Do liver metastases from gastric cancer contraindicate aggressive surgical resection? A 14-year single-center experience
2020-12-18TakefumiYazawaTomohideHoriHidekazuYamamotoHidekiHaradaMichihiroYamamotoMasahiroYamadaMasakiTaniAsahiSatoYasuyukiKamadaRyotaroTaniRyuheiAoyamaYudaiSasakiMasazumiZaima
Takefumi Yazawa, Tomohide Hori, Hidekazu Yamamoto, Hideki Harada, Michihiro Yamamoto, Masahiro Yamada, Masaki Tani, Asahi Sato, Yasuyuki Kamada, Ryotaro Tani, Ryuhei Aoyama, Yudai Sasaki, Masazumi Zaima
Abstract
Key Words: Liver metastasis; Metastatic tumor; Gastric cancer; Hepatectomy; Liver resection; Surgery
INTRODUCTION
Advanced gastric cancer (GC) with liver metastasis is often characterized by multiple and bilobular metastases and may be associated with extrahepatic metastatic lesions.Hence, many physicians consider that radical surgery is contraindicated for these patients.According to the Japanese treatment guideline for GC[1], a smaller number of liver metastases without unresectable factors (i.e., extrahepatic metastases) may be an indication for radical surgery if strict curability (i.e., graphical and surgical R0,according to the Japanese classification of gastric carcinoma[2]) will be accomplished.
The evidence regarding the indications for liver resection (LR) for liver metastases from GC (LMGC) is limited, and much of the research on this topic consists of singlecenter retrospective studies with a small sample size[1,3].These studies suggested that the surgical curability of LR and a smaller number of LMGC were important factors for prognostic outcomes after LR[3]and that aggressive LR for LMGC may prolong survival in carefully selected patients who undergo radical surgery for the primary GC[3].A later study showed that a solitary metastasis was an important independent factor for prognostic outcomes after LR[4].Three or fewer LMGC in highly accurate imaging studies is an indication for LR, and the 5-year overall survival (OS) rate is approximately 0.3 in this population[1,5,6].The effectiveness of LR for patients with synchronous or metachronous LMGC is still controversial[3]; therefore, any patients with synchronous or metachronous LMGC may be candidates for LR[3].Several factors have been clearly detected as important independent predictive factors for postoperative recurrence and/or prognostic outcomes after LR[4-19], and the actual 5-year OS rate reportedly ranges from 0 to 0.37[5-19].
As described above, the surgical approach may have therapeutic potential for carefully selected patients with LMGC[3].We have aggressively recommended LR for these patients.In the present study, we retrospectively evaluated our patients with LMGC who underwent both a radical surgery for the primary GC and LR for LMGC during the past two decades.We present our institutional indications and contraindications for LR in patients with LMGC.From the viewpoint of clinical oncology, the goal of this study was to identify the most important predictive factors for postoperative recurrence and/or prognostic outcomes after LR.
MATERIALS AND METHODS
Evaluation and assessment of oncologic findings, clinical therapies, and actual responses
In this study, the oncologic findings were evaluated and reported according to the Japanese classification of gastric carcinoma [i.e., tumor depth (T factor), cytology (CY factor), pathological lymphatic invasion (ly factor), and pathological vessel invasion (v factor)][2].Additionally, clinical therapies and actual responses were assessed and described according to the Japanese treatment guideline for GC[1][i.e., intentional lymphadenectomy (D number), graphical or surgical curability (R number), and clinical response after chemotherapy].
Institutional indication for LR for LMGC
The indications and contraindications for LR for LMGC at our institution are summarized in Table 1.There were no restrictions in the distribution of metastases(unilobular or bilobular), cytology (CY0 or CY1), or timing of metastasis (synchronous or metachronous).
The indications for LR were as follows: (1) The primary GC could be curatively resected by radical gastrectomy with intentional lymphadenectomy; (2) All LMGC could be resected with no metastasis in the remnant liver; (3) The volume of the remnant liver was graphically estimated as > 40% against the whole liver; (4) The number of LMGC was ≤ 5, though the size of the LMGC was not limited; and (5) No unresectable site was observed.In brief, the curability of LR (i.e., graphical and surgical R0[2]) is of utmost importance, and we accomplish curability whenever possible.
