The silicothermic reduction of magnesium in fl wing argon and numerical simulation of novel technology
2020-12-18YusiCheChoZhngJinxunSongXiojiShngXipingChenJilinHe
Yusi Che,Cho Zhng,Jinxun Song,Xioji Shng,Xiping Chen,Jilin He,∗
aHenan Province Industrial Technology Research Institute of Resources and Materials,Zhengzhou University,Zhengzhou 450001,China
b School of Material Science and Engineering,Zhengzhou University,Zhengzhou 450001,China
c School of Energy and Environment,Anhui University of Technology,Ma’anshan 243002,China
Received 25 September 2019;received in revised form 7 December 2019;accepted 24 December 2019 Available online 2 July 2020
Abstract The silicothermic reduction of magnesium was investigated by the non-isothermal thermoanalysis in f owing argon,while the traditional investigations of silicothermic process for magnesium reduction were carried out under vacuum conditions.Firstly,the thermal gravimetric(TG)and derivative thermogravimetric(DTG)characteristic of briquettes prepared with calcined dolomite,ferrosilicon and f uorite were characterized by the thermogravimetric analyzer(TGA)at different heating rates.The intrinsic chemical kinetic mechanism was identifie as a formal chemical reaction with the Nth order type which showed apparent activation energy E and reaction order n were 290.168kJ mol−1 and 1.076,respectively.Then,a novel technique of magnesium production without vacuum was put forward and a three-dimensional unsteady numerical model incorporating the chemical reaction,radiation,heat conduction and heat convection was established and simulated,which was verifie by Pidgeon process and novel technology.The numerical results showed that the cycle time of the novel technique could be reduced when the argon temperature was higher than 1343K and the argon entrance velocity was over 0.05m s−1.And the effect of the argon temperature on reduction degree was much larger than that of entrance velocity.© 2020 Published by Elsevier B.V.on behalf of Chongqing University.This is an open access article under the CC BY-NC-ND license.(http://creativecommons.org/licenses/by-nc-nd/4.0/)Peer review under responsibility of Chongqing University
Keywords:Kinetics mechanism;Heat convection;Silicothermic reduction;Numerical simulation.
1.Introduction
Magnesium alloys have been widely applied in structural materials,functional materials and additives[1-5],especially in the fiel of biomedicine,automobile production,which have been developing speedily in recent years[6-12].The raw magnesium production,the upstream of magnesium alloys,is mainly rely on the silicothermic reduction process at present[13].Due to advantages of rapid construction,producing high purity magnesium as well as the simple operation and low investment cost,the Pidgeon process has been developed rapidly since be introduced into China[14,15].The Pidgeon process is a thermal reduction process that extracts magnesium from calcined dolomite under a vacuum pressure of 10Pa and a temperature of 1100∼1200°C by the ferrosilicon as a reducing agent and calcium fluorid as a mineralization agent in a horizontally placed reduction pot.Pidgeon process is conducted under vacuum,which results in the process unable to continuous production.As well as reduction pot made of heat-resistant steel with high price and shorts life must be employed.Simultaneously,vacuum equipment is also an important component of the production cost[16].
Therefore,for development of the new low-cost magnesium production technology,several investigators studied the silicothermic reduction of calcined dolomite under nonvacuum condition.Barua and Wynnyckyj[17]reported on reaction kinetics of single spherical pellets under hydrogen in the temperature range of 1070∼1250 °C and found that the overall rates were higher than in vacuum.Kazhikenov and his team[18]suggested that the reduction reaction of silicothermic reduction of magnesium in argon fl w was limited by the diffusion of internal mass transfer.Morsi et al.[19]reported the silicothermic reduction process of dolomite under inert atmosphere was a solid-state reaction controlled by diffusion of reacted species and the apparent activation energy value was 306kJ mol−1.Wulandari and coworkers[20]investigated the kinetic analysis of silicothermic process under fl wing argon atmosphere,and found that the reaction was controlled by the solid-state diffusion of reactants with the Jander and Ginstling-Brounshtein model providing the best representation of the process kinetics.Almost all of kinetic researches of magnesium production in non-vacuum was implemented by isothermal analysis in resistance furnace.
Furthermore,among the numerous research methodologies of magnesium metallurgy,numerical simulation has gradually become an important method in recent years,especially in pilot-scale experimental research and industrial production research.Li et al.[21-23]established the conventional coal combustion model to describe the process of magnesium production in a coal-fire furnace.According to the experimental data,the numerical model was developed and used to predict the magnesium reduction performances in retort.Yu et al.[24]developed a mathematical model to simulate the phenomenon of the heat transfer occurring during a new magnesium reduction process and to predict the temperature distributions,the heating curves,and the total process time.Zhang et al.[25]developed a three-dimensional unsteady numerical approach incorporating the chemical reaction,radiation and heat conduction models and verifie by industrial production data of the magnesium production enterprises.And the method of numerical simulation was applied in research of the novel technology under argon atmosphere.
This paper aims to investigate the kinetic mechanism of the silicothermic reduction of calcined dolomite in fl wing argon by TGA,and to obtain the appropriate chemical reaction kinetic model.According to the chemical reaction characteristic of silicothermic in f owing argon and the feature of Pidgeon process,a novel process without vacuum was developed.And combing with the numerical simulation method put forward by our research group[25],a real three-dimensional physical model was established,which could simulate the actual arrangement of briquettes crowded layer in the reduction pot.Then a three-dimensional unsteady numerical approach model incorporating the chemical reaction,radiation,heat conduction and heat convection was built base on the new process.Finally,the effect of convection heat transfer and argon characteristic on magnesium reduction process was identifie by this numerical model.
2.Materials and methods
The materials used in experiment were in accordance with the Pidgeon process,including calcined dolomite,ferrosilicon and fluorite and the chemical compositions were listed in Table 1,respectively.
