Fermentation of mulberry leaves with Cordyceps militaris enhanced anti-adipogenesis activity in 3T3-L1 cells through down-regulation of PPAR-γ pathway signaling
2020-11-10LuGuoJumSoonKangYoungHoonParkBeongIlJeDaeYounHwangWooHongJooYoungWhanChoi
Lu Guo, Jum Soon Kang, Young Hoon Park, Beong Il Je, Dae Youn Hwang, Woo Hong Joo, Young Whan Choi,4✉
1Department of Horticultural Bioscience, Pusan National University, Miryang 50463, Republic of Korea
2Department of Biomaterials Science, Pusan National University, Miryang 50463, Republic of Korea
3Department of Biology and Chemistry, Changwon National University, Changwon 51140, Republic of Korea
4Life and Industry Convergence Research Institute, Pusan National University, Miryang 50463, Republic of Korea
ABSTRACT
Objective: To establish an efficacious and efficient fermentation method of enhancing the anti-adipogenesis effect of mulberry (Morus alba) leaves using Cordyceps militais.
Methods: Dried mulberry leaves, dried mulberry leaves with 50% raw silkworm pupa and raw silkworm pupa were fermented with Cordyceps militais for 4 weeks at 25 ℃, after which the dried mulberry leaves and fermented product were extracted with 70%ethanol and subjected to high performance liquid chromatography(HPLC).The contents of cordycepin, pelargonidin, chlorogenic acid, iso-quercetin and caffeic acid were determined.We then used the 3T3-L1 cells to investigate whether extracts of fermentation enhanced anti-adipogenesis activity in vitro.
Results: HPLC showed that fermentation changed the contents of cordycepin, pelargonidin, chlorogenic acid, iso-quercetin and caffeic acid.Furthermore, fermented dried mulberry leaves with 50% raw silkworm pupa had a better efficacy of anti-adipogenesis than dried mulberry leaves, fermented dried mulberry leaves and fermented silkworm pupa and inhibited triglycerides accumulation and glucose consumption.Additionally, fermented dried mulberry leaves with 50% raw silkworm pupa inhibited PPAR-γ signaling.
Conclusions: Fermentation with Cordyceps militaris enhanced antiadipogenesis efficacy of mulberry leaves.
KEYWORDS: Mulberry leaves; Cordyceps militaris; Fermentation;3T3-L1 cell; Anti-adipogenesis; PPAR-γ
1.Introduction
In recent decades, obesity has become one of the most common medical problems all over the world[1].Some scientists ascribe obesity to increasing pre-adipocytes adipogenesis in vivo[2].As a pre-adipocyte cell line, 3T3-L1 plays an important role in antiadipogenesis research and anti-obesity drug screening in vitro[3].The 3T3-L1 cells has been widely used to research the adipogenesis when induced with insulin, dexamethasone and rosiglitazone[4].
Mulberry leaves has been used to improve the symptoms of fever,sore throat, cough, and protect the liver and improve eyesight in traditional medicine[5].Recent studies have shown that mulberry leaves contain substantial amount of bioactive compounds, such as pelargonidin, chlorogenic acid, iso-quercetin and caffeic acid[6-8].Pharmacological studies have shown that mulberry leaves extracts exerted anti-diabetic activity, improving hypolipidemic activity,anti-atherogenic activity and anticancer activity[9-12].Chang reported that polyphenol-enrich extract of mulberry leaves inhibited pre-adipocyte differentiation to adipocytes in vitro, but the effective concentrations were 0.25 mg/mL and 0.5 mg/mL[13].
Cordyceps (C.) militaris is a well-known tonic mushroom which is used to improve lung and kidney function in traditional medicine[14].Modern pharmacological studies revealed that C.militaris exerted anti-obesity and anti-adipogenesis effects[15,16].Previous studies reported that cordycepin, isolated from C.militaris, exerted antiobesity activity in high-fat diet induced rats[15,17].C.militaris can be cultured in the laboratory using artificial culture medium.Biological activities were changed or enhanced after organisms (silkworm pupa mung beans, Radix astragali and chickpeas) were fermented with C.militaris[18-20].As mentioned above, polyphenol-enrich extract of mulberry leaves has been reported to exert anti-adipogenesis effects when at very high concentrations; therefore, it may be possible to find a method to enhance anti-adipogenesis efficacy of mulberry leaves using C.militaris.