In contrast, the contraindications for LR were as follows: (1) Five or more metastases were present; (2) Peritoneal dissemination was observed; and (3) An extrahepatic unresectable site was present.In brief, the incurability of LR (graphical or surgical remnant of metastasis) was a strong contraindication for any type of hepatectomy.
Therapeutic strategy for LMGC in our institution
The therapeutic strategy for LMGC at our institution is summarized in Figure 1.If LMGC were resectable, LR was initially performed and adjuvant chemotherapy was subsequently introduced.If LMGC were resectable but involved some difficulties for LR (e.g., higher number and larger size of liver metastases), chemotherapy was initially introduced as neoadjuvant chemotherapy (NAC) and LR was performed thereafter only in patients with a positive response (i.e., complete response, partial response, or stable disease according to the Japanese classification of gastric carcinoma[2]).If LMGC were unresectable, systemic chemotherapy was introduced.LR was performed as conversion surgery only in patients who fulfilled our institutional indications.
Patients
During the 14-year period from January 2006 to January 2020, a total of 1086 patients with GC underwent surgical treatment in our institution.Thirty patients who underwent LR for LMGC were enrolled in this study.We retrospectively evaluated our own results after LR (i.e., clinical course, pathological findings, postoperative recurrence, and prognostic outcomes).
In all cases, thoracoabdominal enhanced computed tomography were routinely performed for checking extrahepatic diseases.Positron emission tomography/computed tomography was also used.
Ethical approval
This retrospective study was approved by our institution’s ethics review committee forclinical studies.The study was performed in accordance with the ethical guidelines of the Declaration of Helsinki.Informed consent was obtained from all patients before enrollment.
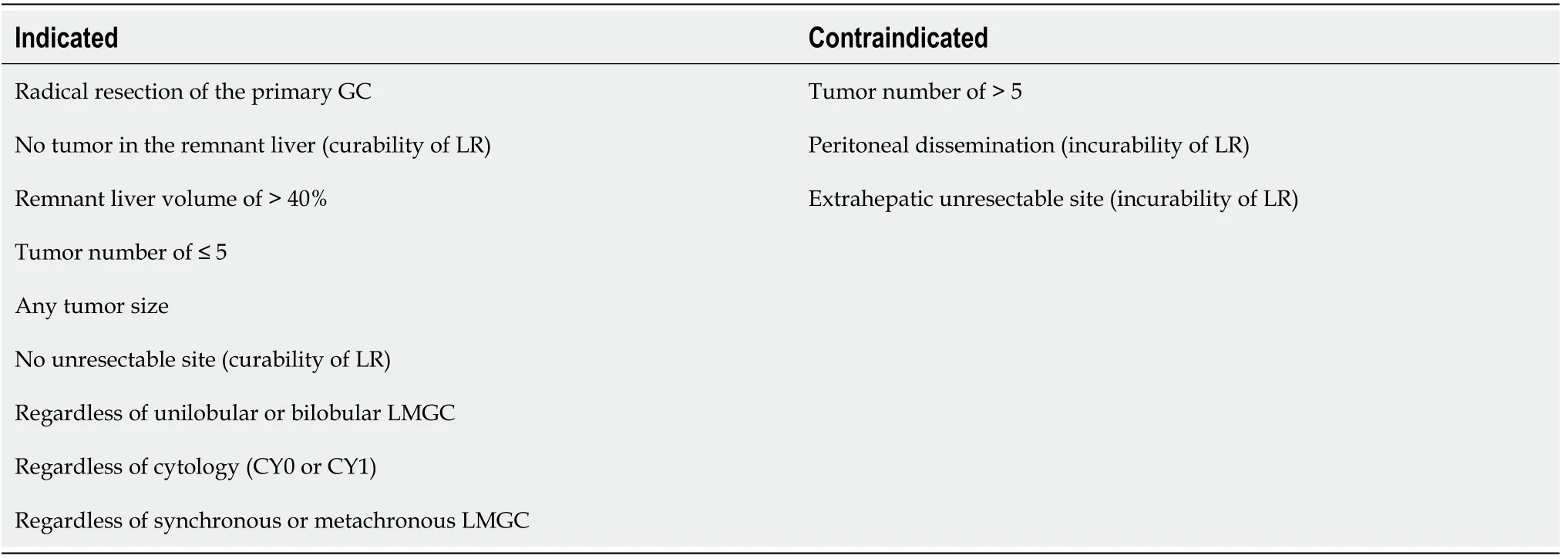
Table 1 Indication and contraindication for liver resection for liver metastases originated from gastric cancer.

