonelike oxide layer is formed on the Nb when it reacts with hydroxide,that helps in osteointegration[36].However,so far only few research[36,37]are available in open literature to estimate the corrosion performance of Mg-Nb biomaterials in biological environments.In the present work,an attempt has been made for the development and characterization of Mg based BMs with improved corrosion performance using conventional sintering technique.
2.Materials and Method
This section deals with the details of material selection,fabrication procedure with its mechanism,energy-dispersive X-ray(EDX)and XRD analysis of fabricated samples.
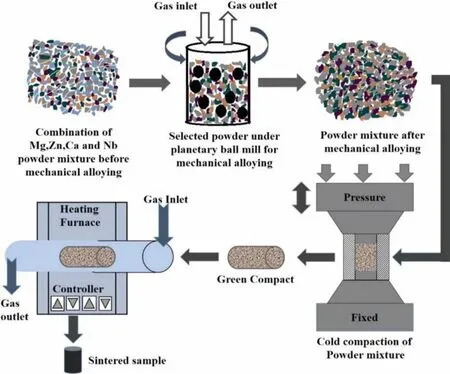
Fig.1.Illustration of the methodology for the development of Mg based BM.
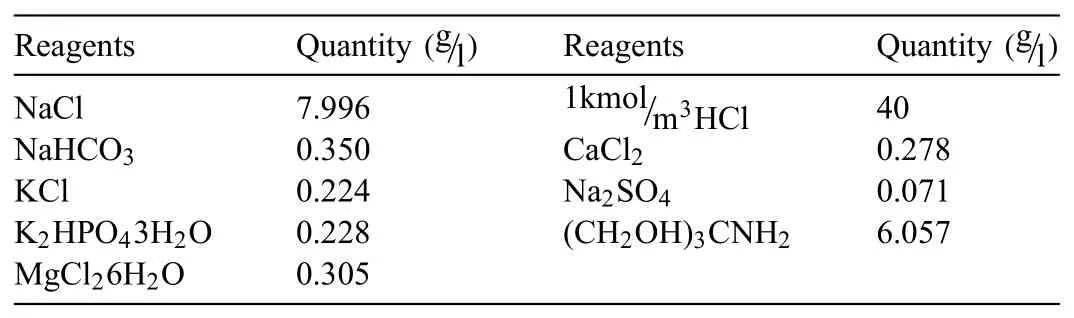
Table 1Chemicals used to prepare simulated body flui(1L,pH 7.4).
2.1.Materials
For the development of Mg BMs,three different materials namely Zn(325 mesh,purity≥99.8 %),Ca(6 mesh,purity≥99.5 %)and Nb(325 mesh,purity≥99.8 %)were added to Mg(325 mesh,purity≥99.8 %).The materials were purchased from Alfa Aesar-United States.Chemicals used for preparation of simulated body flui using solution of one litre has been shown in Table 1[38].
2.2.Fabrication procedure of Mg based BMs

Fig.2.Two step sintering cycle used for the sintering of Mg based BM.
Fabrication of Mg-Nb based BMs were accomplished using powder metallurgy route as illustrated in Fig.1.It began with the mixing of different percentage of Nb to Mg3Zn1Ca in planetary ball mill(Insmart M-780,India)by taking ball to powder ratio 20:1.A homogenous mixture of selected powders were achieved by rotating planetary ball mill at 200 rpm for 1 hrs[39].Purging of existing air was done by fl wing pressurized inert argon gas from the inlet of vessel to prevent any oxidization of powder during milling.Furthermore,the milled powder was uniaxially pressed in a cylindrical die at different compaction pressure(100-500MPa)by using hydraulic press with dwell time of 8-10 min.A green compact of 10mm diameter and 15 mm height was obtained.Thereafter,green compact was sintered without applying any external pressure in tube furnace.Two step sintering(TSS)technique followed by subsequent natural cooling was used to facilitate the grain refinemen and improvement in the material properties[40].Fig.2 illustrates the TSS cycle used for fabrication of Mg based BM using conventional tube sintering.To obtainthe fully dense sample,removal of the volatile fraction and smoothing of pores channel was proceeded by initiating TSS with heating the sample at low temperature 380°C for a certain period and then at higher temperature 640°C[41].After sintering no post processing was needed for the sample.

Table 2Process parameters and their respective values.
The sintering process used in the present work is a category of two step pressure less sintering process,though TSS was performed without applying any external pressure during sintering.The mechanism of TSS process can be understood in two stages.In frst stage,TSS initiates heating of the sample at lower temperature while the second stage ends with heating the sample at higher temperature.Heating at lower temperature promotes elimination of fine particles and precoarsening of sample[42].Elimination of fine particles causes delay in initial densificatio at frst stage leads to reduction in densificatio variation and promotes the establishment of highly dense regions in the initial densifi cation stages.Therefore,a smaller density variations present in the developed samples,expediting more homogeneous microstructure.The fina step of sintering process resulted in increased material densificatio processed by grain growth and colligation of powders[42].The grain size growth was driven by sintering forces that comes by reduction in free surface energy.The free surface energy is reduced by increment in the surface area by replacement of solid-vapor interface to solid-solid interface with adjacent particles that initiated in the form of neck curvature[43].The neck curvature produced a stress on the grain boundary which caused material to fl w from the grain boundary to the neck[44].Moreover,the irregular shaped particles were preferred as they give better interlocking and hence promote the sintering of compacts[45].
EDX analysis has also been carried out on the developed Mg BMs to detect the elements present in the sintered samples.XRD pattern of the developed Mg based BMs is shown in Fig.3(a),which depicts that no phase or impurities are present in the sample of developed Mg BM except the parent elements.Moreover,a fairly uniform distribution of Nb particulates in Mg3Zn1Ca can be seen by circular marks in the SEM image as presented in Fig.3(b).EDX analysis confirme the presence of the parent elements in Mg based BM with the evidence for a fairly distributed Nb particulates in pure Mg3Zn1Ca alloys(ref Fig 3(c)).The combined EDS/SEM analysis of developed Mg based BM have been shown in Fig.3(d).A fairly uniform distribution of all the elements in the Mg matrix can be seen in the Fig.3(d).
3.Preliminary experimentation
Based on preliminary experimentation,process parameters,their responses and outcome from the pilot experiments have been discussed in this section.The current work initiated with pilot experiments having f ve process parameters,namely Nb(wt%),compaction pressure,sintering temperature,heating rate and soaking time were chosen.Fig.4 illustrates the cause behind the selection of parametric range for the development of Mg based BM.An increment in sintered density and UCS were found by increase in the green compaction pressure,heating rate,and sintering temperature.Therefore,the range of compaction pressure selected for the experimentation was from 100 MPa to 500 MPa as at compaction pressure lower than 100 MPa the output was not significan and compaction pressure greater than 500MPa were unable to reach owing to die limitation.Also,a heating rate of 100°C/hrs to 500°C/hrs was chosen for the experimentation,as below 100°C/hrs the process was extremely slow and beyond 500°C/hrs were diffi cult to achieve because of machine limitations.The sintering temperature was kept in the domain of 440°C to 640°C for the experimentation as below 440°C,almost negligible variation in output result was found and beyond 640°C,melting of the sample was observed.The range of the soaking time chosen for the experimentation was from 2 hrs to 10 hrs as soaking time below 2 hrs was not significan while soaking time above 8 hrs showed a reduction in results.Furthermore,Nb percentage selected for the experimentation was in between 3 to 15% owing below 3% Nb,results obtained was not significan while Nb percentage above 12% resulted in undesirable results.
Based on the primary requirement for the bone substitute,the sample was characterized for obtaining the responses.After knowing the development possibility of Mg based BM,Mg based BM was fabricated at random parameters tabulated in Table 2.Cylindrical(10×15 mm2)samples were fabricated as per the ISO 13314[46]to perform compression test.A constant transverse speed of 1 mm/min was used on Universal testing machine(Instron 5582,100 kN capacity)during compression test of fabricated samples.Each sample was prepared in the set of three for compression test and an average of the results were taken in the compressive stress vs strain curve.Also,for comparison purpose,pure Mg and Mg3Zn1Ca were fabricated at same process parameters and compared with developed Mg3Zn1Ca5Nb as depicted in Fig.5.
The theoretical density of the developed Mg based BMs were evaluated according to the rule of mixtures technique.Initially,theoretical density of Mg3Zn1Ca was also calculated according to rule of mixture thereafter the theoretical density of Mg based BMs were calculated by considering the theoretical density of both Mg3Zn1Ca(1.78 g.cm−3)and Nb(8.57 g.cm−3).The experimental density of Mg based BMs were measured based on the Archimedes'principle at ambient temperature in deionized water as per ASTM B962-17 standard[47].The sintered density of developed Mg3Zn1Ca5Nb sample was also calculated and a promising result was found.