The preparation process of briquettes was showed in Fig.1.First,dolomite(CaCO3·MgCO3)was crushed into particles size of 5mm to calcine for 1.5h at 1100 °C,and the decomposition reaction followed as Eq.(1),
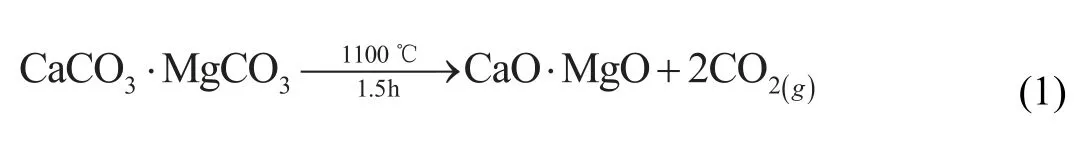
To measure the hydration activity of calcined dolomite(CaO·MgO)should be carried out instantly,and to powder and screen could be done simultaneously.The calcined dolomite,ferrosilicon and fluorit were sieved with 100 mesh,respectively.With Si/2MgO mol ratios of 1.2 and CaF2mass fraction of 3%,all powders were evenly mixed and moulded with the forming pressure of 150MPa.The briquette with a thickness of 0.72mm was divided into small masses to test.
According to the silicothermic reduction[26]and composition of reactants,if the magnesium reduction reaction can be proceed,the inner of briquette will work as Eq.(2),
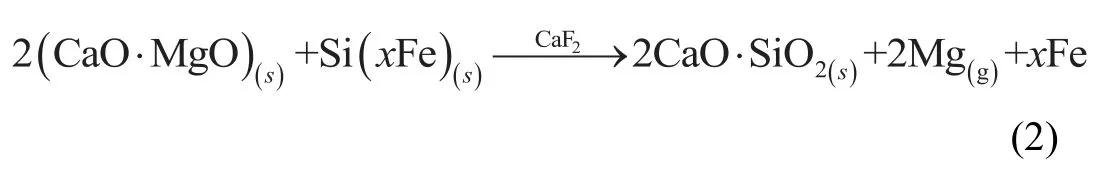
Where the subscriptsandgare solid and gas,xis stoichiometric coefficient
ICP-OES analyzer(iCAP 7200,Thermo Scientific America)and simultaneous thermal analyzer(STA 449 F3 Jupiter,Netzsch,Germany)were used for testing the chemical compositions and the chemical reaction characteristics of briquette,respectively.
In this study,purge gas and protective gas were both argon with purity of 99.999%,and TG plate sample carrier was selected to carry sample.The pressure of argon feed was adjusted at 0.05MPa and the temperature was set from 473 to 1573K.Some experimental parameter and mass of briquette listed in Table 2.
The magnesium conversion obtained by mass change of before and after the reaction is expressed as follows,

whereαis conversion.Mbeforeis the mass of briquette before reduction.Mafteris the mass of briquette after the reaction.ω% is theoretical content of magnesium element in briquette.
3.Results and discussion
3.1.Experimental results and kinetic analysis
Fig.2 shows TG/DTG curves of briquettes determined by STA at different heating rate of 10,15,and 20K min−1.The three obvious mass losses on every TG curve and three peaks on every DTG curve are attribute to three chemical reactions during the heating process.As it is known,calcined dolomite absorb moisture from the air to become Ca(OH)2and Mg(OH)2,and the effect of the former more obvious than the latter.Besides,exposing Ca(OH)2and Mg(OH)2in the air fora long time,the former absorbing CO2further from air to become CaCO3is more easily than latter to generate MgCO3.In Fig.2,the frst mass loss event or peak presenting at the temperature of 620K is apparently lower than the decomposition temperature of carbonate.Thus the most likely cause for the firs peak is the decomposition of Ca(OH)2.The second mass loss event or peak,which has a less obvious tendency to appear at about the temperature of 850K,is caused mainly by the decomposition of calcium carbonate.Moreover,different degrees of metamorphism cause different mass loss changes in the three curves.The platform region between the second and the third peak on DTG curves demonstrates that there is no other reaction.The third mass loss or peak,caused by formation of magnesium vapor,reveals the law of chemicalreaction within briquette,which is the chemical reaction characteristic focused on in this paper.
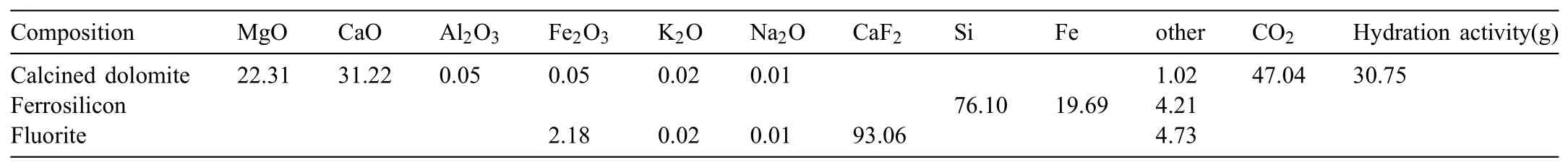
Table 1The composition and calcining index of calcined dolomite(wt%).
