Microstructural evolution of Mg-Al-Re alloy reinforced with alumina fiber
2020-12-18LiLiDejingLiXioqinZengAlnLuoBinHuAnilShevLinglingGuWenjingDing
Li Li,Dejing Li,Xioqin Zeng,∗,Aln A.Luo,Bin Hu,Anil K.Shev,Lingling Gu,Wenjing Ding
a Shanghai Jiao Tong University,Shanghai,China
b The Ohio State University,Columbus,OH,United States
c General Motors China Science Lab,Shanghai,China
d General Motors Global R&D Center,Warren,MI,United States
Received 5 June 2019;received in revised form 28 July 2019;accepted 29 July 2019 Available online 20 July 2020
Abstract Few studies were reported on the phases'relationships of AE44(Mg-4.0Al-4.1RE-0.3Mn,wt.%)and its composites.In this work,AE44 alloy and Saff l(δ-Al2O3)/AE44 Metal matrix composite(MMC)were both prepared by slow shot high pressure die casting(SS-HPDC)technology and their phase constitutions were all studied in detail using experimental techniques combined with CALPHAD(Calculation of Phase Diagram)modeling.The results revealed that the alloy consists of theα-Mg matrix,Al11RE3 intermetallic phase,and one trace phase Al3RE,while the composite contains fi e major phases:α-Mg,δ-Al2O3,Al3RE,MgO and Mg2Si,and two trace phases of Al2RE and Al11RE3,respectively.Al11RE3 is partly derived from Al2RE,while Al3RE is a product of the peritectoid reaction between the two precipitates.The presence of MgO and Mg2Si is due to the interfacial reaction between the SiO2 binder in the fibe preforms and the molten magnesium during infiltration The use of SiO2 binder in the preform manufacturing was limited/minimized to reduce the MgO formation in the MMC casting process,which can be detrimental to the fatigue performance of the MMC materials.© 2020 Published by Elsevier B.V.on behalf of Chongqing University.This is an open access article under the CC BY-NC-ND license.(http://creativecommons.org/licenses/by-nc-nd/4.0/)Peer review under responsibility of Chongqing University
Keywords:Mg-Al-RE;Magnesium composite;Slow shot high pressure die casting;Microstructure;CALPHAD;AlxREy.
1.Introduction
Metal matrix composites(MMCs)are important emerging materials for structural applications in automotive and aerospace industries due to their high Young's modulus,excellent elevated temperature,improved wear resistance and reduced coefficien of thermal expansion[1,2].Compared to continuously reinforced MMCs,discontinuously-reinforced MMCs have attracted more attention due to their isotropic behavior,ease of fabrication and low cost[1].Furthermore,the light metal alloy matrix provides weight reduction for improved fuel economy and vehicle handling performance.An important area for consideration is the application of discontinuously reinforced MMCs in automotive engine systems[2].
Al-matrix MMCs have been used in a few automotive engine applications,such as the Prelude cylinder bores[3]and cylinder engine bores[4].Mg-matrix MMCs would offer additional mass savings compared with Al-matrix MMCs.A creep-resistant magnesium alloy was considered as the matrix to ensure its resistance to temperature.Several alloy systems,such as Mg-Al-Ca/Sr,Mg-Al-Si and Mg-Al-RE,have shown good creep resistance at or above 150°C[1,2,4-8].Among these,a new alloy Mg-4Al-4RE(AE44)[8]has shown excellent elevated temperature creep,tensile strength,and was used in the production of magnesium engine cradles and selected as the matrix alloy in this MMC development.
Mg-based MMCs can provide improved wear resistance and stiffness when suitable reinforcements are introduced.In previous work[9],the mechanical and tribological properties of theδ-Al2O3/AE44 MMC were characterized,and the interfacial characteristics between the Saffi fiber and the AE44 alloy were also investigated.The results showed thatδ-Al2O3/AE44 MMC has the potential to satisfy the requirements for cylindrical bore materials in automotive engine applications.
In addition to the chemistry of the matrix alloy and the reinforcement choice,the choice of the manufacturing process to produce the MMC components is important.Squeeze casting is generally used to infiltrat preforms of reinforcement fiber to manufacture MMC components,however,its low productivity and high cost limit its widespread applications to produce MMCs such asδ-Al2O3/AE44[8].A new process named Slow Shot High-Pressure Die Casting technology(SS-HPDC)was thus developed[9].The SS-HPDC process offers a potential low-cost alternative to manufacturing small engine blocks containing magnesium MMC bores by reducing the plunger shot speed to ensure steady cavity fil and preform infiltratio without damaging the preforms.This process distinguishes itself from conventional high pressure die-casting(HPDC),a high-productivity process used in the production of most aluminum and magnesium power-train components in the automotive industry.Ultimately,the properties of an MMC material depends primarily upon the phases and microstructural constituents present,which are related to the matrix alloy,reinforcement and manufacturing process selected.
A fundamental understanding of the phase relationships is needed for designing MMCs for engine applications.Although the phase constitution of AE44 alloy under different manufacturing process conditions has been extensively studied[10-29,46-51],the microstructure in the presence of reinforcement and the corresponding mechanism behind are still not fully understood.
In this study,Mg cylinder blocks with Saffi fibereinforced bores were successfully cast using the SS-HPDC process.These locally reinforced magnesium composites are a novel class of ultra-light materials that promise extensive weight reduction coupled with tribology improvements restricted to a local area where it is only needed.Microstructure characteristics such as the probable interfacial reactions between the matrix and preforms,phase identificatio and solidificatio sequence were investigated using various experimental techniques assisted by CALPHAD modeling.
2.Experimental procedure
The materials used for MMC casting trials were AE44 magnesium alloy and Saffi alumina fibe preforms.Table 1 shows the chemical composition of commercial AE44 ingots,as measured by inductively coupled plasma atomic emission spectroscopy(ICP-AES).The chemical and physical characteristics of the preforms bought are shown in Table 2,and their dimensions are shown in Fig.1.