Adipogenesis and lipid accumulation are regulated by many transcriptional factors.Peroxisome proliferator-activated receptor-γ(PPAR-γ), a member of the PPARs family of nuclear hormone receptors, is essential in regulation of adipogenesis both in vitro and in vivo[21].Studies have revealed that PPAR-γ regulates adipocyte fatty-acid binding protein (A-FABP), fatty acid synthase(FAS), lipoprotein lipase (LPL), and 3-hydroxy-3-methyl-glutarylcoenzyme A reductase (HMGCR), which play crucial roles in lipids synthesis and accumulation[22-24].Moreover, PPAR-γ regulates glucose uptake through regulation of the expression of glucose transporter type 4 (Glut4)[25].
In this study, we aimed to find a new method of enhancement antiadipogenic effect of mulberry leaves.To accomplish this, dried mulberry leaves with or without silkworm pupae were fermented with C.militaris for 4 weeks at 25 ℃, after which the dried mulberry leaves and fermented product were extracted with 70% ethanol and subjected to high performance liquid chromatography (HPLC)to measure the contents of cordycepin, pelargonidin, chlorogenic acid, iso-quercetin and caffeic acid in dried mulberry leaves (ML),fermented mulberry leaves (FML), fermented mulberry leaves with 50% silkworm pupa (FMLP50), and fermented silkworm pupa (FP).We then used 3T3-L1 cells to study whether fermentation enhanced anti-adipogenic activity in vitro.
2.Material and methods
2.1.Reagents
Antibodies and RIPA buffer were purchased from Santa Cruz Biotechnology (Santa Cruz, Paso Robles, CA, USA).Triton X-100 was obtained from Bioshop (Burlington, Ontario, Canada).A commercial triglyceride quantification kit and a glucose quantification kit were supplied by Asan Pharmaceutical (Seoul,Korea).BioRad protein assay reagent, 4× loading buffer and protein marker were purchased from BioRad (Hercules, CA, USA).Rosiglitazone, dexamethasone, oil red O and other chemicals were bought from Sigma-Aldrich (St.Louis, MO, USA).
2.2.C.militaris preparation
C.militaris was procured from the Rural Development Administration in Korea (Jeonju, Jeollabuk-do, Korea) and was maintained on potato dextrose agar (PDA, Becton Dickinson, New Jersey, USA) slants.The slants were incubated at 25 ℃ for 1 week and then stored in a 4 ℃ freezer.The cultures were incubated in 300 mL Erlenmeyer flasks containing 100 mL of PD medium using a rotary shaker at 150 rpm for 5 d at 25 ℃.
2.3.Fermentation and extraction
The botanical identity of the mulberry (Morus alba) trees (Accession No.: PNU-ML001) (latitude: N 35°45′41″, longitude: E 128°80′72″,elevation: 37 m) was authenticated by one of the authors (Y.W.Choi).Mulberry leaves were collected from mulberry trees in July 2016.Then mulberry leaves were dried at room temperature and stored in a-20 ℃ freezer.Silkworm pupae (Bombyx mori) were purchased from Jinbo Genernal Trading Co.Ltd.(Busan, Korea).A total of 300 g of dried mulberry leaves, 300 g of silkworm pupa, mixture of 150 g of dried mulberry leaves and 150 g of raw silkworm pupa were put into an autoclavable vinyl bag.Then 600 mL distilled water was added to each bag.The samples were sterilized using an autoclave (Hyundai Co., Ltd., Seoul, Korea) at 121 ℃ for 30 min.After cooling to 25 ℃,prepared C.militaris suspension (5%, v/ w) was added into mulberry leaves and maintained at 25 ℃ for 4 weeks.Fermented dried mulberry leaves, fermented silkworm pupa, and fermented mixture of 150 g of dried mulberry leaves and 150 g of raw silkworm pupa were named as FML, FP and FML50 respectively.In addition, dried mulberry leaves not inoculated with C.militaris were used as ML.
After fermentation, the ML, FP, FMLP50 and FML were extracted with 10 times of volume of 70% ethanol using a sonicator at room temperature for 3 times, 1 h for each time.Whatman No.2 filter papers were used to filter the extracts for each time.Subsequently,extracts of each time were combined and concentrated using a reduce-pressure rotary evaporator system (Heidolph Instruments Co.,Ltd., Schwabach, Germany) at 45 ℃ and then stored at −18 ℃.