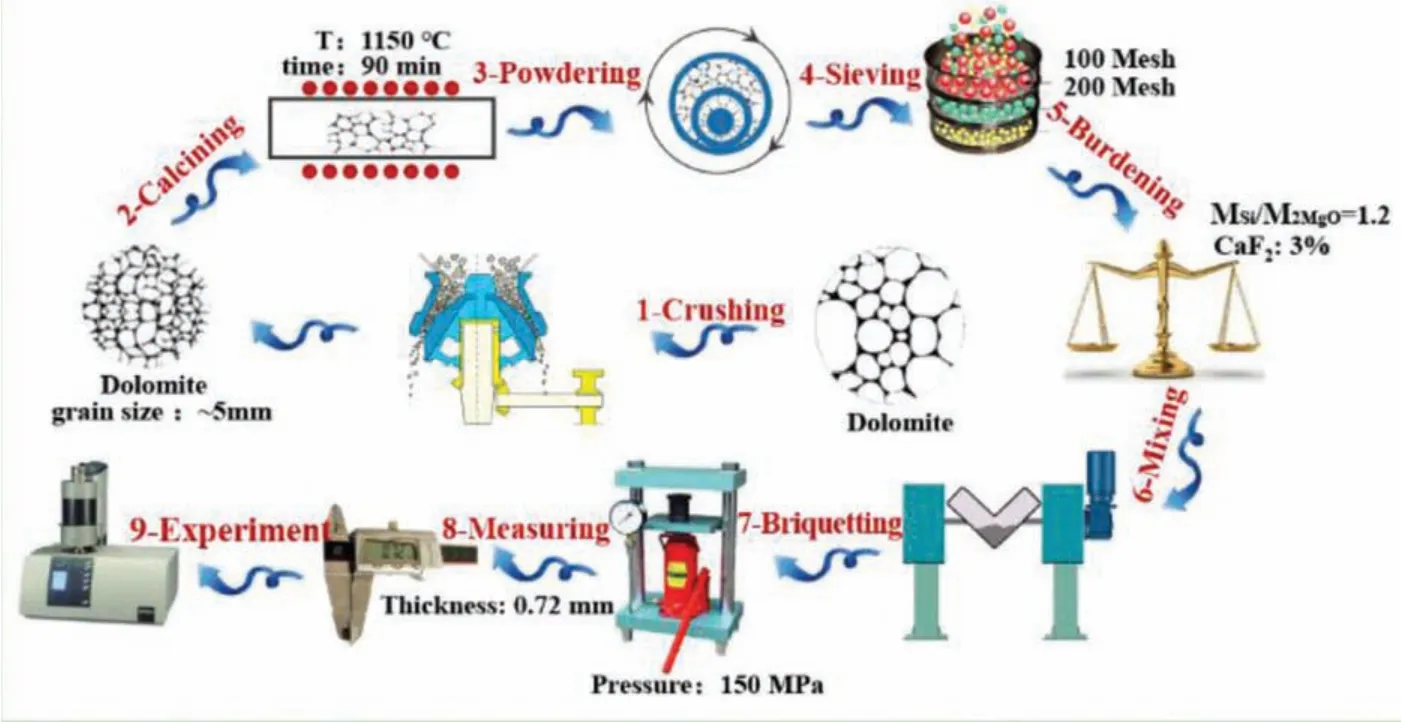
Fig.1.Preparation process of briquette.

Fig.2.The TG and DTG curves of briquettes in argon fl w at different heating rate.
Table 2Experimental parameters and the mass of sample.
The silicothermic reduction of calcined dolomite is belong to solid phase reaction,where the kinetic mechanisms are commonly described by the formal chemical reaction,diffusion,phase boundary reaction and nucleation and growth.According to the method proposed by Bagchi et al.[27],if mechanism functions are selected reasonably,apparent activation energyEand pre-exponential factorAcalculated from differential and integral equations of the same mechanism function are approximate.Thus,the kinetics mechanisms can be analyzed from TG curves at different heating rate in Fig.2.
Two logarithmic forms of integral and differential for analysis of non-isothermal kinetic data are given by Eqs.(4)and(5),
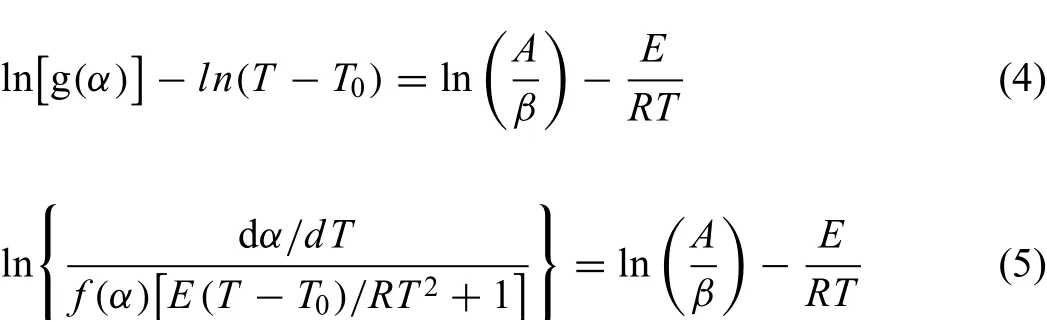
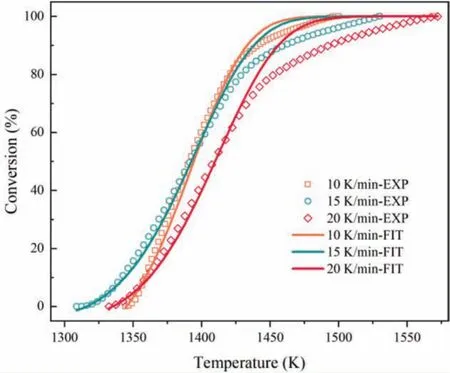
Fig.3.Comparison of experimental data and simulated data at heating rate of 10,15 and 20K min−1.
Whereg(α)is the mechanism function in integral form,Tis the reaction temperature,K.T0is the initial reaction temperature,K.Ais the pre-exponential factor,s−1.βis heating rate,K min−1.Eis the apparent activation energy,J mol−1.R is molar gas constant,8.314J mol−1K−.f(α)is the mechanism function in integral differential form.