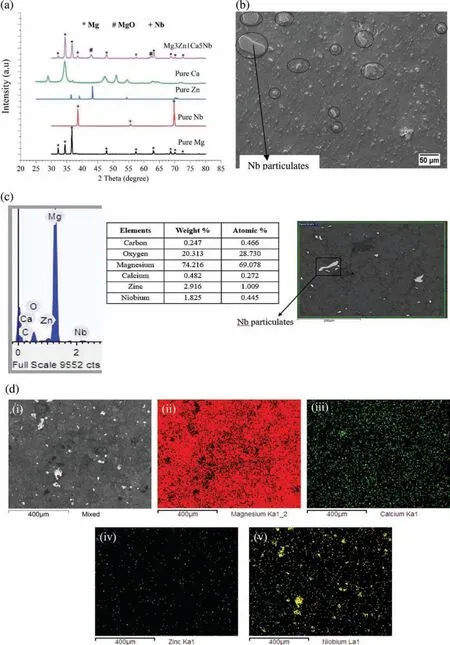
Fig.3.(a)XRD of the developed Mg based BM sample,(b)SEM image of developed Mg3Zn1Ca5Nb sample,(c)EDX of developed sample at experimental points and(d)Combined SEM/EDS map analysis of the Mg3Zn1Ca15Nb,where:(i)SEM micrograph of the investigated area;(ii)EDS elemental map of Mg;(iii)EDS elemental map of Ca;(iv)EDS elemental map of Zn;(v)EDS elemental map of Nb.
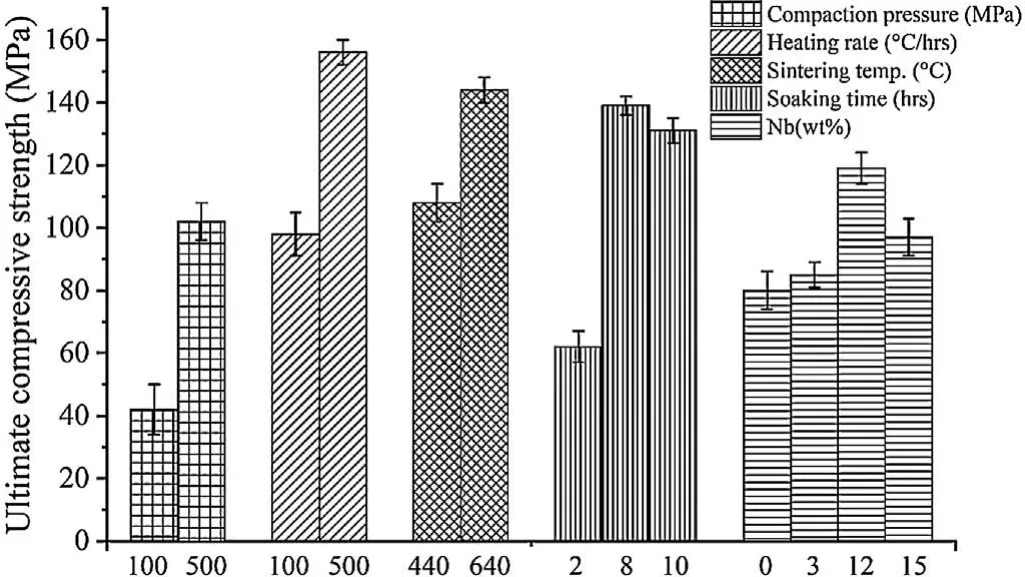
Fig.4.Illustration of pilot experiments parametric range.
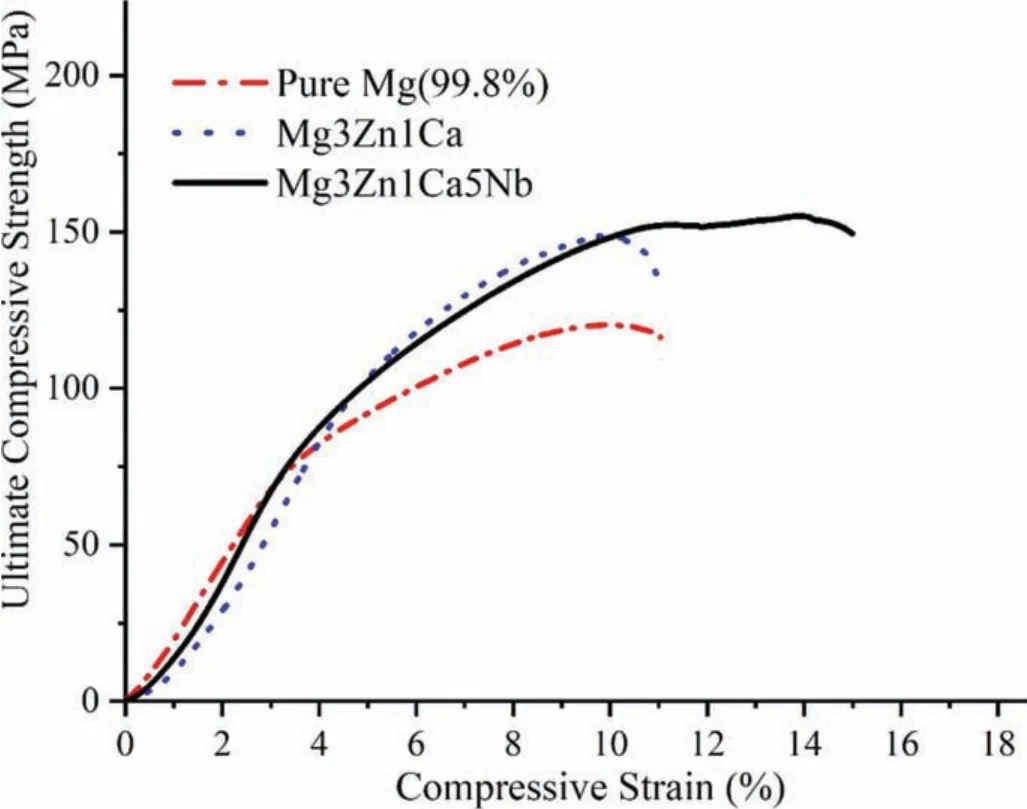
Fig.5.Comparison of compressive stress vs strain curve between pure Mg,Mg3Zn1Ca and Mg3Zn1Ca5Nb samples.
3.1.Corrosion Study
Next to obtaining the significan compressive strength of developed Mg3Zn1Ca5Nb over pure Mg,corrosion test has been performed on the Mg3Zn1Ca5Nb sample using Biologic potentiostat(Model:SP 150).To perform the electrochemical measurement,a standard 3 electrode cell configuratio was employed,where graphite rod,saturated calomel electrode(SCE)and Mg based BM sample were used as counter electrode,reference electrode and working electrode respectively.The electrolyte solution to carry out the electrochemical measurements was simulated body flui(SBF)with pH=7.4.Laboratory grade regents as shown in Table 1 and deionised water were used to prepare the SBF solution.Surface area used for the sample was 1cm2.After stabilizing the Open Circuit Potential(OCP)for 15-20 min,the developed samples were potentiodynamically polarized in the range of−200 mV to+200 mV(vs.SCE)at a scan rate of 1 mV·s−1.Corrosion tests were repeated thrice for the developed Mg3Zn1Ca5Nb sample.As per ASTM G5-14[48],in-vitro corrosion properties of developed sample was evaluated by performing electrochemical measurements.Tafel fi technique,using corrosion analysis toolbox(EC Lab software,v 10.1×)was used to obtain the corrosion parameters.Potentiodynamic polarisation curve of the Mg3Zn1Ca5Nb,fabricated at random set of parameters was compared with pure Mg and Mg3Zn1Ca as illustrated in Fig.6.The detailed description of sample preparation for corrosion testing and its mechanism has been discussed later.
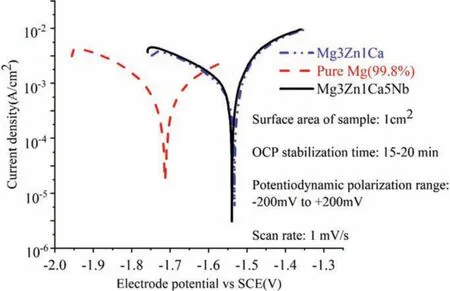
Fig.6.Comparison of potentiodynamic polarisation curves between Mg3Zn1Ca5Nb,pure Mg and Mg3Zn1Ca.

Table 3Process parameters with their respective levels.
4.Planning of experiments using design of experiment
The experiments for fabrication were planned as per central composite design(CCD)to analyse the effect of powder metallurgical process parameters namely percentage Nb,compaction pressure,heating rate,sintering temperature and soaking time on the ultimate compressive strength(UCS)and sintered density.The parametric range chosen for the experimentation was picked up according to machine constraints and the outcome of the pilot experiments.In the framework of experimental investigations,f ve levels of every single factor were chosen as tabulated in Table 3.