Figure 1 Therapeutic strategy for liver metastases originated from gastric cancer. Japanese classification of gastric carcinoma: definitions of PD and PR. LMGC: Liver metastases originated from gastric cancer; LR: Liver response; NAC: Neoadjuvant chemotherapy; PR: Partial response; PD: Progressive disease.
Statistical analysis
All results are shown as mean ± standard deviation or median (range).Survival rates were calculated using the Kaplan–Meier method, and the log-rank test was used for between-group comparisons.The Mann–WhitneyUtest andχ2test were used to compare unpaired continuous or discontinuous variables between two groups.Logistic regression analysis was used to identify important predictive factors for poor prognostic outcomes.The effect of each factor on OS was evaluated by multivariate cyclooxygenase regression analysis.All calculations were performed using SPSS software (SPSS Inc., Chicago, IL, United States).APvalue of < 0.05 was considered statistically significant.
RESULTS
Clinical and pathological findings of primary GC
This study included 23 men and 7 women.Their GC was localized at the corpus (M portion) (n= 13), the antrum and pylorus (L portion) (n= 10), and the fundus (U portion) (n= 7)[2].The greatest dimension was 63.3 ± 29.2 mm, and the primary GC pathologically disappeared after NAC in one patient (i.e., complete response).
Pathologically, 22 patients had well/moderately differentiated tubular adenocarcinoma (tub1/tub2); 4 had poorly differentiated adenocarcinoma (por); and 1 each had papillary adenocarcinoma (pap), mucinous adenocarcinoma (muc),endocrine cell carcinoma, and hepatoid adenocarcinoma.No signet-ring cell carcinoma(sig) was observed.One differentiated adenocarcinoma secreted alpha-fetoprotein.
In the pathological assessments, the tumor depth (T factor) was evaluated as T3 (ss)(n= 16), T4b (si) (n= 6), T4a (se) (n= 5), and T1b (sm) (n= 2).No tumor was observed in the one patient with a complete response after NAC.Vessel invasion (positive v factor) was observed in 28 patients.Twenty-one patients had lymphoid invasions(positive ly factor), and 25 patients had metastatic lymph nodes (LNs) (positive N factor).Cytology (CY factor) was positive in one patient, and peritoneal dissemination(P1[2]) was observed in two patients.
Surgical treatment of primary GC
Radical surgery with intentional lymphadenectomy (D2 or D2+[1]) was performed for the primary GC.Total gastrectomy, distal gastrectomy, and pancreaticoduodenectomy was performed in 16, 13, and 1 patient, respectively.The stage of the primary GC at the time of surgery was IV in 16 patients, IIIA in 6 patients, IIB in 3 patients, IIIC in 3 patients, IB in 1 patient, and IIA in 1 patient.
Timing of LMGC
Radical surgeries including gastrectomy and lymphadenectomy were performed for the primary GC in all patients.Seventeen patients had metachronous LMGC and 13 had synchronous LMGC (Figure 2).
NAC, LR, and adjuvant chemotherapy
Seven patients with metachronous LMGC underwent LR, and two of these seven patients subsequently received adjuvant chemotherapy (uracil and tegafur or S-1).Four patients with LMGC received chemotherapy [S-1, trastuzumab, ramucirumab, S-1 + paclitaxel, S-1 + cisplatin (CDDP), irinotecan (CPT-11) + CDDP, or capecitabine +oxaliplatin], and LR was performed thereafter.The response to NAC was a partial response in two patients and stable disease in two patients.Four of these 10 patients received adjuvant chemotherapy (S-1 or CPT-11 + CDDP).Six patients received no chemotherapy (Figure 2).
Ten patients with synchronous LMGC underwent LR, and 9 of these 10 patients thereafter received adjuvant chemotherapy (S-1).Three patients with synchronous LMGC received chemotherapy (S-1 + oxaliplatin, S-1 + paclitaxel, or S-1 + CDDP), and LR was performed thereafter.All patients had a partial response to NAC.None of these three patients received adjuvant chemotherapy.One patient with synchronous LMGC received no chemotherapy (Figure 2).