On the basis of Eqs.(4)and(5),the common mechanism functions of solid-phase reaction are selected for computation.The apparent activation energyEand the pre-exponential constantAcalculated from differential and integral equations in all possible reasonable functions are compared,and the result shows that the intrinsic chemical kinetic mechanism is the formal chemical reaction with the Nth order type,the correlation R2is the greatest with 0.99801.The apparent activation energyEis 290.168kJ mol−1,reaction ordernis 1.076 and pre-exponential factorAis 8.25 log(s−1).
By substitutingg(α)of Nth-order chemical reaction into Eq.(6)gives:
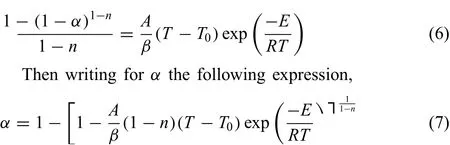
Substituting kinetic parameters ofE,Aandninto Eq.(7),the f tted curves at different heating rate could be plotted.Then comparison of experimental and simulated values follows as Fig.3.
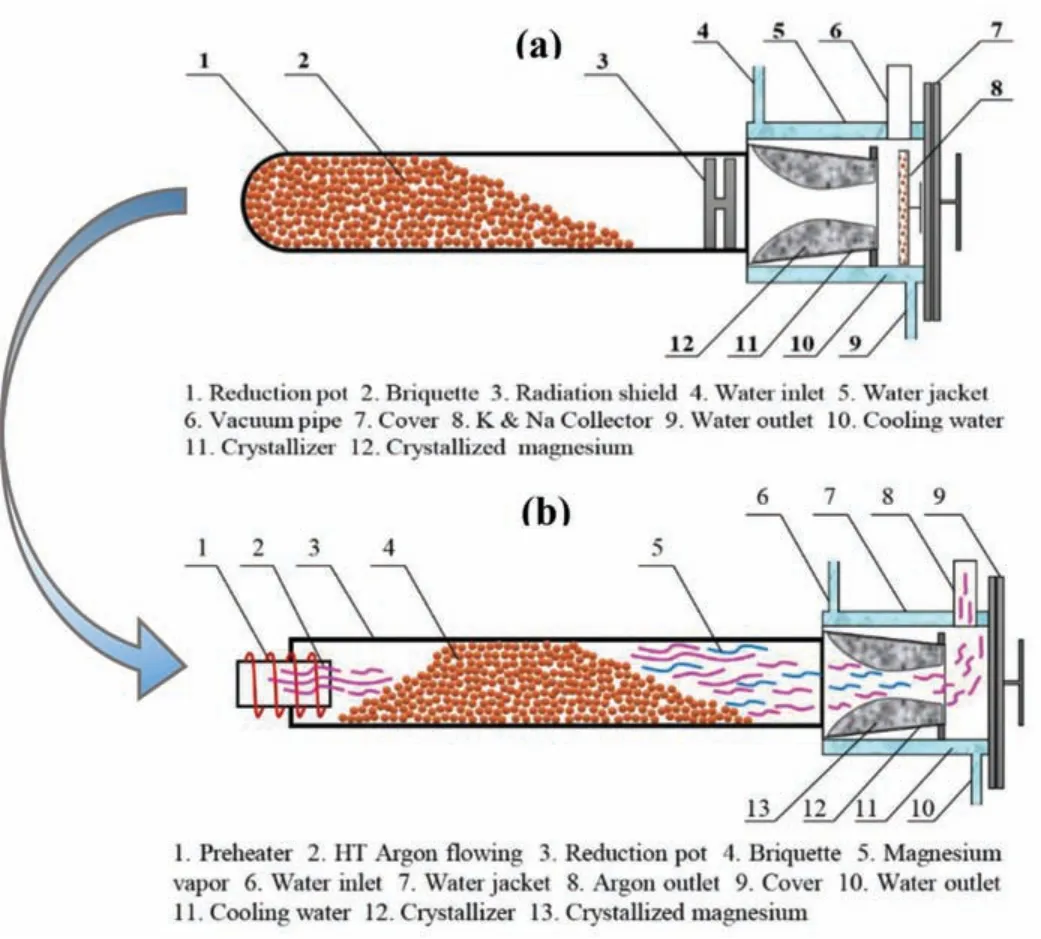
Fig.4.The schematic diagram of structure and working principle of reduction pot.(a)The Pidgeon process;(b)The novel technique.
As Fig.3 dedicates that when the temperature is below 1413K or conversion is less than 70%,the experimental curves matche with simulated curves perfectly.Whereas,the fitte values are higher than experimental values.However,in all kinetic mechanism functions,the best-fittin model is the formal chemical reaction with the Nth order type and the greatest correlation R2.The results are basically the same as Xu and Yuan[28],and they present shrinking unreacted core model with quasi interfacial reaction by analysis of experimental results.To some extent,we deduce that the reaction is likely to be a continuous two step or multi-step controlled dynamic process,but it requires in-depth studies and more evidences.In addition,there are many factors that influenc the results of the experiment,such as the shape and mass of sample,the degree of moisture absorption of sample and others[29-31].Too small mass and manually segmented of sample,so it is difficul to ensure that the mass and shape of each sample is identical.Therefore,the regularity of the experimental results is not perfect.
3.2.Numerical results and discussion
3.2.1.Physical model
The schematic diagram of structure and working principle of reduction pot by Pidgeon process and new process were shown in Fig.4.
The Fig.4(a)shows that the briquettes occur reduction reaction under high temperature and vacuum in the reduction pot to generate the magnesium vapor,and the magnesium vapor passes through the briquettes layer and fl ws into the crystallizer to form crystallized magnesium,and crystallizer maintains the constant temperature by cooling water.In Fig.4(b),the high temperature argon fl ws into reduction pot from one side and passes through the briquettes,then entrains the magnesium vapor produced in reaction region into magnesium crystallizer.When the mixed gas of argon and magnesium vapor fl ws through the crystallizer,the magnesium vapor is condensed into crystallized magnesium cause of low condensation temperature,and argon eliminated from the other side of pot finall.