2.4.HPLC analysis
A total of 200 mg extracts (ML, FP, FMLP50 and FML) were dissolved in 8 mL distilled water, then filtered through a 0.45 μm syringe-driven filter (Millipore, Darmstadt, Germany) and 10 μL of each sample was subjected to an Agilent Technologies 1100 series Infinity HPLC system (Agilent Technologies, Santa Clara, CA,USA) with a DAD detector for quantitative analysis of pelargonidin,chlorogenic acid, iso-quercetin, cordycepin and caffeic acid.A 5 μm Luna C18(2) column (150 mm × 4.6 mm; Phenomenex, Torrance,CA, USA) was used to separate the samples at 30 ℃ and bioactive compounds were detected at 254 nm.The mobile phase is 0.025%formic acid in distilled water (solvent A) and 100% acetonitrile(solvent B).The elution conditions were 20 min (0% B), 30 min(15% B), 40 min (20% B), 50 min (80% B) and 55-60 min (100% B)at 0.5 mL/min.The compounds in the samples were identified and quantified by comparing retention times and UV spectra with standard compounds.
2.5.Cell culture
3T3-L1 cells were bought from KCLB (Korea Cell Line Bank,Seoul, Korea) and cultured in DMEM (Gibco, Gland Island, NY,USA) with 10% FBS (Welgene, Seoul, Korea) (culture medium).Cells grown to full confluence in culture medium in 48-well plates.Forty-eight hours later, cells were incubated with culture medium containing 10 μM rosiglitazone, 10 μg/mL insulin and 1 μM dexamethasone (differentiation medium, DM) for 8 d.The DM was refreshed for every 48 h.Additionally, ML, FML, FMLP50 and FP extracts were dissolved in DMSO as stock solution.Stock solution of samples and DMSO were added to the DM at dilution of 1:100 throughout the induction period to observe their effects on 3T3-L1 pre-adipocytes differentiation.
2.6.MTT assay
3T3-L1 cells were seeded in 96-well plates with DMEM medium or DM and grown to full confluence, then extracts were added at different concentrations.On day 8, 50 μL of MTT solution(2 mg/ mL) was added to every well and plates were put in incubator for another 4 h.After removal of incubation medium, 200 μL DMSO(GENEray, Shanghai, China) was used to dissolve formazan crystal for each well and then measured at 570 nm using an ELISA reader(BioTek Instruments, VT, USA).
2.7.Glucose content in medium
Cells were incubated with DM at presence of DMSO or extracts in 48-well plates as described in cell culture method.On day 8,a commercial glucose quantification kit was used to detect the glucose concentration in medium of each well according to the user instructions.
2.8.Oil red O staining
On day 8, after washing with PBS, cells were fixed using 4%paraformaldehyde (TCI, Tokyo, Japan) at room temperature for 30 min.Then, cells were stained by oil red O at room temperature for 30 min.Subsequently, dye was discarded and plates were washed two times by 70% EtOH.Then pictures were taken using a Motic microscope (Moitc, Xiamen, China).
2.9.Measurement of OD value
To evaluate the degree of differentiation, 150 μL isopropanol(GENEray, Shanghai, China) was used to dissolve the oil red O for each well.Next, 100 μL aliquots from each well were transferred to a new 96-well plate.The OD values then were measured at 500 nm using an ELISA reader (BioTek, Winooski, VT, USA).
2.10.Intracellular triglycerides (TG) content
Cells were incubated with DM at presence of DMSO or extracts in 12-well plates as described in cell culture method.After cells were washed with PBS, triglycerides of each well were extracted with 1%triton X-100.Then a commercial quantification TG kit was used to quantify TG content according to the user instructions.
2.11.Quantitative real-time polymerase chain reaction(PCR)
The 3T3-L1 cells were cultured in culture medium (control),or induced in DM or DM with FMLP50 (5 μg/mL, 10 μg/mL,20 μg/mL) for 24 h or 8 d.Total RNA was extracted using the Trizol reagent (Takara, Kyoto, Japan) according to the user instruction.After removal of genomic DNA using DNase I, 3 μg sample of RNA was subjected to first cDNA synthesis using a cDNA synthesis kit (Thermo, Madison, WI, USA).After which,gene expression was analyzed using an ABI SepOnePlus Real-Time PCR system (Applied Biosystems, Waltham, MA, USA).The primer sequences were: mouse PPAR-γ sense primer 5′-TGCTGTATTTGAATCCGACGTT-3′, mouse PPAR-γ anti-sense primer 3′- GCTCTTTAGAAACTCCCTTGTCATG-5′; mouse GAPDH Sense primer 5′-CATCAAGAAGGTGGTGAAGC-3′,mouse GAPDH Anti-sense primer 5′-CCTGTTGCTGTAGCCGTATT-3′.The expression level of PPAR-γ were normalized using GAPDH as an internal control.