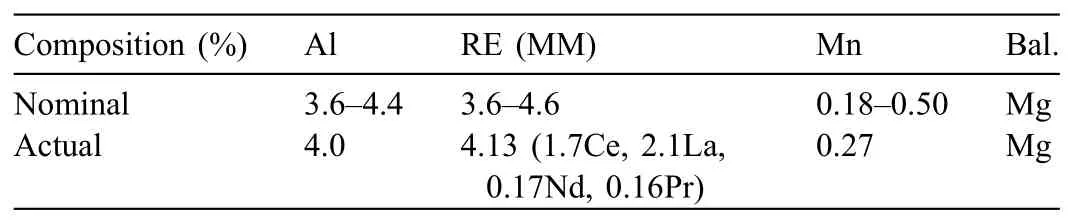
Table 1Composition(by weight)of AE44 alloy∗.
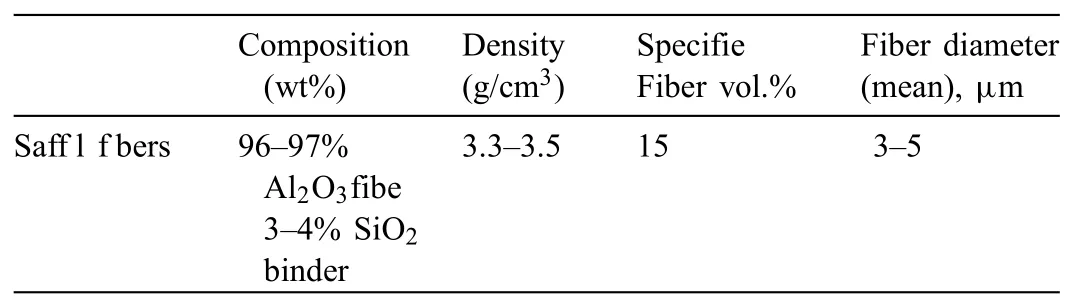
Table 2Saff l f ber properties for MMC preforms.
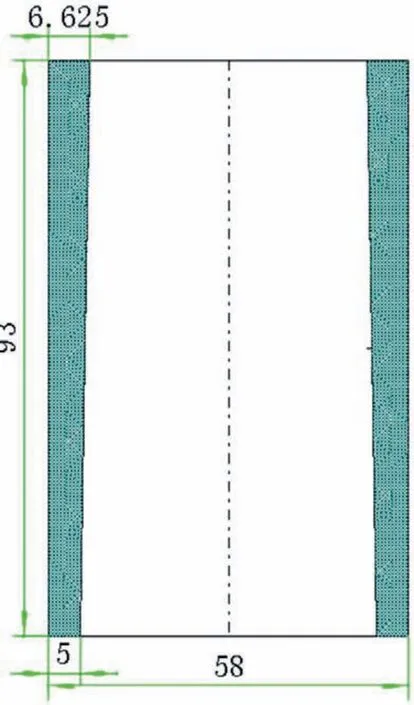
Fig.1.Preform dimensions and geometry(unit,mm).
The SS-HPDC trials were conducted using a cold-chamber die casting machine(UBE 650T HPDC).The plunger speed in the intensificatio phase varied from 100 to 250cm/s with a 90MPa intensificatio pressure on the melt(760°C).The Saffi preforms and the engine block die were preheated to 760°C and 275°C,respectively.The preheated preforms were placed in the die,which was then closed,after which molten magnesium was transferred at 760°C to the short sleeve and pushed into the die cavity.All melting and handling of molten magnesium alloy was done under an atmosphere of CO2with 1vol.% SF6.More process details on the SS-HPDC technique were published in an earlier[9].
The samples for microstructure observation were cut from the cylinder castings and then ground using 220,320,500,and 800 grit SiC papers and polished using 6μm and then 1μm diamond paste.Rinsing at each stage was done with 0.04μm SiO2suspension solution for slowing the surface corrosion rate of samples.

Fig.2.Cross-section of the AE44/δ-Al2O3 MMC engine block.
The microstructure of the matrix alloy and MMC samples was examined using an optical microscope(OM),and a scanning electron microscope(SEM)equipped with an energy dispersive X-ray spectrometer(EDX),SIRION 200/INCA OXFORD SEM.
Elemental analysis and phase identificatio were performed with an EDX at an accelerating voltage of 15kV,while Xray diffraction(XRD)was conducted with an Ultima IV XRD with D/teX Ultra detector operating at 40kV and 30mA with Cu Kαradiation.The measurements were performed by continuous scanning for 2h from 10° to 90° with a step size of 0.01°.The count time per step was more than 1s in order to increase the signal detective probability and to detect the phases that could be present in small amounts.A JEM2100F transmission electron microscope(TEM)operated at 200kV was used to evaluate the phases present at the interface observed.This was coupled with selected area electron diffraction(SAED)and energy dispersion spectroscopy(EDS).The TEM specimens were cut with a linear cutting machine and mechanically ground to a thickness of 50μm,and then thinned by the ion milling.Firstly,were milled until a hole opening up at the middle around 1.5-3 h of 6KeV,then followed by 0.5-1 h at 2-3KeV and last 0.5-1 h at 0.5-1KeV.All of these steps were done at±5°.The phase composition in the AE44 matrix alloy and MMC samples were also predicted with CALPHAD modeling by assessing the Al-Ce and Mg-Al-Ce systems,using Pandat8.1 code and the PanMg2012 database.
3.Results and discussions
Fig.2 shows cross-section images of the AE44 magnesium alloy reinforced withδ-Al2O3fiber taken from the cylinder block made by the SS-HPDC process[9].The end(top)of the casting showed some preform compression,but unlike the thicker preform studied earlier[9],no shrinkage pores were presented in the casting.The SEM image in Fig.3 shows the microstructure in both the unreinforced matrix alloy and the fibe infiltrate area.The process parameters were optimized to obtain sound castings;for example,it was found that an intensificatio pressure of at least 90MPa was required to minimize the likelihood for forming defects such as pores if the preform was thicker than 5mm.

Fig.3.SEM image showing the microstructure of the matrix alloy and the MMC.
3.1.Microstructure of unreinforced AE44
Fig.4 shows that the typical microstructure of unreinforced AE44 alloy,which has an average grain size of approximately 30μm(measured according to ASTM E112-2004),and there is a large amount of lamellar and feather-like phases distributed at grain boundaries.The EDX(Fig.4(c)and(d))microanalysis results reveal that both the lamellar phase and the feather-like phase contain La,Ce,Nd and Al with an Al/RE ratio close to 11:3(after the solid solute effect deducted),and the XRD pattern in Fig.5 further shows that the intermetallics belong to Al11RE3(Al11Ce3or Al11La3).It is hard to distinguish Al11RE3types because of the similar structure and minor differences of d values in their PDF cards.