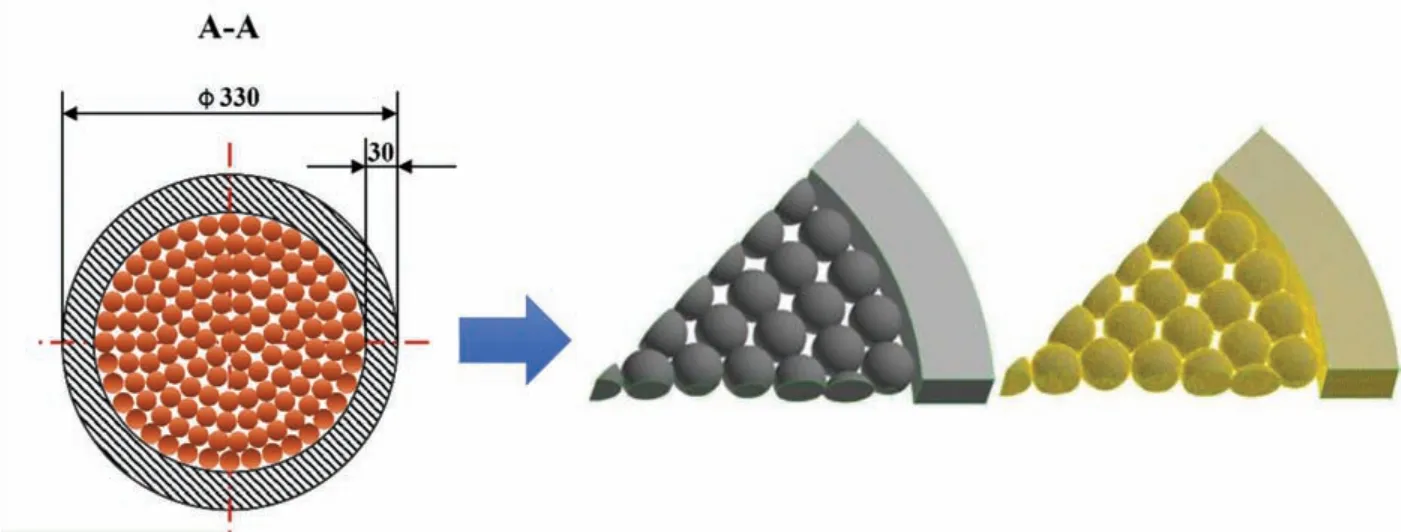
Fig.5.Cross sectional of reduction pot and schematic diagram of the three-dimensional computational regions and the grid system.
In Pidgeon process,the elliptical spherical briquettes are in a random arrangement in reduction pot with an outer diameter of 330mm and wall thickness of 30mm.The same size of reduction pot and briquettes is employed in the new process of our work.To calculate conveniently,the elliptical spherical briquettes in industrial production of Pidgeon process were simplifie into spherical ones by volume,and an eighth of cross section and one briquette layer in height was taken as the computational domain.The briquettes were arranged in a random order,which is coincided with the actual industrial production conditions.Then the physical model of the novel technology basing on Pidgeon process was developed.
3.2.2.Numerical simulation
Fig.5 shows the reduction pot and briquettes,as well as the three-dimensional computational regions and the grid system.A series of meshes were tested to take the grid independence into account and independent simulation results were achieved when the grid number reached 7.5×105.
For the briquette,the governing equation for the threedimensional numerical model in a polar coordinate system can be described as follows,
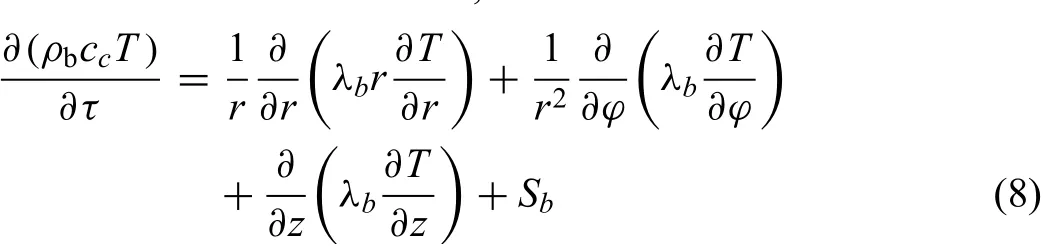
whereρbis the density of briquette,kgm−3.cbis specifi heat capacity of briquette,J kg−1.λbis the thermal conductivity of briquette,Wm−1K−1.Tis the temperature of briquette,K.Sbis the chemical reaction heat source,is also related to rate of chemical reaction of Eq.(8),and could be calculated by Eq.(9),

WhereMbis the maximum magnesium production per unit volume and the value is 12,587.9mol m−3according the ratio of reactants.φbis the thermal energy required per mole of magnesium in the reduction reaction,J mol−1,and the expression ofφbcould be given as follows:

dα/dτis the change in rate of reduction degree with time and it can be expressed as,
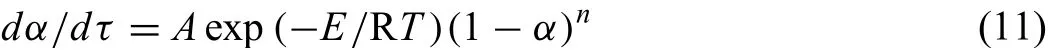
By substituting kinetic parameters value ofE,Aandninto Eq.(11),
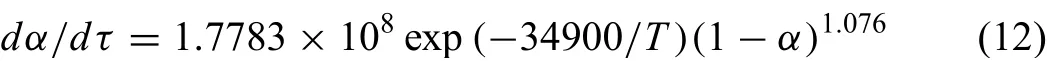
In the process of silicothermic reduction of calcined dolomite at high temperature,thermal radiation plays an important role as the same as heat conduction[32-34].In this work,the Discrete Transfer radiation model(DTRM)in Fluent was suitable to describe the model which was the radiative heat transfer between briquettes,briquettes and pot wall.The verificatio of the accuracy of DTRM model and the method of using correctly the model has been accomplished before.In this improved silicothermic reduction process,the high temperature argon fl ws into reduction pot and crosses the briquettes.Thus,the convection heat transfer between argon and briquettes could be calculated by[35],

WhereAbis the surface area of the briquette,m2.TAris the temperature of argon,K.hconis the convection heat transfer coefficien calculated from the correlation,
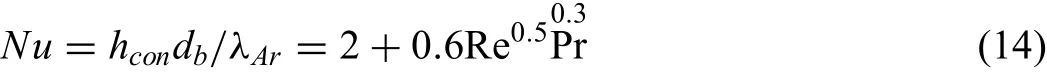
WhereNuis the Nusselt number,dbis the diameter of briquette which was assumed to remain constant while the density of briquette changed during the reduction process,m.λAris the thermal conductivity of argon,W m−1K−1.Reis the Reynolds number based on the briquette diameter and relative velocity.Pr is the Prandtl number of argon.