Surgical procedures and curability of LR
The mean age at the time of LR was 67.4 ± 8.4 years.Partial hepatectomy was performed in 16 patients, and the other 14 patients underwent major hepatectomy (e.g., lobectomy and segmentectomy).The major hepatectomies were left lobectomy (n= 5), right lobectomy (n= 2), extended right lobectomy (n= 1), right lobectomy +partial hepatectomy (n= 1), right anterior segmentectomy + lateral segmentectomy (n= 1), right posterior segmentectomy + partial hepatectomy (n= 1), extended left hepatectomy (n= 1), left hepatectomy + partial hepatectomy (n= 1), and lateral segmentectomy + partial hepatectomy (n= 1).
Curative LR (graphical and surgical R0[2]) was achieved in 29 patients.Curative LR was not achieved in one patient because of bone metastasis, but postoperative radiation therapy was performed in this patient.
Characteristics of LMGC
The size and number of LMGC were graphically and macroscopically assessed.The median greatest dimension of LMGC was 30 mm (range, 5-120 mm), and seven patients had a tumor size of > 5 cm.The median number of LMGC was 2 (range, 1-6),and one patient had > 5 LMGC postoperatively.Seventeen patients had multiple LMGC, and 13 patients had a solitary liver metastasis.Twenty-two patients had unilobular LMGC and eight had bilobular LMGC.
Survival after initial LR
The median follow-up duration was 33.7 mo [range, 0.8 (death of unrelated causes) to 157.1 mo].Three patients with no recurrence after the initial LR died of unrelated causes during follow-up.

Figure 2 Actual treatments for synchronous and metachronous liver metastases originated from gastric cancer.Seventeen patients had metachronous liver metastases originated from gastric cancer (LMGC) and 13 patients had synchronous LMGC.Seven patients with metachronous LMGC underwent liver resection (LR), and two of these seven patients subsequently received adjuvant chemotherapy.Four patients with metachronous LMGC received chemotherapy,and LR was subsequently performed.A partial response to neoadjuvant chemotherapy was achieve in two patients.Four of these 10 patients received adjuvant chemotherapy.Ten patients with synchronous LMGC underwent LR, and 9 of these 10 patients subsequently received adjuvant chemotherapy.Three patients with synchronous LMGC received chemotherapy, and LR was subsequently performed.All responses to neoadjuvant chemotherapy were categorized as partial responses.1One case of conversion was involved.LMGC: Liver metastases originated from gastric cancer; LR: Liver resection.
The actual OS curve after the initial LR is shown in Figure 3A.The 1-, 2-, 3-, and 5-year OS rate was 0.78, 0.63, 0.48, and 0.48, respectively.Among the 15 patients who died of oncological causes related to their GC (excluding 3 patients who died of unrelated causes), the median survival period after the initial LR was 16.8 mo (range,3.7–84.0 mo).
The actual recurrence-free survival curve after the initial LR is shown in Figure 3B.The 1-, 2-, 3-, and 5-year recurrence-free survival rate was 0.49, 0.28, 0.28, and 0.28,respectively.Among the 21 patients with recurrence, the median recurrence-free period after the initial LR was 8.6 mo (range, 1.2-18.0 mo).
Postoperative recurrence after initial LR and additional surgeries
Although no recurrence was observed in 9 patients (30.0%), recurrence appeared after the initial LR in 21 patients (70.0%).When these 21 patients with recurrence were asymptomatic and ambulant, the target sites of recurrence after the initial LR were the liver (12 patients, 40.0%), LNs (8 patients, 26.7%), lung (4 patients, 13.3%), and peritoneum (2 patients, 6.7%).The target sites of the first recurrence after the initial LR were the liver (n= 8), LNs (n= 5), lung (n= 2), liver + lung (n= 2), liver + LNs (n= 2),peritoneum (n= 1), and liver + LNs + peritoneum (n= 1) (Table 2).
Additional surgeries were performed in nine patients with recurrence after the initial LR.These additional surgeries were LR (n= 3), LN dissection (n= 2), lung resection (n= 2), LR + LN dissection (n= 1), and LR + lung resection (n= 1).A total of eight LRs were repeated in five patients with liver recurrence (Table 2).Additionally,four lung resections were repeated in three patients with lung recurrence.One patient received additional LR, and moreover, this patient underwent lung resections twice(Table 2).