Thus,the AE44 alloy microstructure obtained here mainly consists ofα-Mg grains and Al11RE3intermetallic phase at grain boundaries.The XRD results in this study are slightly different from those reported for AE44 with Ce-rich mischmetal elements[10],but similar to those of the AE44 alloy with mischmetal substituted by La[11-13].Further,TEM images show a bow-shaped particulate mainly Al11La3(Fig.6)and a lamellar phase mainly Al11Ce3(Fig.7).Trace phases were also detected by TEM,and Fig.8 shows some evidence of Al3La phase in the microstructure.This is different from the phase composition of AE44 cast by sand-cast technology,where large grains ofα-Mg solid solution,needle-shaped precipitates of the Al11RE3phase,polyhedral precipitates of the Al2RE phase and Al10RE2Mn7phase were founded[18]but coincide with the phase composition obtained via experiments[27].
3.2.The microstructure ofδ-Al2O3/AE44 MMC

Fig.4.(a,b)SEM images;and(c)-(d)SEM combing EDX spectrums showing the microstructure of the SS-HPDC AE44 matrix alloy.

Fig.5.X-ray diffraction pattern of AE44 matrix alloy.
Fig.9 shows a typical SEM microstructure of the AE44 alloy reinforced with Saffi fiber(δ-Al2O3/AE44 MMC).As shown in Fig.9(a),the fiber remain predominantly in a planar-random arrangement in the composites and are surrounded by whiskers.The whiskers are observed not only on the fiber(Fig.9(b))but also in the matrix grain interior(Fig.9(c)).Fig.9(b,d)illustrate that the whiskers may grow on the fiber with an appearance that is mostly blocky and a few having a lamellar shape.This is different from the microstructure of unreinforced AE44 where the second phase particles(Al11RE3)are mainly located in the inter-dendritic boundaries.EDX analysis indicates that the lamellar phases are Al-and RE-rich with a RE:Al ratio of about 11:3,while the rectangular phases are also Al-and RE-rich with a RE:Al ratio of about 8:3.
To better understand the element distribution around the fibers element mapping was done and its result is shown in Fig.10,which shows that Al,La,and Ce are rich around the fiber consisting of Al and O,with SiO2binder.This result,along with the XRD pattern in Fig.11,suggest that the RE-containing rectangular-shaped belong to Al3RE,while the lamellar phases belong to Al11RE3.In addition,the element mapping also reveals that no Al appears but some Si is present in the Mg matrix(Fig.10)indicating that Al is low in solid solution,while SiO2is reduced by Mg as there is no Si in the original AE44 alloy.Thus,there may be other reaction products except for RE-containing phases.This is consistent with the XRD results there showing both MgO and Mg2Si in the reinforced AE44 samples which has been reported[8,58].
Fig.12 shows the morphology of second phases around aδ-Al2O3fibe.Several phases were marked from number‘1'to‘5'to identify their crystal structure.Selected area electron diffraction patterns(SAED)in TEM show that the blocky phase marked as region“1”is Mg2Si(Fig.13)with a CF12 crystal structure(Fm3m).The prismatic morphology phase,marked as“2”,is identifie as Al2La with a CF12 structure(Fig.14),and the lamellar phase marked as“3”is Al11Ce3with an oI28 structure(Immm71),in agreement with many previous reports[34-35,54].The rectangular-shaped phase labeled as“4”(Fig.15)indicates some crystallographic relation among those compounds(Al11Ce3,Al3Ce:(0 4 2)//(0 2 1),[1 0 0]//[1−1 2];Al2Ce,Al3Ce:(2 0−1)//(1 1 0),[0 1 0]//[1−1 2]).The region marked as“5”(Fig.16)also shows a relationship between Al11La3and Al2La of((1−2 0)//(0 1 2),[2 1 1]//[2−2 1]).
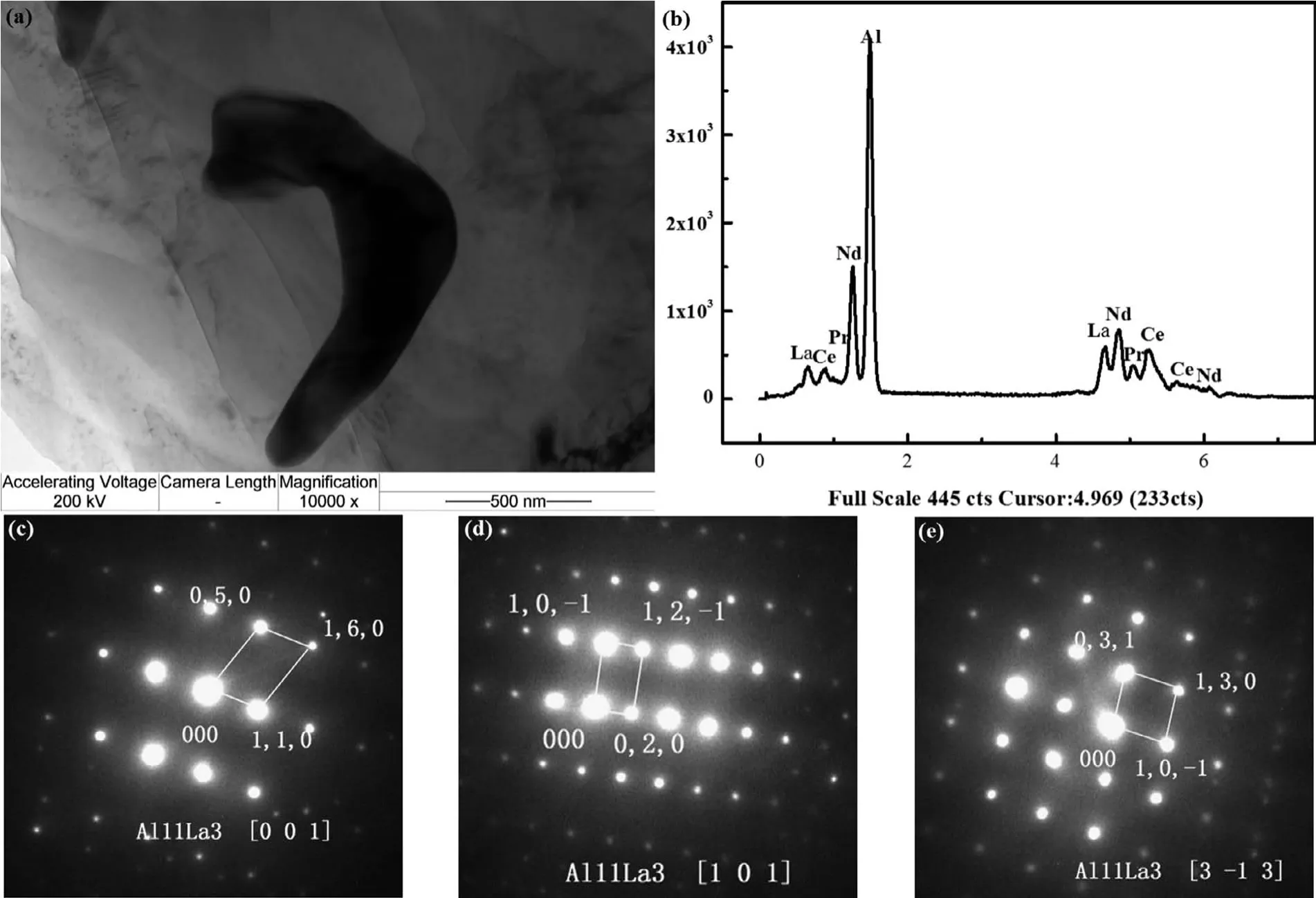
Fig.6.(a)TEM bright fiel image;(b)EDX spectrum;and(c-e)SAED patterns([0 0 1]zone,[1 0 1]zone,[3-1 3]zone axis,respectively)of Al11La3 precipitates in unreinforced AE44 alloy.