Among three type of CCD,such as rotatable,spherical and face cantered,rotatable CCD is more preferred as it predicts more accurate results.Nevertheless,as the experimental points obtained with rotatable CCD provided the fraction values of the input parameters.Therefore,these parameters such as,compaction pressure on compaction machine and sinter-ing parameters(heating rate,sintering temperature and soaking time)on conventional sintering machine were difficul to achieve due to the machine constraints.Therefore,in order to get the data points which can be achievable at compaction machine and conventional sintering machine,the CCD was design as per the alpha value for the axial experimentation run.The value of alpha was taken as 2,which describes the CCD as face cantered.Moreover,Central composite design(CCD)was opted to proceed the experimentation,as it offers quadratic and interaction effect of process variables on the response by executing minimal number of experiments[43,49,50].The methodology provides a well-structured collection of runs to minimize the experimental time and costs.The set of 32 experiments withαvalue 2 was performed based on the response surface methodology equation in following form
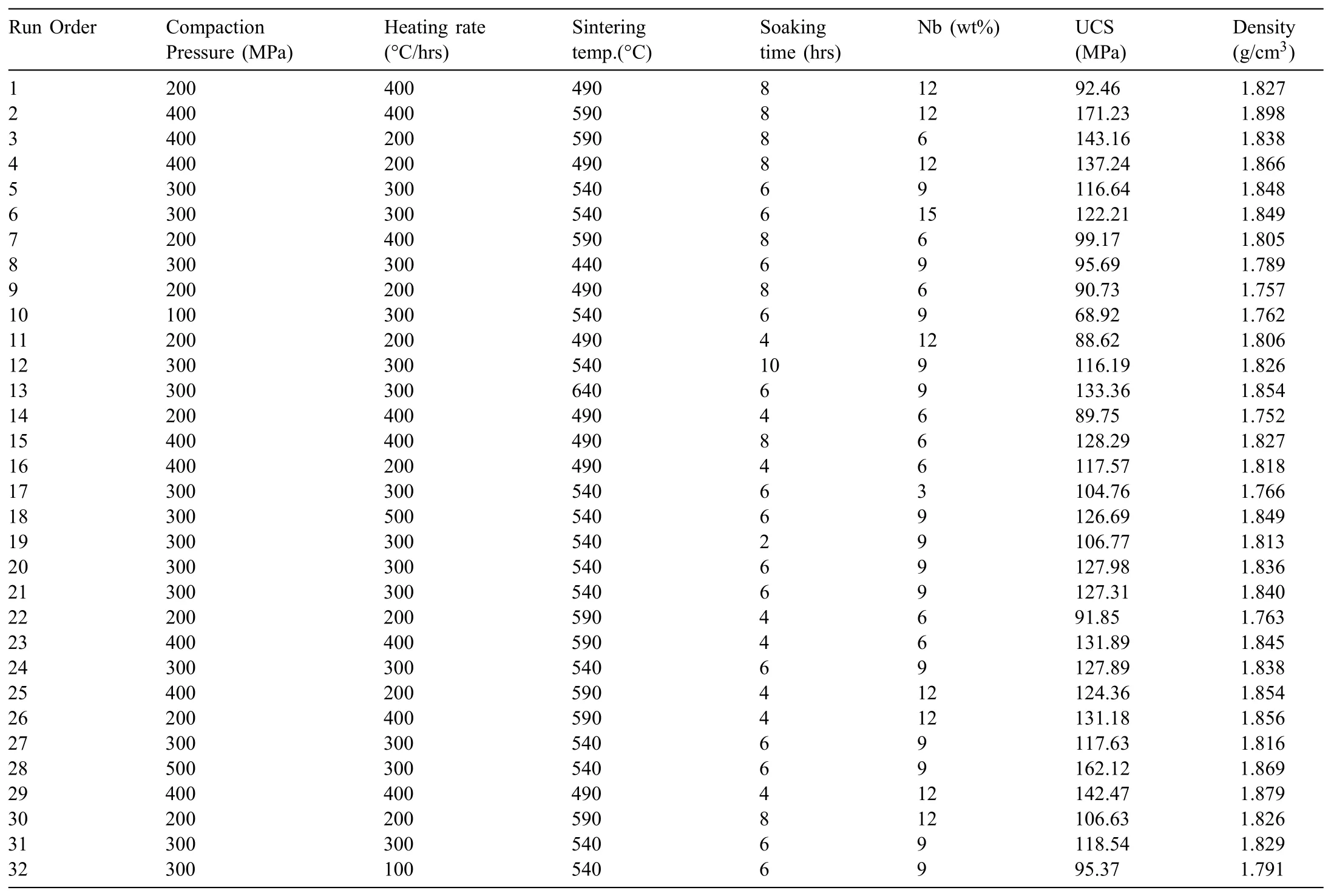
Table 4Experimental log with process parameters and their corresponding responses.
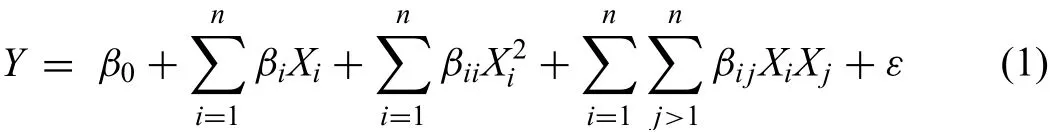
where,Y:Response variable;n:Number of variables;
βi,βiiandβij:Constants;ε:Random error;
Xi:Process factor consisiting frst order
X2i:Process factor consisiting second order
XiXj:Process factors consisting interaction terms
5.Statistical analysis of experimental data
In an effort to study the effect of process parameters,the ANOVA was opted for the experimental data shown in Table 4.Based on RSM design,the experimental runs combined with output responses are also presented in Table 4.Acceptability of the obtained model were verifie(at 95%confidenc level)by comparing the obtained Fregressionwith the standard Fstandardvalue(for both model and lack of fit)In the very beginning,every insignifican expressions(P-value>0.05)of the ANOVA were excluded successively.The ANOVA tables obtained next to the exclusion of insignifican expressions have been tabulated as Tables 5 and 6,respectively.Eqs.(2)and(3)present the statistical equation for the responses with interaction terms.
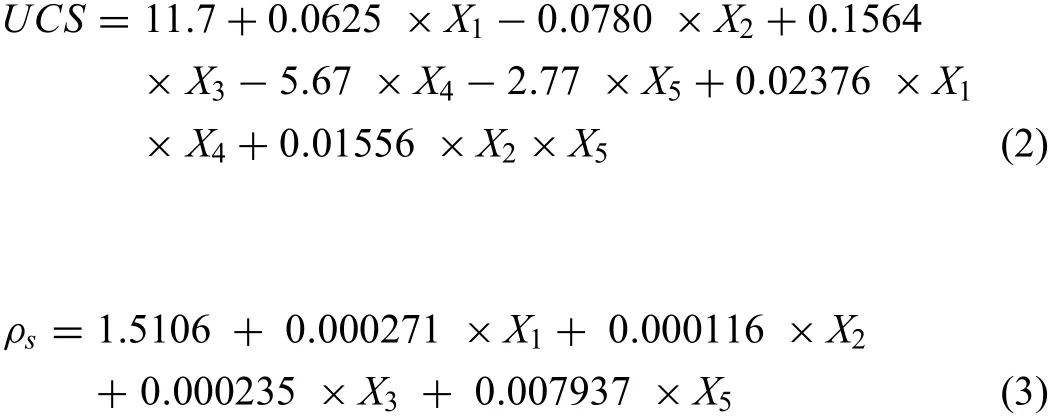
The R2value for every responses(<100 %)represents the presence of error in the experimental data owing to the existence of noise in the experiments.As a consequence of this error,it is complicated to predict the responses precisely;although a range can be predicted.Hence,in the favour of evaluating the range of responses,Eq.(4)is used.
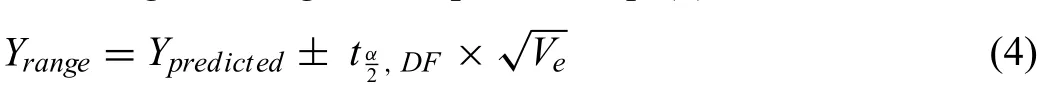

Table 5ANOVA table for experimental UCS by considering only significan terms.