Each additional surgery achieved curability (graphical and surgical R0).Among the 21 patients with recurrence, the 9 patients who underwent additional surgeries for recurrence showed significantly longer survival than the 12 patients who did not undergo additional surgeries (P= 0.0014) (Figure 4).

Table 2 Postoperative recurrence after the initial liver resection and additional surgery
Important predictive factors for prognostic outcome after initial LR
Previously reported important predictive factors for postoperative recurrence and/or the prognostic outcome after the initial LR[4-19]were analyzed in the present study and are shown in Table 3.In the univariate analysis, serosal invasion (i.e., pathological T factor) was significantly different from the other factors (P= 0.0249).The multivariate analysis showed that this factor was an independent predictor of a poor prognostic outcome after the initial LR (P= 0.0249).
No predictors of postoperative recurrence after initial LR
Previously reported important factors for postoperative recurrence and/or the prognostic outcome after the initial LR[4-19]were analyzed in the present study and are shown in Table 4.In the univariate analysis, no factors showed statistical significance.We found no predictive factors for postoperative recurrence after the initial LR.
DISCUSSION
Optimal surgeries for GC have been previously investigated.Intentional dissection of regional LNs (D2 Lymphadenectomy) is currently considered to be the standard lymphadenectomy technique for advanced GC[1].Extended lymphadenectomy of paraaortic LNs does not improve the postoperative survival rate[20], and splenectomy for extended lymphadenectomy should be carefully chosen[21].NAC before radical surgery is recommended for treatment of advanced but resectable GC, especially in patients with massive metastases in regional LNs[22,23].
The therapeutic strategy for stage IV GC has been previously discussed[24-32].Improved prognostic outcomes have not been reported for surgeries that result in incurability (i.e., R1 or R2[2]), and these surgeries are currently contraindicated for patients with GC at unresectable sites[24,27].The surgical curability is crucial for patients with stage IV GC, and adjuvant chemotherapy after curative surgery (i.e., graphical and surgical R0) is strongly recommended in this patient population[1].In our two patients with peritoneal dissemination, there were no distant disseminations.Their disseminations were localized nearly at the primary GC (i.e., P1[2]), and graphical and surgical R0 was accomplished by peritonectomy in each patient.
Paradoxically, aggressive surgeries that achieve curability (i.e., graphical and surgical R0) may have potential benefits for patients with stage IV GC[5,8,12,33].The surgical techniques of LR are well established, and medical devices for LR have beenwell developed.Hence, major or extended hepatectomy is a safe and feasible treatment option for liver disease[34].Aggressive LR for LMGC may prolonged survival in carefully selected patients[4-19].From the viewpoint of preoperative prediction of postoperative recurrence and/or poor prognostic outcomes, many surgeons have focused on the most important surgical indications for LR in patients with LMGC(Table 5): The timing of LMGC[6,13,15,17,18], the greatest dimension of LMGC[5,8-11], the multiplicity of LMGC[10,12,15-17], the occupation of LMGC[11-14], the number of LMGC[4,5,8],the curability of LR[5,8,12], serosal invasion[5,9], lymphatic invasion[8,10], vessel invasion[8],LN metastases[19], induction of chemotherapy[13], and pathological differentiation[17].Reliable reports published during the past two decades are summarized in Table 5.

Table 3 Important factor for prognostic outcome after the initial liver resection

Table 4 Univariate analyses for postoperative recurrence after the initial liver resection

Table 5 Important factors for liver resection in patients with liver metastases originated from gastric cancer
The reported 5-year OS rate ranges from 0 to 0.37[5-19], and the rate in the present study was 0.48 (Table 5).Although the effectiveness of LR for LMGC remains controversial, the present results suggest that the OS rate after LR seems to be acceptable and that aggressive LR may be indicated in carefully selected patients with LMGC.