Fig.7.(a)TEM bright-fiel image;(b)EDX spectrum;and(c-e)SAED patterns([5 1 1]zone,[1 0 0]zone,[3 1 0]zone axis,respectively)of the Al11Ce3 precipitates in unreinforced AE44 alloy.

Fig.8.(a)TEM bright-fiel image;(b)EDX spectrum;and(c-e)SAED patterns([−8 7 1]zone axis,[−3 1 3]zone axis,[1 0 1]zone axis,respectively)of the Al3La precipitates formed in AE44.

Fig.9.The microstructure of the AE44/δ-Al2O3 composite sample by SEM.
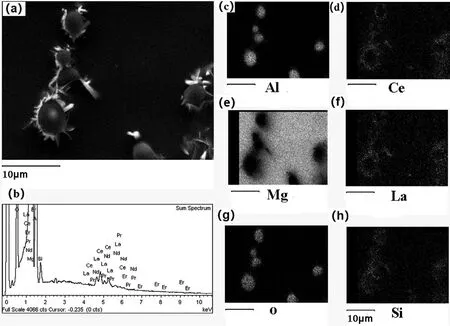
Fig.10.EDS element mapping showing the distribution of Al,Ce,Mg,La,O,Si in the AE44/δ-Al2O3 composite.

Fig.11.X-ray diffraction pattern of AE44/δ-Al2O3 composite.

Fig.12.TEM micrograph showing the morphology of second phase particles around aδ-Al2O3 fibe.
In summary,the reinforced AE44 alloy consists of fi e different phasesα-Mg,δ-Al2O3,Al3RE(rectangular,blocky shape),MgO and Mg2Si(blocky shape)and two trace phases Al2RE(prismatic morphology,)and Al11RE3(lamellar like).The orientation relationships among these phases are provided.
3.3.Formation of AL-RE phases

Fig.13.TEM micrograph and SAED pattern showing the formation of Mg2Si.
It is right of the RE-containing compounds other than other phases such as Mg17Al12enhancing the temperature usage zone of AE series alloy comparing to AZ magnesium.Thus,to clear the formation sequence of AlxREycould give guidance of the suitable choice of process technology of this series alloy or composites according to the concrete requirements of the mechanical property.As we know,AlxREywith specifi Al/RE ratio corresponds to a unique melting point.Though the melting point of each type is higher than that of Mg17Al12,it is better for us to avoid the lower melting point AlxREy,then the higher temperature usage zone would be utmostly enlarged.
3.3.1.Formation of AL-RE phases in unreinforced AE44
Extensive research has been recently reported on the microstructure and phase constitution of AE44 under different processing conditions[10-29].Kielbus and co-workers investigated the second phases(in addition toα-Mg grains)in the microstructure of AE44 alloy before and after hotchamber die casting,and reported Al11RE3(globular,lamellar and acicular),Al3RE(particulate)and Al8CeMn4phases before die casting,but only Al11RE3and Al2RE phases after die casting[14-15,19-21].It was also reported[21]that the phase constitution of AE44 alloy changes with casting wall thickness(cooling rate)of the HPDC process:Al11RE3and Al2RE were found to be the dominant intermetallic compounds in the 5mm thick areas,while Al2.12RE0.88was additionally detected in the 1mm thick areas.Zhang conducted a series of careful investigations in a cold chamber die casting machine with AE44 alloy using different types of RE additions[10-13,22-25]and reported that AE44 consisted ofα-Mg grains,Al11RE3(acicular and lamellar precipitates)and Al2RE(blocky particles).The intermetallic compounds and their fractions were shown to be determined by the kind of rare earth elements added:for example,Al2La was not observed when Ce-rich mischmetal was substituted by pure La[10-13].Above all the refs,though different RE intermetallics have been reported,the existence of Al11RE3phase seems to be consistent and was often to be reported in lamellar or acicular shape distributed along theα-Mg grain boundaries depended on casting geometry and process parameters.Regarding the other rectangular or globular particles,the appearance probability varied depending on the process parameters and the die dimension.In this study,the AE44 alloy obtained by SS-HPDC mainly containedα-Mg grains,Al11RE3particulates,and trace amounts of Al3RE,as illustrated in Figs.5-8,coinciding well with the report in ref.[28].