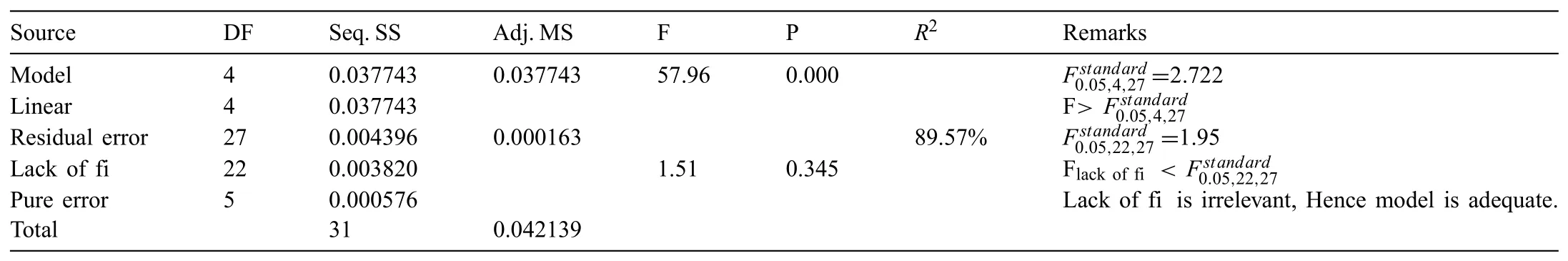
Table 6ANOVA table for experimental sintered density by considering only significan terms.
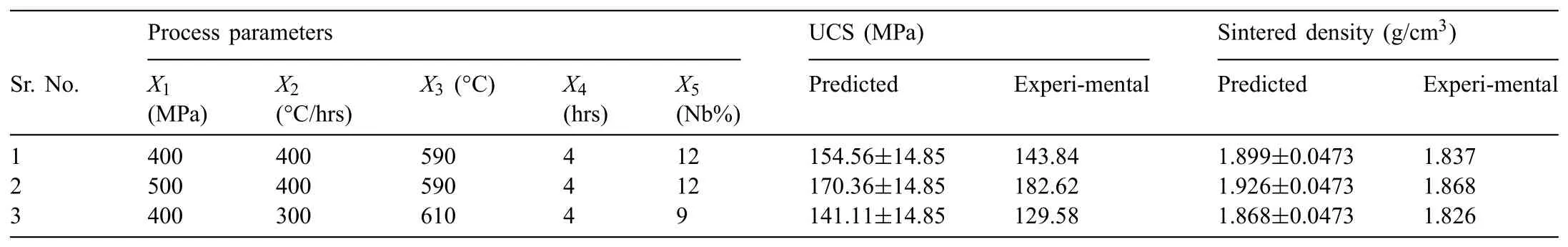
Table 7Confirmatio experiments.
whereYrange: Range of responses,Ypredicted:Predicted responses from model,:Statistical t value with significancand degree of freedom(DF)andVe:Variance of error of the model.
Therefore,95 % confidenc level was used to fin out the range of the models for ultimate compressive strength and sintered density as listed in Table 7.
6.Results and discussion
Fig.7(a)represents the fabricated Mg based BM cylindrical sample,and Fig.7(b)represents the sample before and after the compression test.All the developed samples were failed in compression test by splitting into two parts.The fractured surfaces(ref.Fig.7)were inclined at about 45° to the loading direction in all the samples.The fractured surface of pure Mg shows dominant brittle mode fracture.A combined shear and brittle mode of fractures is obtained by the addition of Nb to Mg matrix.Further increase in Nb increases the failure strain and the ductility accordingly[39].The microstructure of the fabricated Mg based BM is depicted in Fig.7(c).The mean grain size of the Mg based BM was obtained with image processing tool ImageJ,and the grain size obtained thereof was 25μm.The decrease in average grain size of the particles may be the reason for the improvement in the mechanical and anti-corrosive properties of Mg based BM[51].To prevent any hindrance in sintering,the oxides fil were mechanically destructed during compaction of the powder mixture.As high compaction pressure results in a maximum shear stress concentration between the particles which causes rapture of oxides fil and establishment of fresh contacts between metallic particles[52].Moreover,mechanical milling yields the irregularity of Mg and reinforced particulates.This assists in joining of particles together more firml than spherical particles and subsequently leads to a superior mechanical integrity without any phase formation[53].
6.1.Effect of process parameters on ultimate compressive strength and sintered density
Figs.8(a),(b)and 9(a),(b)illustrate the main effect plots and percentage contribution of parameters on the UCS and sintered density respectively.It could be noticed from Fig.8(b)that the compaction pressure has higher influencon UCS followed by heating rate and sintering temperature.Similarly,from Fig.9(b)it can be seen that soaking time has a marginal influenc on the sintered density while all other parameters except soaking time show a significan influenc over the sintered density.The individual effect of the parameters on UCS as well as sintered density is discussed further in the next subsections.

Fig.7.Illustration of(a)Fabricated Mg based BM(b)before and after compression test and(c)Microstructure of fabricated Mg based BM.
6.1.1.Effect of compaction pressure
It is very obvious form the Fig.8(a),a rapid increment in UCS was obtained by linear increment in compaction pressure.It may be due to the elimination of the oxide layers from the powder surface during compaction process.Initially,a higher pressure leads to high stress concentration between the powders particles which in direct contact with each other.This results in the deformation of the powder particles,rapture of the oxide film and generation of new contacts between metallic particles.Subsequently,raising the compaction pressure may lead to formation of effective bonds between the existing powder particles.Furthermore,the fresh metallic interface developed by compaction are fused together in the subsequent sintering process and turned into strong intermetallic bonds between the Mg particles.Moreover,irregular shaped particles could also act as a key factor in increment of UCS because they give better interlocking of particles on applying high pressure and hence promote the sintering of compacts.This results in elimination of residual pores presented in the compacted sample and turned into highly dense product whereas,this strong intermetallic bond effectively withstands high compressive load.
6.1.2.Effect of heating rate
According to many reports,sintering using low heating rate on compacted samples offer lower densificatio thus results in lower strength.At lower heating rate,many particles remains in compact form and welding spots between grains are not formed.While high heating rate improves the densificatio in compacts by formation of more adhesion and welding spots[54].Fig.10(a)and(b)elucidate the SEM images of the sample sintered with 500°C/hrs and 100°C/hrs respectively.It is clear from the Fig.10 that increasing heating rate induces reduction in porosity and improves both the sintered density and compressive strength of developed Mg based BM.With lower heating rate sample gets more time to receive the heat which results in softening of Mg matrix and allows to settle down and agglomerate the heavier particles(Nb)by leaving large number of pores within the matrix.While,samples sintered at high heating rate allows the fair distribution of reinforced Nb particles in the entire region of the sample with minor porosity.Uniformly distribution of Nb particles in the sample could also be a cause in improvement of UCS because these reinforcement restrict the movement of dislocations between particles whenever subjected to compressive load.
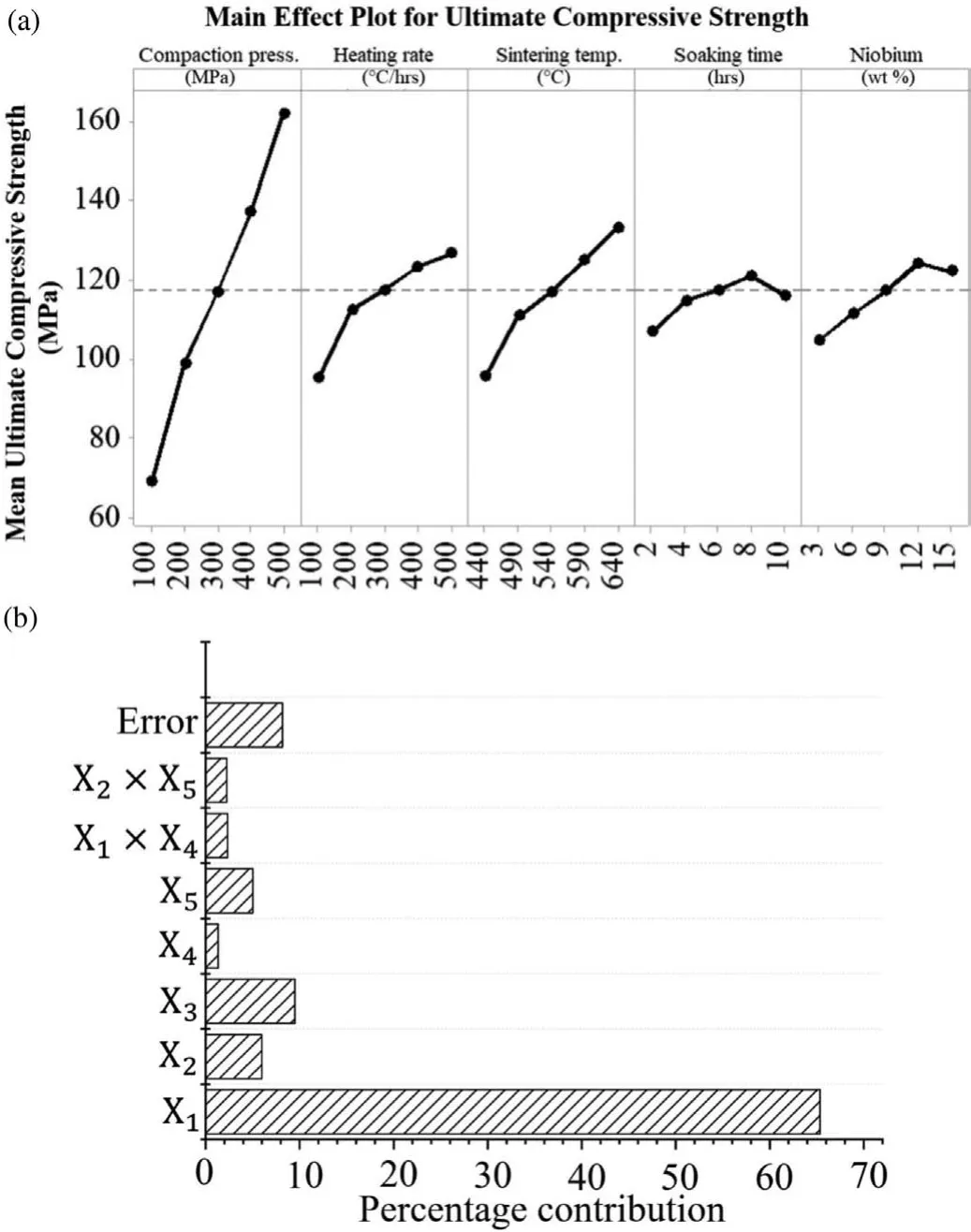
Fig.8.(a)Main effect plot and(b)percentage contribution of parameter for UCS.