During silicothermic reduction process,the density,thermal conductivity,specifi heat and emissivity of the briquettes change over the extent of the reduction.To obtain more accurately results[36],the extent-dependent properties were used in our numerical calculation process.Due to convection transfer conducted between argon and the briquettes surface,the temperature of argon may vary significantl in the process of fl wing.Therefore,temperature-dependent density,thermal conduction and heat capacity of argon must be considered in the numerical calculation process.Table 3 summarizes the extent-dependent thermophysical properties of briquette and the temperature-dependent thermophysical properties of argon.
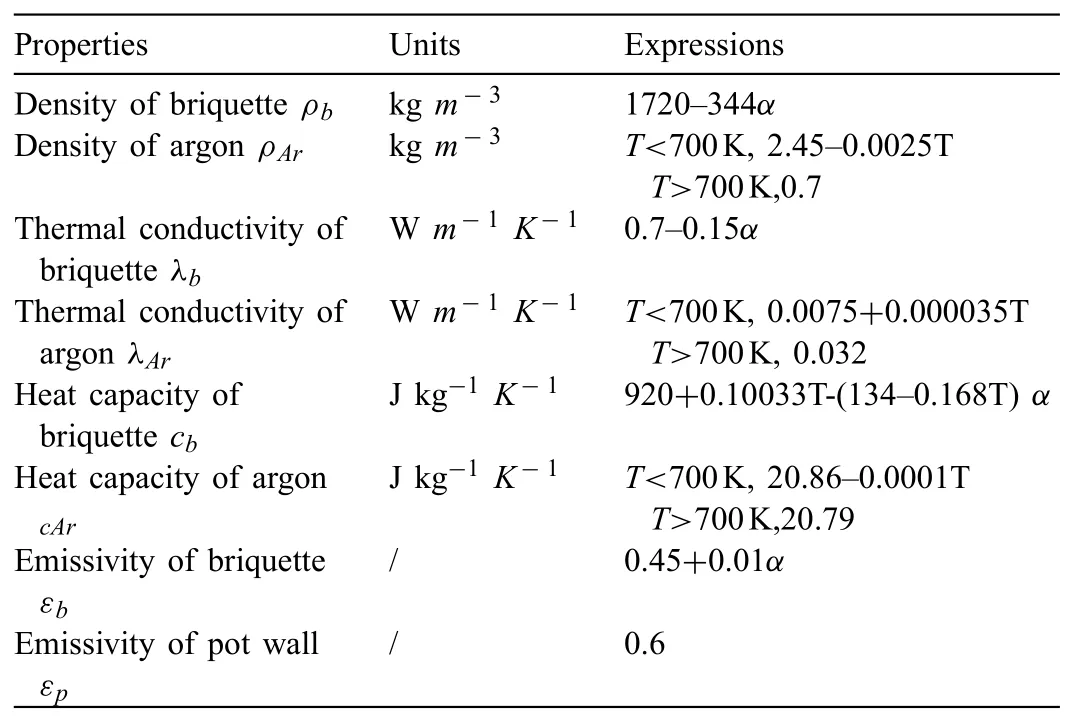
Table 3Thermophysical properties used in the numerical calculation process.
Considering the symmetry of the calculation region,the boundary conditions are listed as follows,
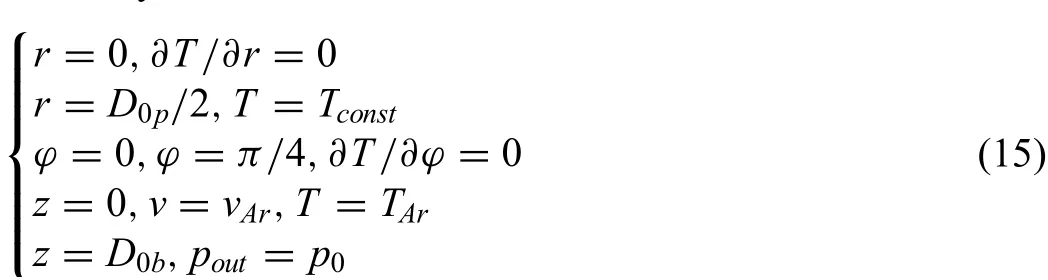
Where D0p,D0bare the diameter of the reduction pot,briquette,respectively,m.Tconstis the temperature of the outer wall of the reduction pot,K.vAris the entrance velocity of argon,m s−1.TAris the temperature of argon,K.poutandp0are outlet pressure and atmospheric pressure,respectively,Pa.