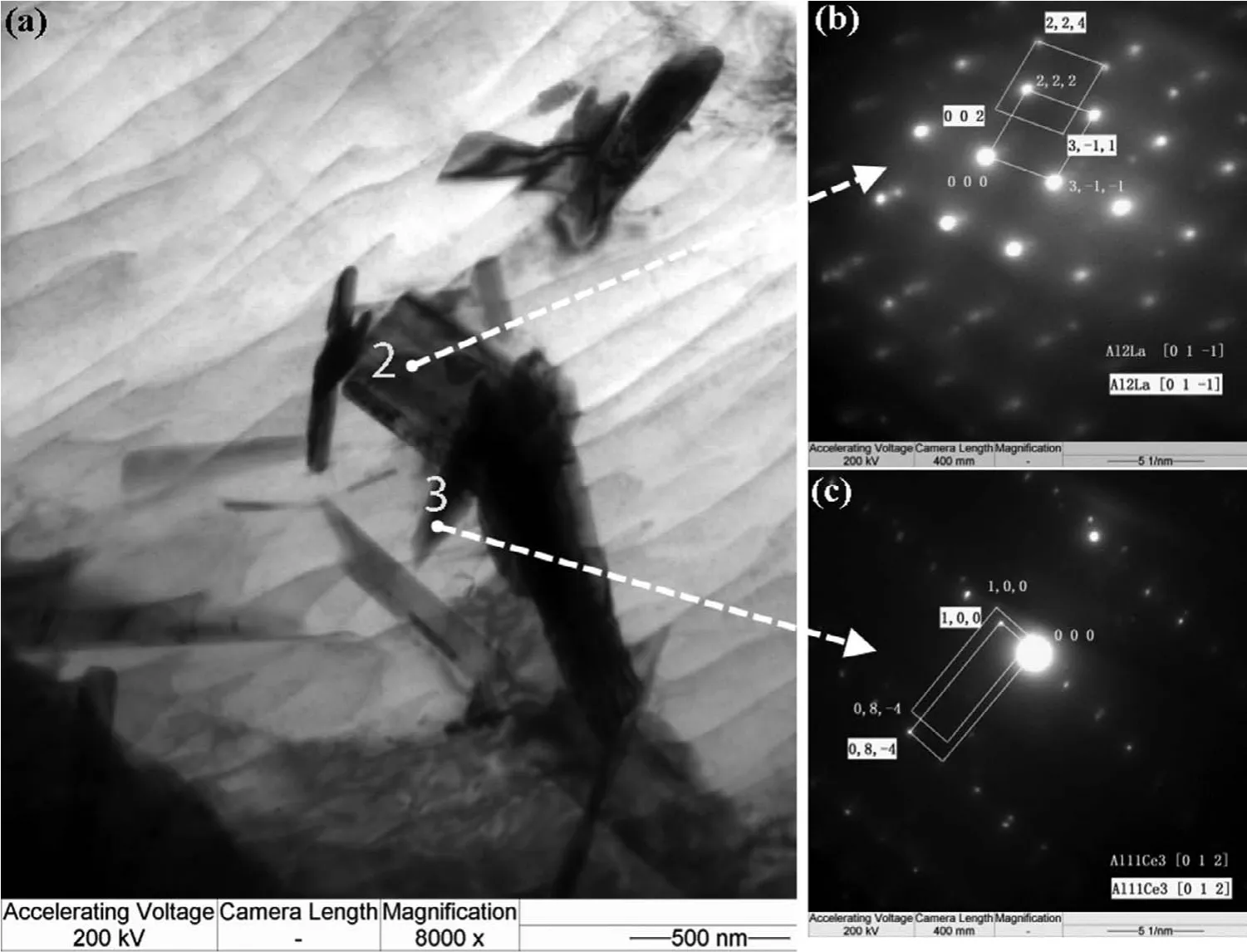
Fig.14.Morphology of second phases(a)and SAED images(b)Al2La(c)Al11Ce3.
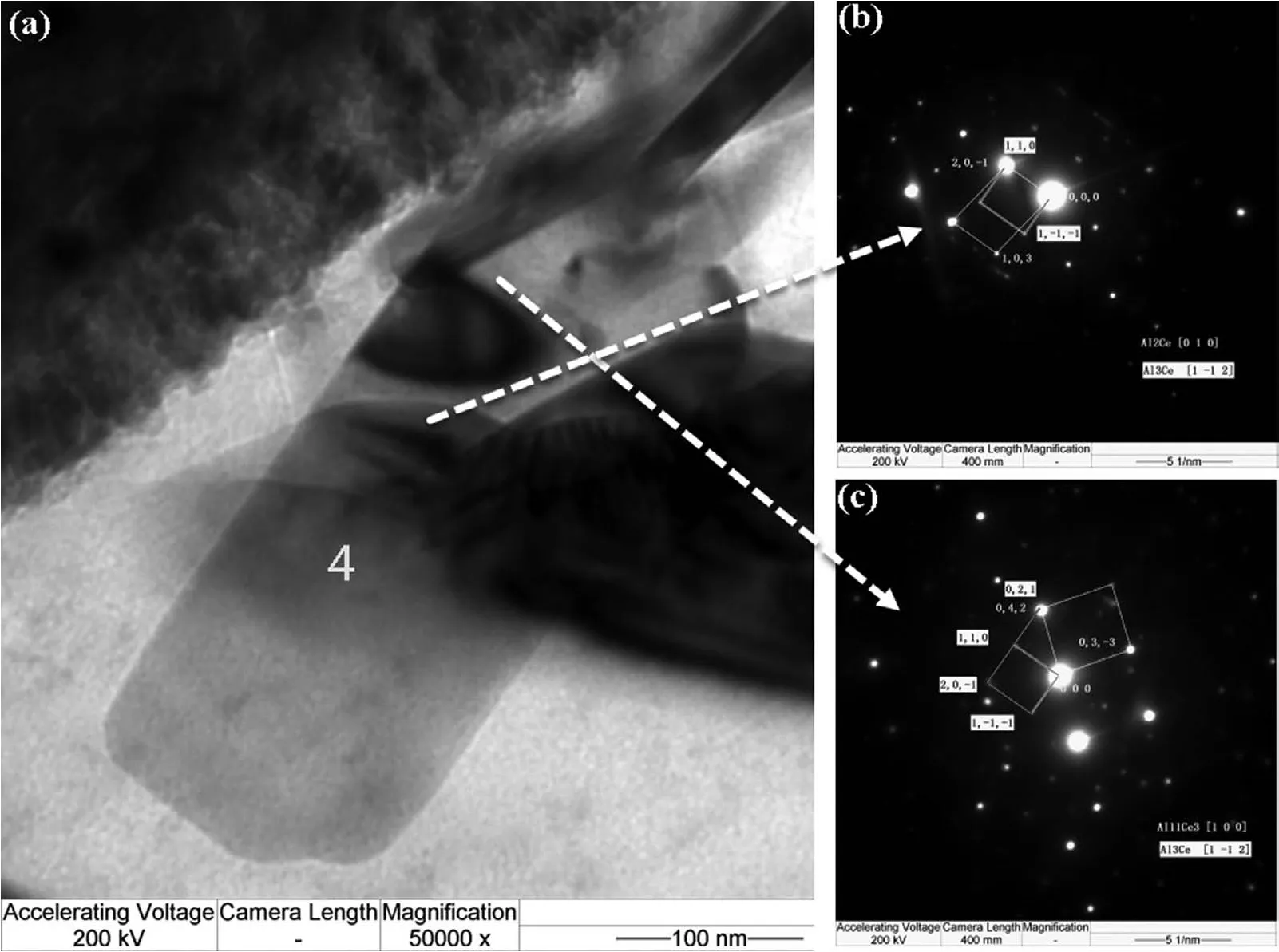
Fig.15.Morphology of second phases(a)and SAED images(b)Al3Ce and Al2Ce(c)Al3Ce and Al11Ce3.