2.12.Western blot
The 3T3-L1 cells were incubated with culture medium (control),or DM or DM with FMLP50 (5 μg/mL, 10 μg/mL, 20 μg/mL) for 24 h or 8 d.Cells were collected after washing with ice-cold PBS.Total proteins were extracted using RIPA buffer and quantified using BioRad protein assay reagent according to user instruction.Immediately, the proteins were mixed with 4× loading buffer and heated at 95 ℃ for 5 min.Then, 10% SDS-PAGE was used to separate proteins with a protein marker.Afterwards, a semi dry transfer system (BioRad, Hercules, CA, USA) was used to transfer proteins from gel to a PVDF membrane.After blocking with 1%BSA in 0.1% Tween-20 in PBS (PBST), the membranes then were probed with primary antibodies (PPAR-γ, HMGCR, A-FABP, CD36,LPL, FAS and Glut4) at dilution of 1:200 in 3% BSA-PBST for 16 h in a 4 ℃ freezer.Following, PBST was used to wash the membranes 3 times for 5 min each time.Then the membranes were incubated with the corresponding HRP-conjugated secondary antibodies for 1 h at room temperature.After the membranes were washed with PBST three times for 5 min each time, an ECL kit (Thermo Scientific, Rockford, IL, USA) was used to visualize the immunoreactive bands according to the user instruction.Finally, band density was measured using Image J software (Wayne Rasband, NIH, USA).
2.13.Statistical analysis
The Duncan’s Multiple Range Test was performed to analyze the levels of bioactive compounds and OD values of stained oil red O among groups, while the One-way-ANOVA was performed to analyze differences between groups.All data were shown as the mean±SD based on at least three independent replications.P<0.05 was considered as statistical difference.
3.Results
3.1.Fermentation changed content of bioactive compounds
To investigate the optimal addition dosage of silkworm pupa in mulberry leaves, the content of cordycepin, pelargonidin, chlorogenic acid, iso-quercetin, and caffeic acid in ML, FP, FMLP50 and FML were measured using HPLC (Figure S1, Table 1).Cordycepin was not detected in ML, but the silkworm pupa significantly impacted cordycepin content.The maximum content of cordycepin was(5 120.31±393.70) μg/g in FMLP50.However, it was significant lower (1 437.71±22.05 μg/g) in FML.The pelargonidin content of ML, FP, FMLP50 and FML were similar trend with cordycepin.However, fermentation with C.militaris notably decreased the contents of chlorogenic acid, iso-quercetin and caffeic acid when compared with ML group.The highest content of cordycepin and pelargonidin was observed in FMLP50 showing significant differences when compared with others, therefore, FMLP50 was considered as the optimal fermentation method.
3.2.Fermented extracts did not affect cell viability in 3T3-L1 cells
To investigate whether ML, FP, FMLP50 and FP affect cell viability in 3T3-L1 cells, an MTT assay was performed.Figure 1 showed that ML, FMLP50 and FP did not affect cell viability in 3T3-L1 cells at concentrations of 25 μg/mL, 50 μg/mL, 100 μg/mL and 200 μg/ mL and FMLP50 did not affect cell viability in 3T3-L1 cells at concentrations of 5 μg/mL, 10 μg/mL and 20 μg/mL.
3.3.Fermented extracts inhibited pre-adipocytes differentiation
Extracts were dissolved in DMSO and used to investigate the antiadipogenic activity.We observed that 3T3-L1 pre-adipocytes were differentiated to adipocytes after an 8-day differentiation period.As shown in Figure 2A, FP did not affect differentiation in 3T3-L1 cells.Although ML, FML and FMLP50 inhibited differentiation in 3T3-L1 cells, FMLP50 presented a better efficacy than ML and FML (Figure 2B).Specifically, adipogenesis was notably inhibited by ML and FML at concentrations of 100 μg/mL and 200 μg/mL and FMLP50 significantly inhibited adipogenesis at concentrations of 5 μg/mL, 10 μg/mL and 20 μg/mL (Figure 2).