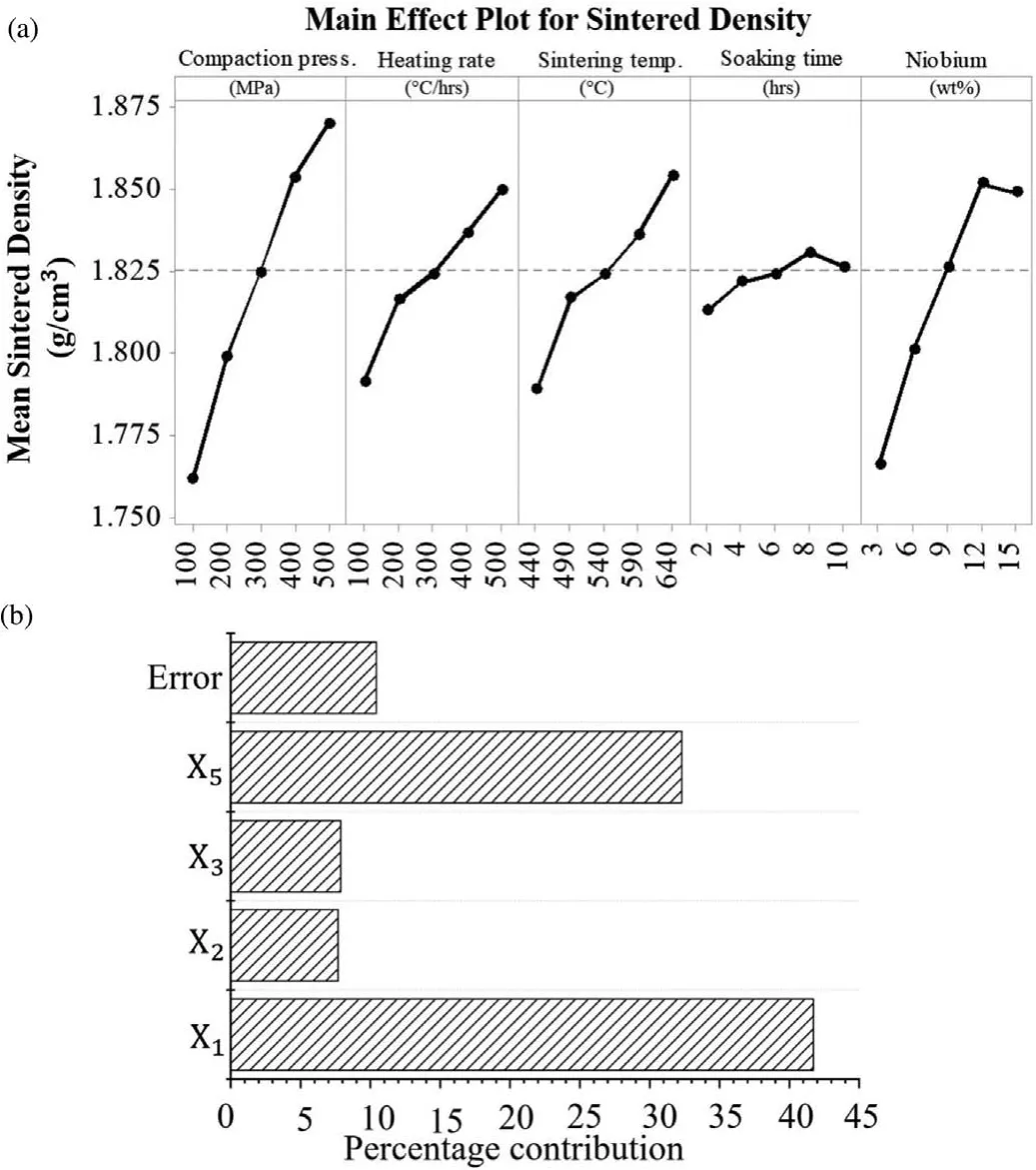
Fig.9.(a)Main effect plot and(b)percentage contribution of parameter for sintered density.
6.1.3.Effect of sintering temperature
Figs.8(a)and 9(a)illustrate the effect of sintering temperature on UCS and sintered density respectively.The effect of different sintering temperature on sinterability of Mg based BM was evaluated,as sintering temperature plays a vital role in sinterability.For this,the powder mixture was compacted in a die by applying very low compaction pressure∼5 MPa.There after the compacted sample was broken into two part and each parts were sintered at different temperature by keeping other parameters constant.Fig.11 exhibits the effect of sintering temperature on the sinterability of compacted sample.From Fig.11 it is evident that sample sintered at higher temperature(640°C)has proper diffusion of particles with minor pores than the sample sintered at lower temperature(440°C).Higher sintering temperature accelerates the particle diffusion,leads to high densificatio and offers strong bonding between particles.Maximum sintered density and ultimate compressive strength is obtained at higher sintering temperature.It could be due to the higher rate of diffusion between particles that leads to reduction in porosity(ref.Fig.11(b))at higher sintering temperature.This observation is similar to other reports mentioned somewhere else[54,55].
6.1.4.Effect of soaking time
Figs.8(a)and 9(a)elucidate the influenc of soaking time on the UCS and sintered density of fabricated samples respectively.A marginal influenc of soaking time on UCS as well as sintered density was observed.Nominal enhancement of approx.10 MPa in UCS was observed with progressive increase in soaking time from 2 hrs to 8 hrs as shown in Fig.8(a).This may be attributed owing to the enhancement in diffusion rate and thus elimination of porosity.Proper diffusion of Mg firml holds the uniformly distributed reinforcement(Nb)particles which produces a highly dense Mg based BM.Moreover,the uniformly distributed reinforcement within the Mg matrix strongly restricts any dislocation under loading condition and demonstrates high UCS.However,sintering temperature near about 620°C a marginal reduction in UCS is observed that starts reducing after further increment of soaking time.This may be due to the softening of Mg matrix which allowed heavier particles(Nb)to settle down by leaving pores inside the matrix and thus resulted in reduction of UCS and sintered density.Moreover,increasing soaking time beyond certain limit,may allow the expansion of isolated pores owing to migration of pores along with grain boundaries resulting in a decrease in UCS and sintered density[43].