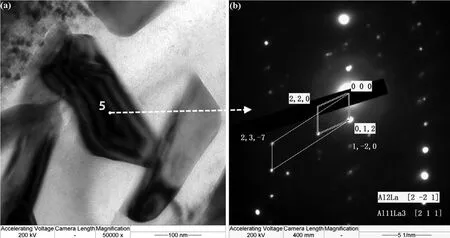
Fig.16.Morphology of second phases(a)and SAED images(b)Al11La3 and Al2La.
The specifi appearance of the RE compounds is related to the local Al:RE ratios in the melt,which is influence by the electronegativity of the elements and the solid solubility of RE in Mg.Also,the phase equilibrium is related to the thermodynamic properties of the system.Thus,factors affecting these parameters can influenc the phase constitution in the fina microstructure.
It is well known that the stability of intermetallic compounds mainly depends on the electronegativity difference of the elements involved.Large electronegativity difference means strong bonding force and easy formation of the intermetallic compound[32-37].Among Mg-Al,Mg-RE and Al-RE systems,the electronegativity difference between Al and RE is the largest,so the formation of AlxREyis preferred rather than the other compounds such as MgxAlyor MgxREy.Apparently different RE elements have different electronegativities,thus the probability of forming AlxREydiffers as reported in the earlier studies[10-13,22-27,55].In this paper,there are four RE elements(La,Ce,Pr,and Nd)in the added Ce-rich mischmetal.The electronegativity of those four elements increases from 1.1 to 1.14 in turn according to the Pauling scale,as can be calculated that the electronegative difference between Al and La is the largest while that between Al and Nd is the smallest,means that Al-La bonding will be preferred leading to the formation of AlxLay.
The alternation of those atomic ratios between free Al atoms and other concrete RE atoms in the melt shouldn't be ignored once the frst AlxLayintermetallic phase forms[37].Given the quantity of free Al,atoms are constant,the ratio of free Al and other RE elements such as Al/Ce or Al/Nd decrease after the formation of primary RE intermetallic and thus favors the formation of high RE content AlxREycompounds such as Al2RE.It means the formation probability of Al2La in this condition is minor since La favor the formation of high Al content intermetallics like Al11La3.However,the probability of Al2Ce coming out in the same situation may be larger than Al11Ce3.This could explain the presence of Al2Ce phase when Ce-rich mischmetal is added,but no Al2La phase when only pure La is added[13].
On the other hand,when the RE atomic number increases,the solubility of RE in Al decreases[32],and the amount needed to form AlxREyincreases.In other words,the solubility between La and Al is larger than that between any other RE element such as Nd and Al.Thus,La will more likely to form Al11RE3,while Nd would form Al2RE in high RE-containing alloys.As we all know,when there is AlxREy,the quantity of the RE to be added into the Mg melt should overcome its solution limitation in Mg in order to leave more free RE atoms in the melt.Therefore,given all the quantities of different RE elements are consistent with that and they are the same,the quantity of La consumed in solid solution is larger than other kind RE elements.Thus leaving the less quantity free La atoms to bond with Al atoms.Therefore,the local ratio of La/Al is smaller and thus easier to form high Al RExAlyintermetallics such as Al11La3other than to form high RE RExAlysuch as La3Al or La2Al.Once the primary Al11La3present,the ratio of free Al atoms and other free RE atoms is then changed into the situation that will favor the formation of high RE intermetallics such as Al2Ce.This has been verifie by experimental observations of Al11La3and Al2Ce phases in AE44[13].
However,the phase constitution of Mg-Al-RE alloys depends not only on the electronegativity and solid solubility of RE in Mg but also on their thermochemical characteristics.RE elements generally show similar thermochemical behavior[30-33];the thermochemical properties of the intermetallics in the Al-Ce system are presented in Table 3.It shows that the probability of Al-Ce intermetallics decreases from Al11Ce3,Al3Ce to Al2Ce and Al2Ce,where the highest melting point AlxCeyphase,seems to be the most unstable and would be presented at the melt frst[34-37].Actually,all those three phases have been observed in various process procedures[10-29].In those reports,Al11Ce3has been observed in almost all studies on AE44 and seem to be the most stable phase,for AE44 with a low fraction of Al11RE3has better high-temperature properties[28,40].Al3Ce with the lowest melting point has rarely been reported and it would relate to the different process parameters which result in different solidificatio rate due to different cooling rate.