Initial conditions:

3.2.3.Results and discussion
3.2.3.1.Comparison with Pidgeon process.As a new silicothermic reduction technique,the improved process presented in this article has not been applied in magnesium industrial production.Although the results of numerical calculation could not be directly validated by industrial data,the improved technique is based on Pidgeon process,and the structure size of reduction pot and briquette used in this threedimensional unsteady numerical model were the same as Pidgeon process.Thus,the calculated results could be compared with the data of Pidgeon process[16,37].To simulate more accurately,the new magnesium industrial production process,the temperature of the outer wall of the reduction pot was set to 1450K,and the velocity and temperature of argon was set to 0.025ms−1and 1343K,respectively.

Fig.6.Comparison of simulation results with the industrial production data of Pidgeon process.
Fig.6 compares the extent of magnesium reduction in a single pot with the industrial production data of Pidgeon process.The results show that the magnesium reduction degree of the new technique is larger than the industrial production data of Pidgeon process in a magnesium production period.In addition,the deviation decreased with increasing reduction time and the magnesium production period of the new technique is still about eight hours.It is clear that the f ow of high temperature argon enhanced heat transfer in the reduction pot,especially during the early stage of the period.In Pidgeon process,heat transfer occurring from layer by layer for the only source of heat was the outer wall of the reduction pot.And there were only two forms of solid conduction and surface radiation heat transfer.In contrast,the new technique provided an additional convection heat transfer between the briquettes on the basis of solid conduction and radiation heat transfer.
It can be expected that the three-dimensional unsteady numerical model,incorporating the radiation,heat conduction,convection heat transfer and intrinsic chemical dynamic models,is reasonable and feasible.Compared with Pidgeon process,the new technique does not require any vacuum equipment and short life heat-resistant steel reduction pot,which leading to the initial investment reducing and providing possibility for continuous production.Based on industrial data of Pidgeon process and numerical simulation results of the new technique,it can be inferred that the magnesium production efficien y could be promoted,and production period could be shorted by increasing the temperature of argon.
3.2.3.2.Effect of argon entrance velocity and temperature on the magnesium production process.Duo to gas fl w rate strongly impacted the convection heat transfer,the effect of argon entrance velocity and temperature on the production process was investigated in this section,and the simulated results were showed in Fig.7.
Fig.7(a)shows the curves of magnesium reduction degree with time under different argon entrance velocity.Setting the temperatures of the outer wall and argon were set to 1450K and 1343K respectively,the results presented that the reduction degree increased gradually with time for each argon entrance velocity,while the extent rise decreased with time.Besides magnesium reduction degree increased with argon entrance velocity although all of the curves showed a similar trend.It is worth mentioning that the improvement of extent is limited when argon entrance velocity is lower than 0.05m s−1.
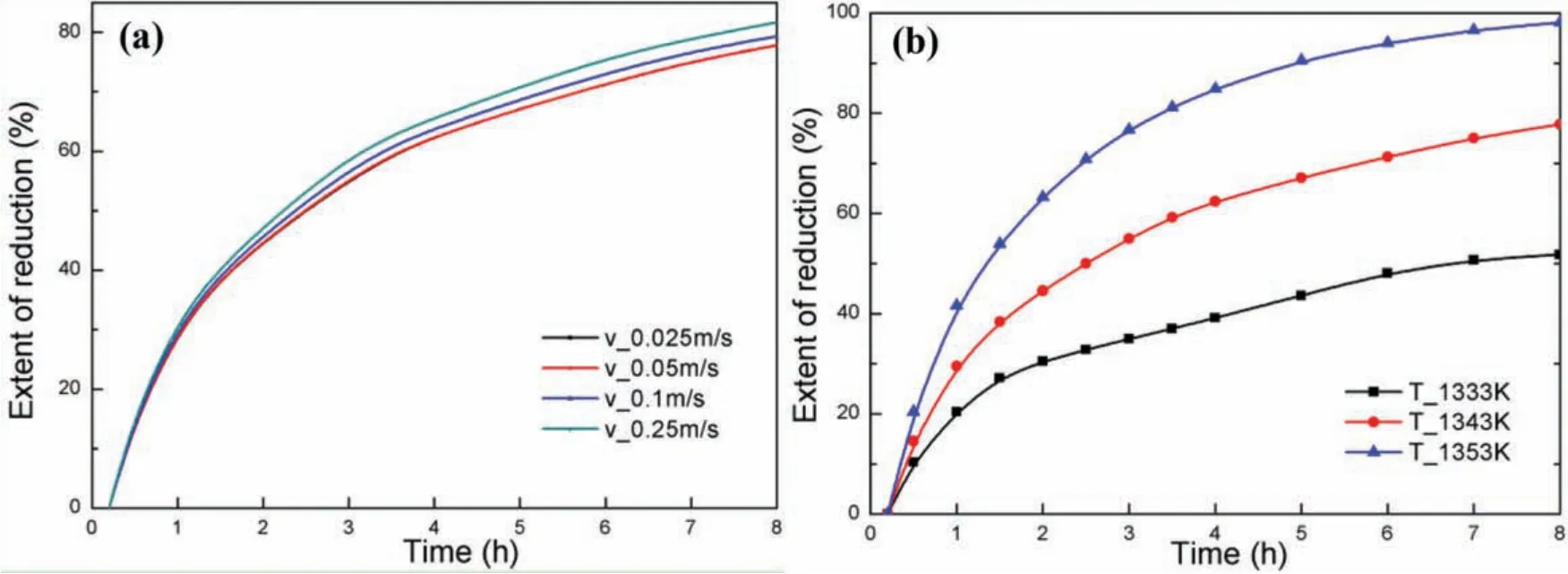
Fig.7.Effect of argon characteristics on magnesium reduction degree.(a)Effect of argon entrance velocity;(b)Effect of argon entrance temperature.