3.4.FMLP50 inhibited triglyceride (TG) accumulation and glucose consumption in adipocytes
Previous studies have reported that PPAR-γ regulates glucose uptake and fatty acid storage[26].In this work, rosiglitazone, a PPAR-γ agonist, was used to induce 3T3-L1 cells differentiationat presence of insulin and dexamethasone.We investigated the influence of FMLP50 on the triglyceride accumulation and glucose concentration in DM using a commercial triglyceride quantification kit and a commercial TG quantification kit respectively.The results indicated that FMLP50 markedly decreased cellular TG content and glucose consumption in 3T3-L1 cells (Figure 3).

Table 1.Content of biological active compounds in ML, FP, MLP50 and FML extracts (μg/g).

Figure 1.Fermented (FML, FMLP50 and FP) and dried mulberry leaves (ML) extracts did not affect cell viability in 3T3-L1 cells.3T3-L1 cells were maintained in culture medium (control) or differentiation medium (DM).DMSO, FP, FMLP50, ML or FML were added to medium in the whole inducing period.In day 8, an MTT assay was used to measure cell viability, results showed ML, FP, FMLP50 and FP did not affect cell viability in 3T3-L1 cells.Values present as mean±SD (n=3).

Figure 2.Fermented extract inhibited adipogenesis in 3T3-L1 cells.3T3-L1 cells were maintained in culture medium (control) and induced adipogenesis with differentiation medium (DM).DMSO, FMLP50, ML, FML or FP were added to medium in the whole inducing period.Cells stained with oil red O for taking pictures (A), after that, stained oil red O were dissolved using iso-propanol for OD value detection (B).Pictures were taken at 200× (A).Values present as mean±SD (n=3).Different letters mean significant differences (P<0.05) among groups by Duncan’s Multiple Range Test.
3.5.FMLP50 inhibited expression of PPAR-γ in 3T3-L1 cells
To reveal the anti-adipogenic mechanism of FMLP50, 3T3-L1 cells were incubated in DM with DMSO or FMLP50 for 24 h and 8 d.Then total proteins and total RNA were extracted to measure the expression level of PPAR-γ which plays an essential role in the differentiation process of 3T3-L1 cells[26].Treatment with 10 μg/ mL and 20 μg/mL FMLP50 for 24 h decreased the expression of PPAR-γ to 72.9% and 51.3% at protein level, respectively, when compared with DMSO (Figure 4A).On day 8, PPAR-γ expression was decreased significantly by approximately 69.6%, 60.4% and 48.5% at protein level in response to treatment with FMLP50 at 5 μg/mL, 10 μg/mL and 20 μg/mL, respectively, when compared with DMSO (Figure 4B).The expression levels of PPAR-γ mRNA were significantly decreased after treatment with FMLP50 on day 1 (Figure 4C) and day 8 (Figure 4D) as well.These indicated that treatment with FMLP50 led to significant decrease of PPAR-γ expression when compared to DM group.

Figure 3.FMLP50 decreased glucose intake and TG content in differentiated 3T3-L1 cells.3T3-L1 cells were maintained in culture medium (control) and induced adipogenesis with differentiation medium (DM).DMSO or FMLP50 was added to the medium at the same time.Medium of each well were collected to determine glucose content (A).TG were extracted using 5% triton X-100 and determined quantity using a commercial TG kit according to protocol (B).Values present as mean±SD (n=3), *as P<0.05, **as P<0.01.