The enthalpy of solidificatio is sensitive to cooling rate[38-39],which affects the solidificatio microstructure via the thermophysical properties.The cooling rate enlarged increases the enthalpy of solidificatio and slightly increases both liquids and solids temperatures.Higher cooling rates can thus benefi creep resistance of AE series magnesium alloys due to the presence of high melting point intermetallic phases AlxREyother than Mg17Al12[47-50,55-56].For a higher solidificatio rate provides a greater driving force for nucleation,not only fosters the formation of main creep strengthening phase Al11RE3[49],but also favors the formation of Al3RE,Al2RE and other metastable phases like Al2.12Ce0.88in AE44 alloy which makes the total RE intermetallic amounts increase[39].As we all know,the cooling rates of different casting processes are usually in descending order as follows:highpressure die casting(HPDC),SS-HPDC,squeeze casting and sand casting.This may explain the deviation of intermetallic types about AE42 alloy[35,41,43,51-53].Also,in our paper,trace amounts Al3La are found by TEM(Fig.8),which is often reported in die-cast microstructures.It should owe to the different process amateurs,for cooling rate in our process SS-HPDC is slower than traditional HPDC.
3.3.2.Formation of AL-RE phases inδ-Al2O3 reinforced AE44
The AE44/δ-Al2O3composites contained fi e different phases and two trace phases,which is different from the composition of the matrix alloy.MgO was detected by XRD in this study and its TEM micrograph was the same as in our earlier report[8],it is likely to come along with the formation of Mg2Si according to the following reactions based on minimizing Gibbs free energy:
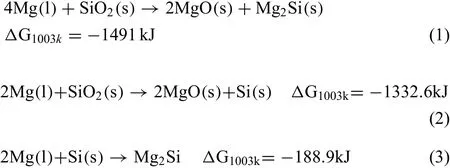
The colloidal SiO2will react with the molten Mg to form two new compounds,MgO and Mg2Si,according to Eq.(1).This interfacial reaction was also reported in earlier investigations on squeezing casting[8].
As discussed earlier,the local Al/RE ratio in the melt and the thermodynamic properties of the second phases are veryimportant.Any factors affecting those two criteria will change the constitution of the microstructure[37-40,41-44].During solidificatio of AE44/δ-Al2O3composites,Mg2Si forms frst,followed by Al2Ce,Mg,and Al11Ce3[34].Al3Ce forms last because it is a reaction product between Al2Ce and Al11Ce3[30-33],as shown in the calculated Al-Ce phase diagram in Fig.17.
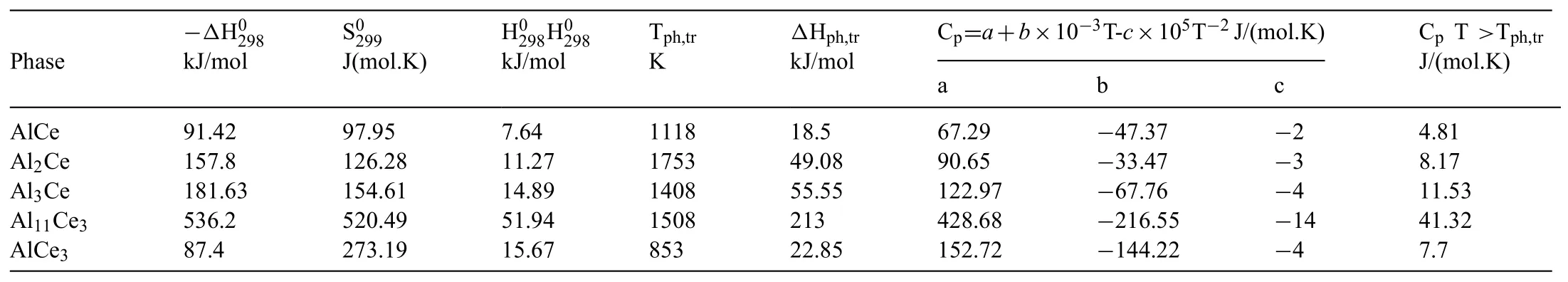
Table 3Thermochemical properties of intermetallics in Al-Ce system[34].
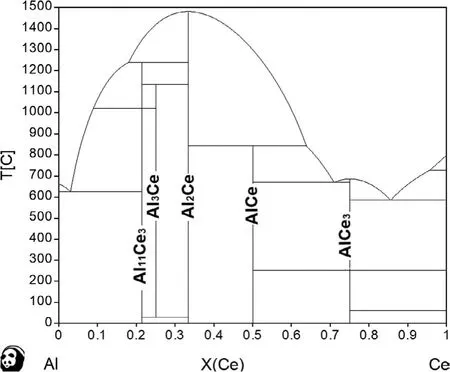
Fig.17.Calculated Al-Ce binary phase diagram.
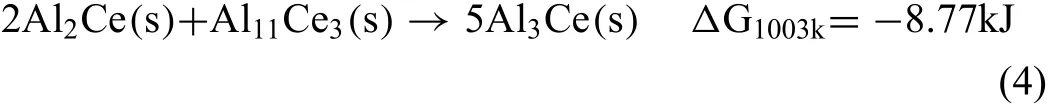
In the high-temperature regime,the fiber along with the Mg2Si particles facilitate the nucleation of Al2Ce by providing heterogeneous nucleation sites.This is evident from the many short whiskers observed around the fiber in Fig.9.Moreover,within the framework of classical nucleation theory,is derived from molecular dynamics method,the nucleation rates not only relate to the curvature dependence of the interfacial free energy but also to the temperature of the melt[41-44].Once infiltrate into the preform,the melt surrounded by fiber cools much slower than without fiber becauseδ-Al2O3has poor thermal conductivity and reduces the cooling rate locally.
Al11Ce3particles can be formed via the decomposition of Al2Ce(L+Al2Ce→Al11Ce3)according to the binary phase diagram Fig.17.This also coincides the calculations of the relative thermodynamic stability of Al11RE3with respect to L(Al)+Al2RE two-phase equilibrium state at elevated temperatures,where Al11RE3is always thermodynamically stable than those two phases[33].The orientation relationship between Al2Ce and Al11Ce3as shown in Fig.16(Al11La3and Al2La:(1-2 0)//(0 1 2),[2 1 1]//[2−2 1]).The more quantity the Al2Ce forms,the bigger probability of the formation the Al11Ce3is.In addition,the formation of primary Al11Ce3(L→Al+Al11Ce3)would also be fostered by the formation of Al2Ce concurrently withα-Mg.That is because around the fibers once the primary nucleation of Al2Ce was completed(heterogeneous nucleation),the free Al to Ce ratio around the fiber increases.For the local Ce free atoms were consumed by bonding with Al atoms.In other words,the Al2Ce surrounding a fibe can lead to higher local Al concentration in the melt after the formation ofα-Mg,resulting in more primary Al11Ce3(Fig.17).Thus,the Al11Ce3phase in this study is the major phase.