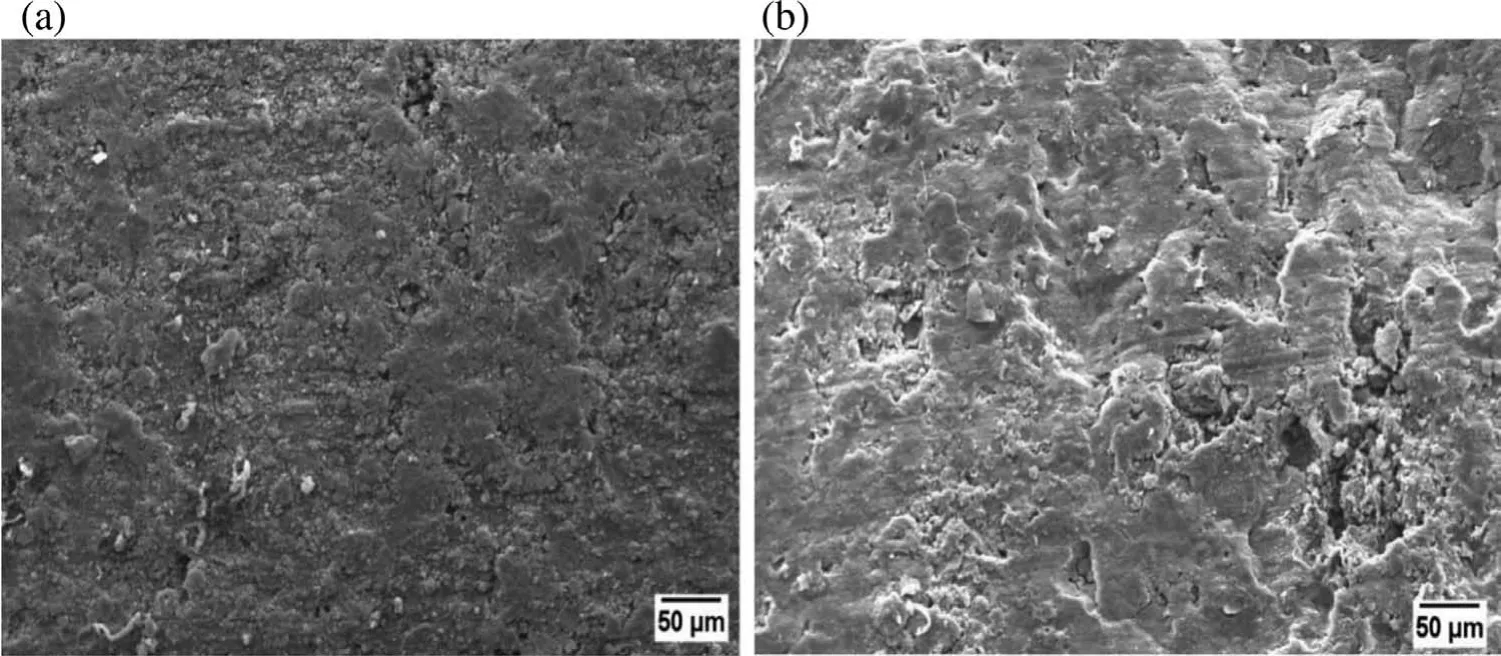
Fig.10.SEM image(at 500X)of samples fabricated at different heating rate(a)500°C/hrs and(b)100°C/hrs.
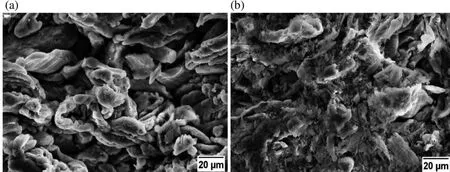
Fig.11.SEM images(at 2000X)of samples sintered at different temperature(a)440°C and(b)640°C.
6.1.5.Effect of percentage Nb
The effect of percentage Nb on UCS and sintered density of fabricated part have been shown in Figs.8(a)and 9(a)respectively.It can be seen from Figs.8(a)and 9(a)that ultimate compressive strength as well as sintered density of developed BM increased with increase in addition of Nb(wt%).However,a reduction in UCS and sintered density are observed by addition of Nb(wt %)beyond 12 %.The initial increment in UCS may be attributed to increase in number of barriers in the way of dislocations.Similarly,an increment in density was observed by addition of Nb till 12 % owing to uniform distribution of these reinforced Nb particles into the Mg matrix.However,as discussed earlier,addition of Nb(wt%)to Mg matrix beyond a specifi limit resulted in reduction of UCS and sintered density which may be owing to the agglomeration of reinforced particles caused by cold welding of reinforcement during milling process[29,53].A significan amount of work has been published by many authors related to the cold welding and agglomeration of ductile particles during milling operation[29,53]The agglomeration of particles,possesses reduction in homogeneity of the reinforcements in Mg matrix,results in deterioration of mechanical properties[29,53].
6.2.Effect of interaction on ultimate compressive strength
A significan interaction between Nb(wt %)and heating rate was observed on UCS after experimental investigations.Fig.8 shows the surface plot and effect of interaction on UCS.It has been noticed that UCS of developed Mg based BM reduced at low heating rate and high weight % of Nb.It is may be due to the fact that,at lower heating rate sample gets more time to receive the heat which results in softening of Mg matrix.Subsequently,it allows to settle down the heavier particles(Nb)by leaving a larger numbers of pores within the matrix resulting in lower UCS.However,the combination of both high heating rate and high Nb(wt %)possesses increased UCS as shown in Fig.8.As discussed earlier that higher hating rate results progression of welding spot and moreover embedding of uniformly distribution of Nb within the Mg matrix.Embedded particles in the Mg matrix could be major cause in improvement of UCS because these reinforcement restrict the movement of dislocations.It can be seen from Fig.8 that UCS was low at high heating rate and low Nb % while UCS was high at high heating rate and high Nb%.As discussed earlier,high heating rate results in improved adhesion quantity and weld spot however,the less number of obstacles against dislocation movement,exhibit less UCS.Whereas,due to the formation of large quantity adhesion and welding spots at high heating rate and increase in number of obstacles to the dislocation movement results in high UCS.Further,UCS was high at low heating rate and at low Nb %while UCS was low at low heating rate and high Nb %.This may be due to the reason that,at low concentration of Nb which possesses less agglomeration of reinforced Nb even at low heating rate and this low agglomeration of reinforced Nb does not influenc the strength of composite[39].Whereas,lower heating rate sample gets more time to receive the heat which results in soften of Mg matrix and allows to agglomerate the higher concentration of reinforced Nb along with larger size pores.
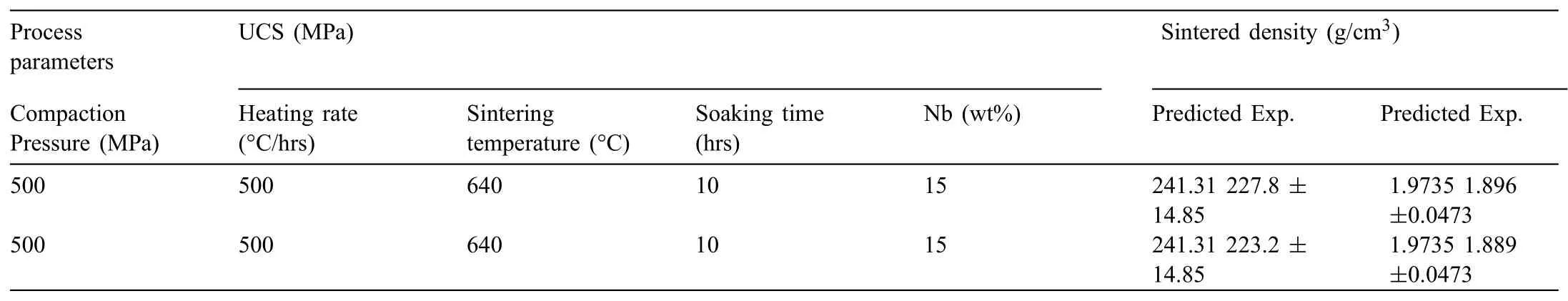
Table 8An optimised set of conventional sintering parameters.