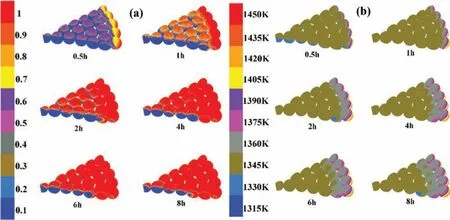
Fig.8.Distribution of magnesium reduction characteristics with time.(a)Magnesium reduction degree with time;(b)Magnesium reduction temperature with time.
Fig.7(b)shows the curves of magnesium reduction degree with time under different argon temperature.The temperatures of the outer wall and argon entrance velocity were set to 1450K and 0.1m s−1,respectively.The reduction degree enhanced with the increasing of argon temperature,and an extent of about 20% was improved as the argon temperature increased by 10K.It should be clear that the effect of the argon temperature on magnesium reduction degree is much larger than that of the entrance velocity.And the efficien y of the new technique is higher than that of Pidgeon process obviously,when the temperature of argon is over 1343K.
The phenomenon above might be attributed to the reason that the high temperature argon could heat the briquettes when the temperature of briquettes was lower than that of the argon.However,when the temperature of briquette exceeded the argon temperature,the high temperature argon turned to a cold source leading to the decrease of temperature.This conclusion could be demonstrated by the exit temperature of argon.when the entrance temperature was set to 1333K,the exit temperature of argon reached 1361K.A wealth of information found in the numerical calculation results indicates that the application of the new technique could shorten the magnesium production cycle time,when the temperature of argon is above 1343K.
Comparing Figs.6 and 7(b),the production cycle of the new technique is approximately only 3−4h when the temperature of argon is 1353K,while the production cycle of Pidgeon process exceeds 8h.This means that the production capacity can be improved more than 100%.
3.2.3.3.Distribution principle of magnesium reduction characteristics with time.The distribution of magnesium reduction degree and reaction temperature with time was simulated in this section,and the results were showed in Fig.8.
Fig.8(a)presents the magnesium reduction degree distribution with time at a constant outer wall temperature of 1450K,argon temperature of 1343K and argon entrance velocity of 0.025m s−1.The reduction process advanced from external to internal and from up to down,is thorough by layer upon layer progressive principle.This character of the new technique is very different from Pidgeon process.Besides,the effect of the heating of high temperature argon on the reduction degree mostly occurred in the firs two hours.The prime reason is that the temperature of argon is lower than that of outer wall.At the initial stage of less than 2h,the temperature of briquettes is pretty low and the high temperature argon heated the briquettes which could promote the chemical reaction.Two hours later,the effect of heating of high temperature argon on the reduction degree become weak as the intake temperature increasing.For some briquettes which temperature is higher than 1343K,the argon transformed from a heat source to a cold source resulting in the increasing amplitude of magnesium reduction degree decreased at the late stage.
Fig.8(b)shows the temperature distribution and its evolution with reduction time at a constant outer wall temperature of 1450K,argon temperature of 1343K and argon entrance velocity of 0.025m s−1.The results show that the temperature of briquettes go up extremely at the initial stage,but the trend become quite slowly at the later stage.The tendency of the temperature distribution is similar to that of the degree.The minimum temperature of the briquettes sharply increases from 300K to 1323K during the firs two hours,then nearly unchanged in the following several hours.The reason may be that there is a large temperature gradient in the early phase,and both of the high temperature argon as well as outer wall of the pot can heat up the briquette.With time progressing,the reaction heat absorption increases extremely and the argon is transformed from the heat source to the cold source when the briquette temperature is higher than 1343K.
4.Conclusion
Silicothermic reduction of magnesium under argon fl wing was investigated by thermogravimetric analysis.The result shows the kinetic mechanism of intrinsic chemical reaction is the Nth order formal chemical reaction.Then according to Pidgeon process,the improved technology without vacuum is established,where the reaction is carried out under argon fl wing.After that,the three-dimensional unsteady numerical model incorporating the chemical reaction,radiation,heat conduction and convection heat transfer is established based on the physical model of the novel technology,and it is verifie by technical data from Pidgeon process and improved technology.
The effects of argon entrance velocity and temperature on the magnesium production process were simulated in the reduction pot,as well as the magnesium reduction degree distribution characteristics and temperature distribution characteristics.The result shows that the silicothermic reduction process of magnesium smelting under the condition of high temperature and argon fl wing is feasible theoretically.The cycle time of this novel technique can be shortened when the temperature of argon is higher than 1343K,and the magnesium reduction degree increases with argon entrance velocity when the argon entrance velocity is over 0.05ms−1.The effect of the argon temperature on magnesium reduction degree is much larger than that of the entrance velocity,implying that raising the argon temperature can improve the magnesium production capacity.The production cycle is only half of Pidgeon process,when the temperature of argon used in the new technique is 1353K.And in novel technology,not only argon gas is used circularly,but also heat of argon is circulation utilization.
Acknowledgement
The study was supported by the National Key R&D Program of China(Grant No.2016YFB0301100)and Anhui Provincial Natural Science Foundation of China(Grant No.1808085QE152).
杂志排行
Journal of Magnesium and Alloys的其它文章
- Microstructural evolution of Mg-Al-Re alloy reinforced with alumina fiber
- Predicting and controlling interfacial microstructure of magnesium/aluminum bimetallic structures for improved interfacial bonding
- Plasma electrolytic oxidation of AZ31 and AZ91 magnesium alloys:Comparison of coatings formation mechanism
- Effects of annealing treatment on microstructure and tensile behavior of the Mg-Zn-Y-Nd alloy
- Microstructure and performance of biodegradable magnesium alloy tubes fabricated by local-heating-assisted dieless drawing
- Comparisons of microstructure homogeneity,texture and mechanical properties of AZ80 magnesium alloy fabricated by annular channel angular extrusion and backward extrusion