The slower cooling rate enables the peritectoid reaction forming Al3Ce as shown in Eq.(4)which consumes most of the Al11Ce3and Al2Ce present according to the Al-Ce phase diagram Fig.17,while Fig.14 also implies some relationship between Al3Ce and these two intermetallics as below:
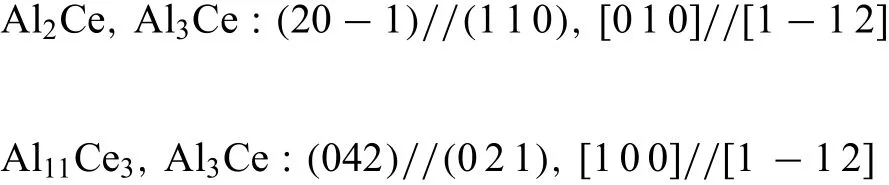
For a better understanding of the phase equilibrium in the Mg-Al-Ce ternary system,an isothermal section at 400°C in the Mg-rich corner was calculated as shown in Fig.18.The original composition of AE44 in this paper is marked as“A”in the ternary diagram.The directions of Ce and Al content are marked by the red arrow lines as“1”and“2”,respectively,while the ratio of Ce and Al shifts towards arrow line“3”.Actually,the constituents fluctuat among the delta-shaped region“0AA2”,which means that three intermetallic phases could be stable at room temperature and the formation of CeMg12can be neglected at high cooling rates.However,the thermal conductivity ofδ-Al2O3is not so low to simulate an equilibrium environment during the reaction process.The peritectoid reaction thus progresses incompletely,and trace amounts of Al11Ce3and Al2Ce are formed at room temperature along with Al3Ce.As for alloy AE44 without fibers its constituent point is in the 400°C section zone composed of Al3Ce and Al11Ce3only.Unlike the composite,no trace Al2Ce was observed in the matrix alloy due to unavailability of nucleation sites and the small concentration fluctuation
The above results are significan in the understanding and control of the microstructure of Mg-matrix MMC materials.The formation of Mg2Si and Al-RE phases(Al3Ce,Al2Ce,and Al11Ce3)is generally beneficia to the high-temperature mechanical properties of the composites,owing to the thermal stability of those intermetallic phases[23-26,33,29]or the amounts of them[45-50,54].The presence of MgO and Mg2Si,due to the interfacial reaction between the SiO2binder in the fibe preforms and the molten magnesium[8,56],can improve the interfacial wetting during the infiltratio process,but excessive amounts of MgO in the interfacial areas can act as crack initiators during fatigue loading[8,56],thus,should be minimized.It is,therefore,recommended that the use of SiO2binder in the preform manufacturing be limited/minimized to reduce the MgO formation in the MMC casting process.
4.Summary
1.Unreinforced AE44 alloy consists ofα-Mg grains and Al11RE3intermetallic particles with trace phases of Al3RE.
2.The formation of RE intermetallic phases is affected by cooling rate.However,high cooling rates favor the formation of Al3RE and Al2RE or other metastable intermetallic phases.
3.Theδ-Al2O3fibe reinforced AE44 alloy(composite)is composed of mainly fi e different types,i.e.,α-Mg,δ-Al2O3,Al3RE,MgO and Mg2Si and two trace type phases,i.e.,Al2RE and Al11RE3.The RE-containing intermetallic phases grow on the fibe surfaces and in the matrix with the following orientation relationship:
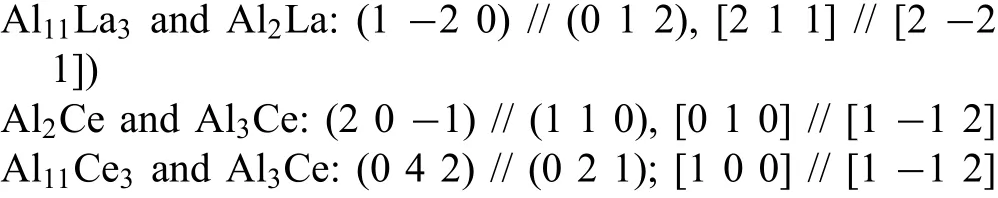
4.The formation ofδ-Al2O3/AE44 interfacial reaction products is in the following sequence:Mg2Si,Al2RE,Mg,Al11RE3,and Al3RE.Al11RE3is partly derived from Al2RE while Al3RE is a product of the peritectoid reaction between Al11RE3and Al2RE.
5.There are four major interfacial reactions in theδ-Al2O3/AE44 composites:1.the reaction between SiO2binder in the preform and molten magnesium during infiltration resulting in two phases MgO and Mg2Si;2.the fiber offer nucleation sites for heterogeneous nucleation of the Al2RE phase;3.the local alloy constitute environment around the fiber can facilitate the formation of Al2RE to favor the formation of Al11RE3,and 4.the heat preservation effect of fiber makes the peritectoid reaction possible forming Al3RE at the expense of Al11RE3and Al2RE phases.
Declaration of Competing Interest
The authors declare that they have no known competing financia interests or personal relationships that could have appeared to influenc the work reported in this paper.
Acknowledgments
This work was co-funded by,The National Key Research and Development Program of China(NO.2016YFB0301002)and General Motors Global Research and Development.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Predicting and controlling interfacial microstructure of magnesium/aluminum bimetallic structures for improved interfacial bonding
- Plasma electrolytic oxidation of AZ31 and AZ91 magnesium alloys:Comparison of coatings formation mechanism
- Effects of annealing treatment on microstructure and tensile behavior of the Mg-Zn-Y-Nd alloy
- Microstructure and performance of biodegradable magnesium alloy tubes fabricated by local-heating-assisted dieless drawing
- Comparisons of microstructure homogeneity,texture and mechanical properties of AZ80 magnesium alloy fabricated by annular channel angular extrusion and backward extrusion
- Effect of Y addition on microstructure and corrosion behavior of extruded Mg-Zn-Nd-Zr alloy