The curability of LR is important for patients with LMGC.In the present study, the initial LR was curative in 29 patients.Adjuvant chemotherapy after curative surgery (i.e., graphical and surgical R0) is strongly recommended in patients with stage IV GC[28-32], and perioperative chemotherapy was introduced in 23 patients.GC patient with synchronous LMGC is categorized as Stage IV, and neoadjuvant and/or adjuvant chemotherapy will be introduced for synchronous LMGC as possible.Although neoadjuvant and/or adjuvant therapies were introduced in all of 13 patients with synchronous LMGC, 6 of 17 patients with metachronous LMGC did not receive perioperative chemotherapy (Figure 2).In these 6 patients with metachronous LMGC,the surgical curability (i.e., graphical and surgical R0) of LR was accomplished in each.Though introduction of perioperative chemotherapy may involve a difficulty due to some reasons (e.g., underlying disorder and performance status), and LR with surgical curability may be beneficial for metachronous LMGC patients who had some difficulty of perioperative chemotherapy.Recurrence after the initial LR occurred in 70% of patients.Notably, however, our results indicate that repeated additional surgeries for recurrence after the initial LR may be beneficial if surgical curability (i.e., graphical and surgical R0) can be obtained (Figure 4).The present results of LR for LMGC may be supported by the combined strategy of aggressive curative surgeries and chemotherapy during the perioperative period of LR.Three of 6 patients with metachronous LMGC who did not receive perioperative chemotherapy were undergone additional surgeries for recurrence after the initial LR, and two of these 6 patients were still alive.Surgical curability is important for LR and additional surgery.LR and additional surgery may be beneficial for these metachronous LMGC patients if surgical curability is obtained, though we believe the combined strategy of aggressive curative surgeries and chemotherapy during the perioperative period of LR comes first for patients with LMGC.

Figure 3 Overall survival and recurrence-free survival after initial liver resection.A and B: Actual curves of overall survival and recurrence-free survival.LR: Liver resection; OS: Overall survival; RFS: Recurrence-free survival.

Figure 4 Survival curves in patients with recurrence.Recurrence after the initial liver resection was observed in 21 patients.Additional surgeries were performed in nine patients with recurrence, and each additional surgery accomplished graphical and surgical R0.These 9 patients had significantly longer survival than the other 12 patients who did not undergo additional surgeries (P < 0.05).LR: Liver resection; OS: Overall survival.
As shown in Table 5, only 14 papers have been previously documented, and almost all of these important papers were written based on retrospective design and/or single-center experience.Sample size were shown in Table 5.This was a retrospective study performed in a single institution and therefore has inherent limitations due to bias and a small sample size.Thus, the conclusions must be interpreted with extreme caution.In 2018, when our sample size was smaller and follow-up term was shorter,the greatest dimension of > 50 mm was an independent predictor of postoperative recurrence after the initial LR in the univariate and multivariate analyses (data not shown).We found no important factors for postoperative recurrence after the initial LR (Table 4), although pathological serosal invasion was still an independent predictor of poor prognostic outcomes after the initial LR (Table 3).
Although resectable LMGC is clinically rare, strict and careful patient selection can lead to prolonged survival in patients with advanced GC.The surgical approach may have a therapeutic potential for LMGC.
CONCLUSION
In conclusion, aggressive LR may be indicated for carefully selected patients with LMGC, and the survival rate after LR seems to be acceptable.
ARTICLE HIGHLIGHTS
Research background
Advanced gastric cancer (GC) often accompanies with liver metastasis.Though many physicians consider that radical surgeries are contraindicated for liver metastases from GC (LMGC).a smaller number of liver metastases without unresectable factors may be an indication for liver resection (LR).
Research motivation
The actual 5-year overall survival (OS) rate was previously documented as 0 to 0.37.Here, we presented the institutional indications for LR for LMGC, evaluated our own results.
Research objectives
In total, 30 patients underwent LR for LMGC during a 14-year period, and we evaluated the clinical, surgical, and oncological findings.
Research methods
In all patients, radical surgery with intentional lymphadenectomy was performed for the primary GC.The median follow-up duration after the initial LR was 33.7 mo.The OS and recurrence-free survival rates after the initial LR were assessed.Also, we identified important factors for prognostic outcomes.
Research results
The 5-year OS and recurrence-free survival rates were 0.48 and 0.28, respectively.The median survival duration and recurrence-free duration after the initial LR were 16.8 and 8.6 mo, respectively.Although recurrence might develop after the initial LR,additional surgeries for recurrence clearly prolong survival.Pathological serosal invasion was an independent predictor of a poor prognostic outcome after the initial LR.
Research conclusions
Our results of LR for LMGC seem acceptable.Pathological serosal invasion is important for poor prognostic outcomes.
Research perspectives
Aggressive LR may be indicated for carefully selected patients with LMGC.