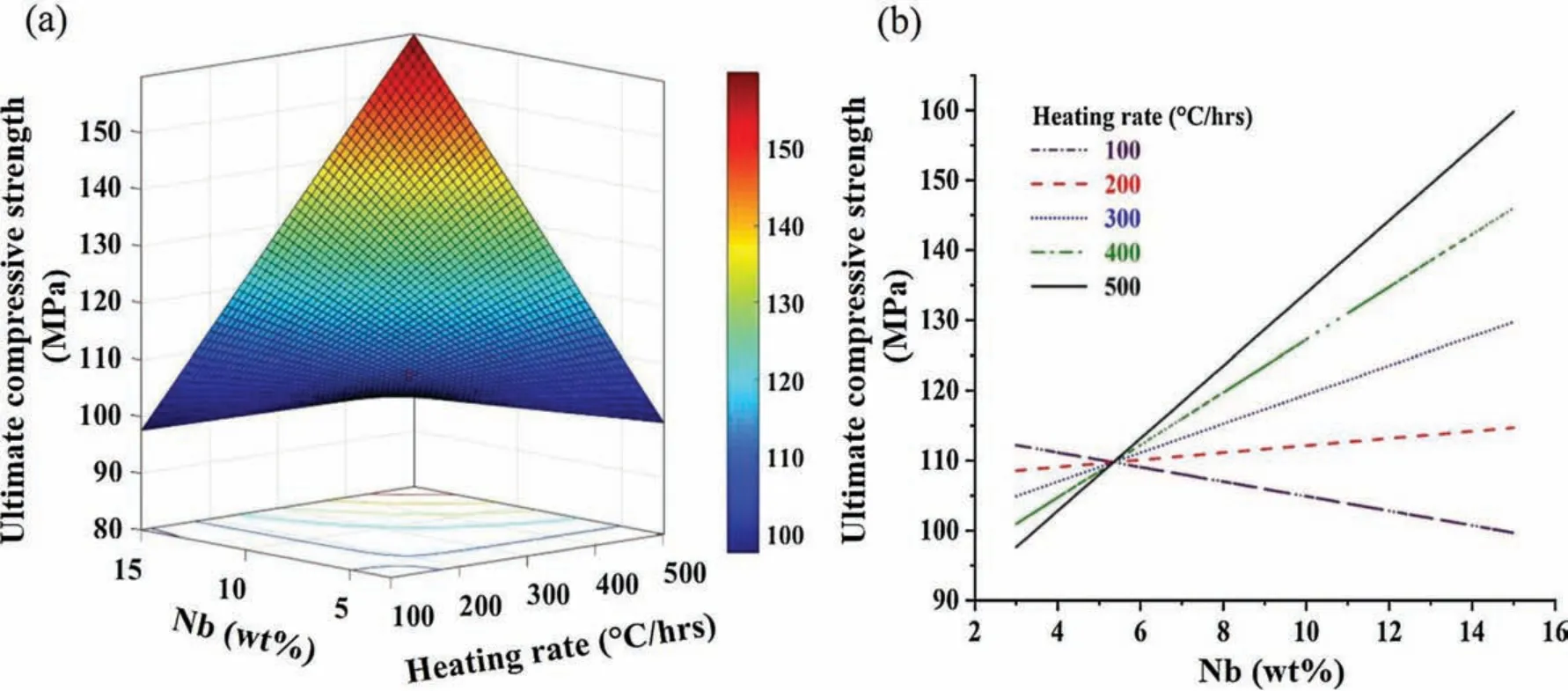
Fig.12.Illustration of Nb % and heating rate interaction effect on UCS using(a)surface plot and(b)interaction plot.
Fig.9 elucidates a surface plot and substantial interaction between compaction pressure and soaking time after experimental investigations.Increase in UCS was found at higher compaction pressure and soaking time.It is because,higher compaction pressure resulted in formation of strong initial bonds between the existing powder particles which gets strong enough by diffusion during sintering.As discussed earlier,higher compaction pressure distrust the oxide layers from particles surface,thus reduces the chances of sinterability hindrance.Moreover,a certain period of soaking time allowed Mg matrix to soften and create a strong hold on the reinforced Nb particles which restricts the dislocation movement when under compressive load,therefore elucidated higher strength.It is evident from Fig.9,that UCS was low at high compaction pressure and low soaking time while at UCS was high at low compaction pressure and at low soaking time.This could be due to presence of micro-cracks that come due to high compaction pressure which may not diffuse at low soaking time.However,at low compaction pressure,the absence of micro-cracks exhibit enough hindrance to dislocations even at low soaking time.Moreover,high soaking time allows the diffusion of micro-cracks which may occur due to high compaction pressure.These diffused micro-cracks joined together and thus exhibit sufficien strength under the influ ence of compression loading.
6.3.Multi-objective optimization
In an effort to get the optimal input parameters of powder metallurgy technique,a multi-objective optimization approach,based on genetic algorithm was implemented by utilizing optimization toolbox in MATLAB(R2017a).The objectives of the current work was to maximize the ultimate compressive strength and sintered density.As the objective functions obtained by toolbox resulted in minimization.So,maximization was obtained by performing inverse of objective functions.The objective combined with constraints for the multi-objective optimization is given below
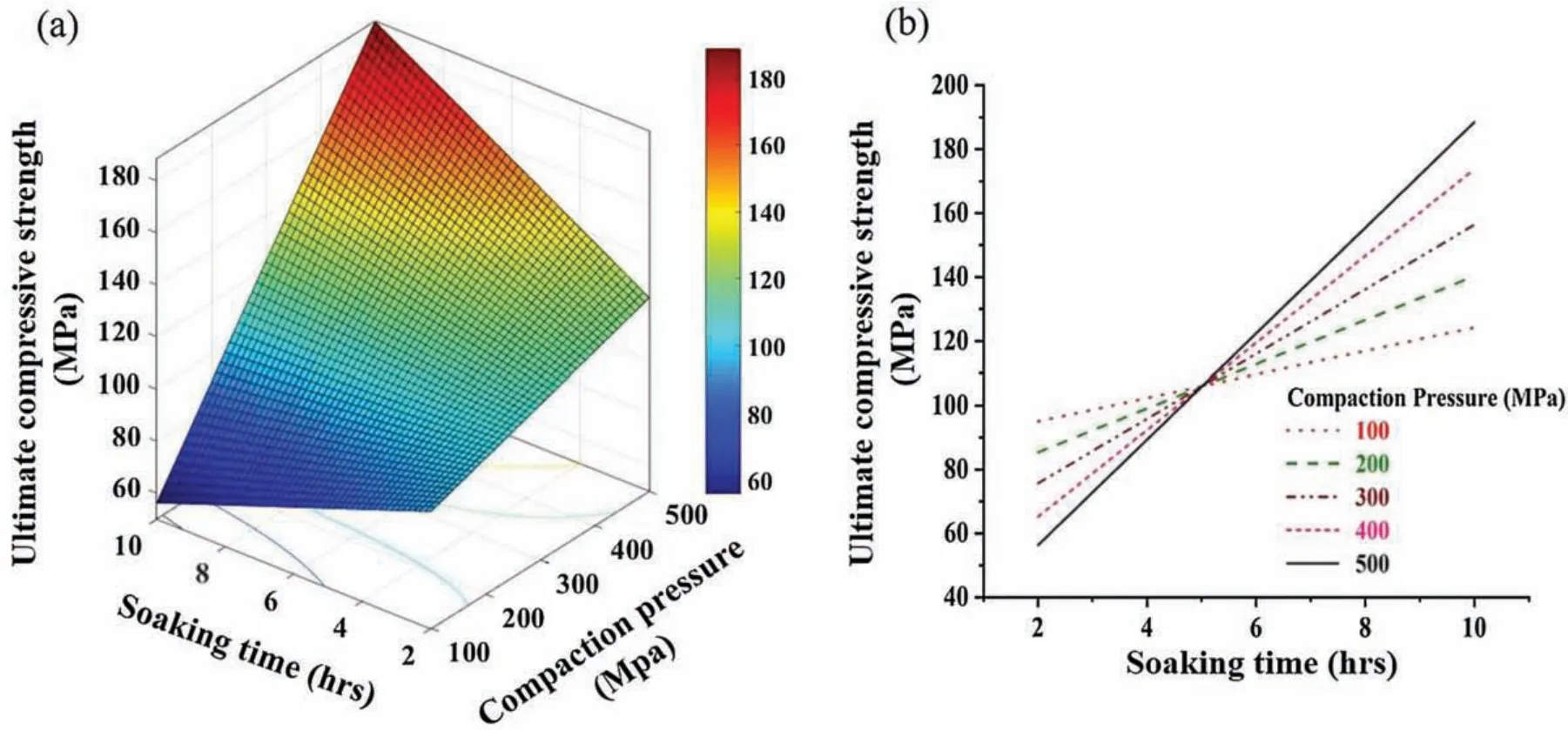
Fig.13.Illustration of soaking time and compaction pressure interaction effect on UCS using(a)Surface plot and(b)interaction plot.
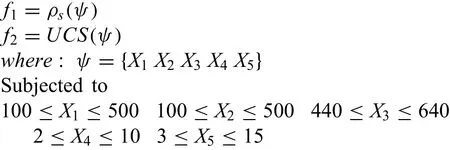
The optimum solutions of the experiments were obtained using multi-objective technique and correlated with the results derived using the genetic algorithm as tabulated in Table 8.
6.4.Potentiodynamic polarization test for developed sample at optimized parameters
In order to evaluate the corrosion stability of developed sample,the potentiodynamic polarization curves of Mg3Zn1Ca15Nb is depicted in Fig.14.The polarization curve of the pure Mg(99.8%),Mg3Zn1Ca and Mg3Zn1Ca5Nb samples are also plotted in Fig.14 for comparison purpose.Almost similar kind of polarization curves were observed for all the tested samples as shown in Fig.14.Moreover,a positive potential shift of the polarization curve was observed for Mg3Zn1Ca15Nb,which indicates the increase in electrode passivity.Substantial increment in positive potential exhibits with 15% Nb addition to Mg3Zn1Ca.The most feasible issue of such increment in potential is the formation of Nb oxide on the surface,which possesses higher anti-corrosive property compared to Mg oxide(EDS mapping:ref.Fig.3(d)).Similar finding for anti-corrosive properties of Nb were observed in the previous work[36,56].A considerably lower value of measured anodic slopes for the Mg3Zn1Ca15Nb is also due to the reduction of the H2gas evolution rate[36].It can be encapsulated that Nb acts as effective barrier,which minimizes the active surface area in the electrolyte without changing the corrosion mechanism.The corrosion current densities were calculated by extrapolating the linear segment of the cathodic and anodic Tafel polarization curves to the corresponding corrosion potential(Ecorr),later on the results obtained are tabulated in Table 9.It is quite clear that lower current densities were obtained for the Mg3Zn1Ca15Nb samples as compared to pure Mg and Mg3Zn1Ca samples.Also,from the present study,it can be concluded that the addition of 15% Nb to Mg alloys leads to reduction of corrosion activity by 32.8%.

Fig.14.Comparison of potentiodynamic polarization curves for optimized sample with pure Mg,Mg3Zn1Ca,and Mg3Zn1Ca5Nb sample.
7.Comparative study
The mechanical and corrosion properties of developed Mg based BM was compared with the cortical bone and prior studies,as shown in Table 10.Some prior arts in the background does not have the disadvantages/problems specified However,general findin in many prior arts is that mechanical properties and corrosion behavior is studied separately.No work reports about both mechanical and corrosion properties for the same samples.In few papers although they studied both but the results are not as promising as the present article.Both mechanical and corrosion behaviors of the Mgbased BM is studied in the present.It was found that developed Mg based BM for orthopedic applications,possessed promising mechanical and biological properties.
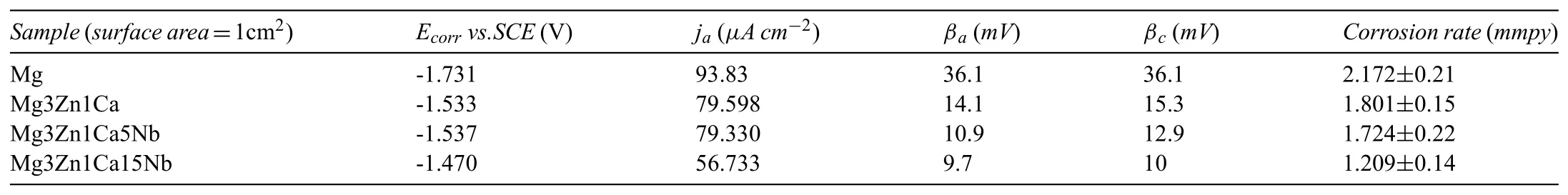
Table 9Corrosion parameters for the developed samples evaluated using Tafel analysis.
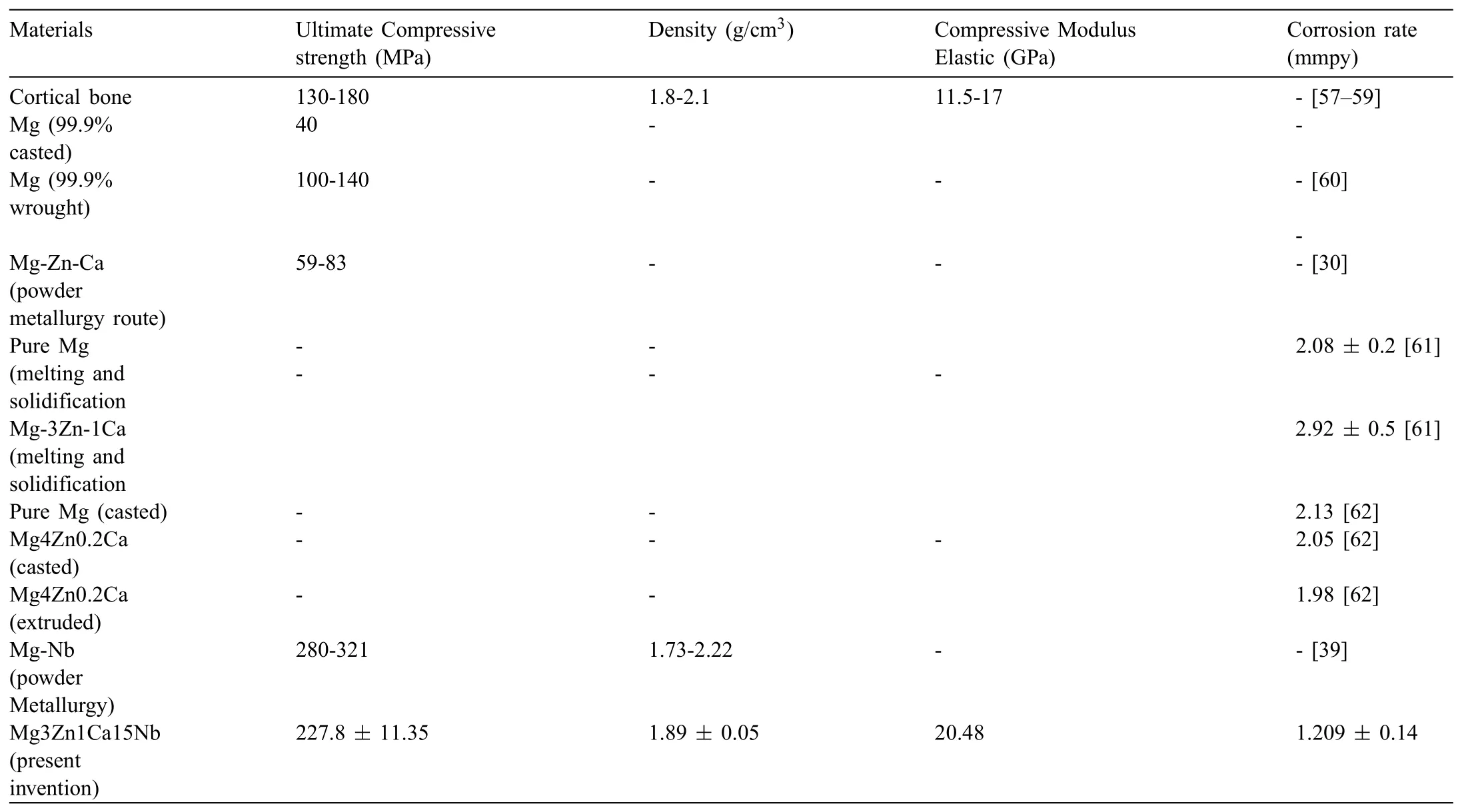
Table 10Comparison of mechanical and degradation properties of developed Mg based BM with prior studies.
8.Conclusions
·A novel Mg based Mg3Zn1Ca15Nb was developed successfully using conventional sintering route with improved mechanical and corrosion properties.It was elucidated from the XRD data that no phase or impurities were present in the developed sample as mandatory for its application the biomedical field
·The results revealed that the ultimate compressive strength was obtained for combination of maximum compaction pressure,maximum temperature and maximum soaking time.The reason for this result was attributed to the variation in sintering mechanism as well as elimination of porosity.Moreover,increase in UCS was found with increase in heating rate owing to enhanced adhesion quality and weld spot.
·The sintered density was found maximum at maximum level of compaction pressure,heating rate and sintering temperature.This was due the improvement in sintering mechanism owing to close packing of irregular particles with each other as well as elimination of obstacles from sintering route by destruction of oxide film from particles surface.Moreover,increase in soaking time did not have much effect in increment in sintered density.While increase in Nb % till certain limit resulted in homogenous mixture which allowed improvement in the sintered density.
·The developed Mg based BM showed a noticeable mechanical property(sintered density=1.89 g/cm3and UCS=227.8 MPa)at optimized sintering parameters(compaction pressure=500 MPa,heating rate=500°C/hrs,sintering temperature=640°C,soaking time=10 hrs,Nb(wt %)=15).
·Incorporation of Nb to Mg alloys led to higher corrosion resistance of the Mg based BM in SBF solution.Corrosion rates of developed Mg based BM decreased to approx.32.8% by the incorporation of 15% Nb to Mg3Zn1Ca alloy.
Declaration of Competing Interest
There is no conflic of interest.
Funding
This work was supported by Department of Science and Technology-Science and Engineering Research Board(DST-SERB),New Delhi,India(Grant reference no.EMR/2017/001550).
杂志排行
Journal of Magnesium and Alloys的其它文章
- Microstructural evolution of Mg-Al-Re alloy reinforced with alumina fiber
- Predicting and controlling interfacial microstructure of magnesium/aluminum bimetallic structures for improved interfacial bonding
- Plasma electrolytic oxidation of AZ31 and AZ91 magnesium alloys:Comparison of coatings formation mechanism
- Effects of annealing treatment on microstructure and tensile behavior of the Mg-Zn-Y-Nd alloy
- Microstructure and performance of biodegradable magnesium alloy tubes fabricated by local-heating-assisted dieless drawing
- Comparisons of microstructure homogeneity,texture and mechanical properties of AZ80 magnesium alloy fabricated by annular channel angular extrusion and backward extrusion