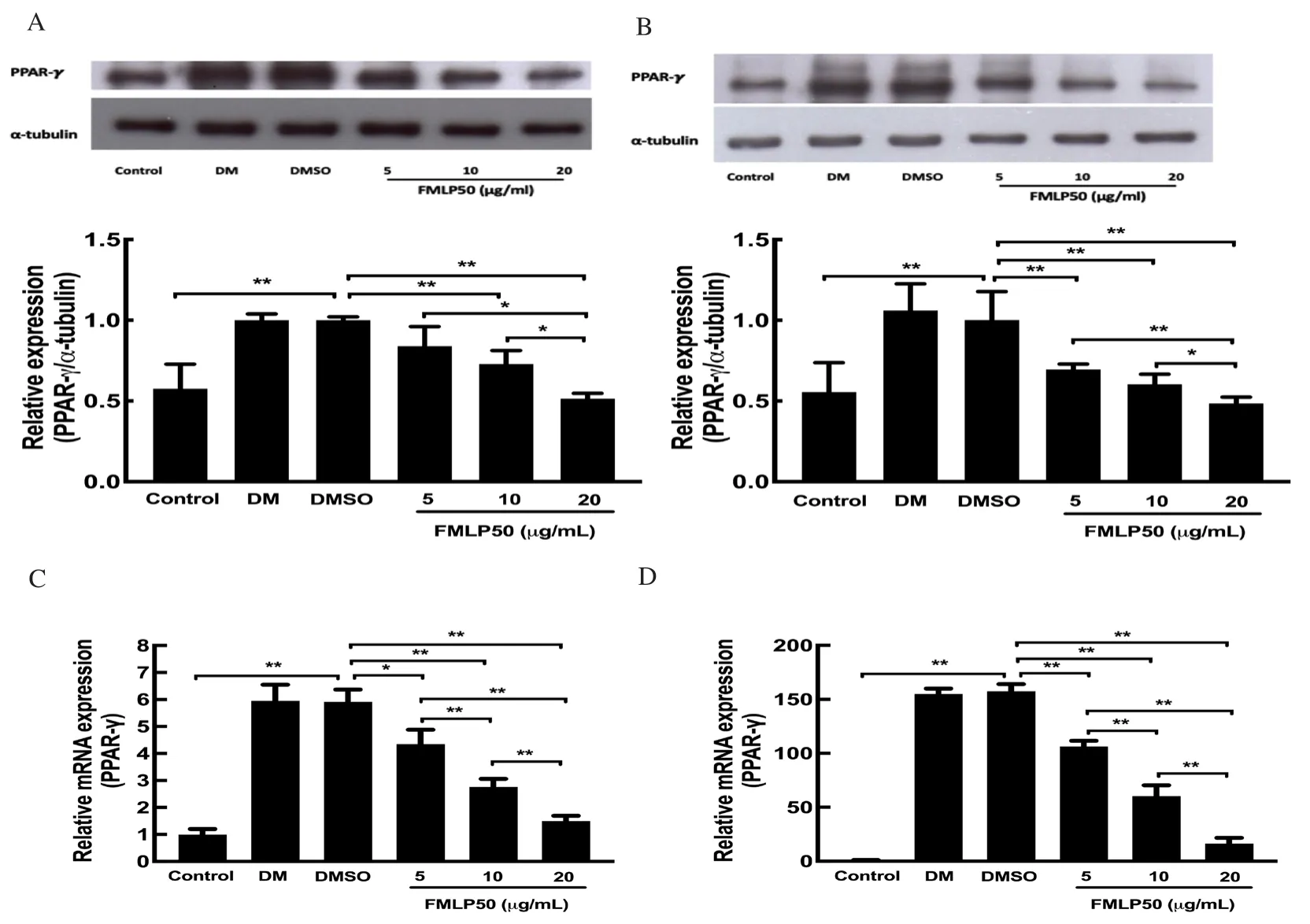
Figure 4.FMLP50 inhibited expression of PPAR-γ at 24 h (A) and 8 d (B).3T3-L1 cells were maintained in culture medium (control) and induced adipogenesis with differentiation medium (DM).The cells were treated with DMSO or FMLP50 for 24 h and 8 d, total proteins and RNA were extracted using RIPA buffer and Trizol reagent, and analysis by western blot and quantitative real-time PCR.The results show that fermented extract inhibited expression of PPAR-γ both at time point of 24 h (A, C) and 8 d (B, D).Values present as mean±SD (n=3), *as P<0.05, **as P<0.01.
3.6.FMLP50 inhibited expression of PPAR-γ’ s target protein in 3T3-L1 cells
The results of western blot and real-time PCR illustrated that FMLP50 suppressed the expression of PPAR-γ on day 1 and day 8.Additionally, the expression of PPAR-γ’s target protein in 3T3-L1 cells were evaluated at the time point of day 8 using western blot assay.The results showed that FMLP50 decreased expression of CD36, A-FABP, FAS, LPL, HMGCR and Glut4 in 3T3-L1 cells(Figure 5).
4.Discussion
Obesity affects people’s life habits all around the world[27].The 3T3-L1 cell line is universally used as an in vitro model for antiobesity drug screening.Rosiglitazone, a PPAR-γ agonist, is widely used to induce differentiation in 3T3-L1 cells at the presence of insulin and dexamethasone[28].These effectors have been confirmed to activate a cascade of transcriptional events that impact the expression of mature adipocytic phenotypes[29].In this work,rosiglitazone, insulin and dexamethasone successfully induced adipogenesis in 3T3-L1 cells (Figure 2).
Mulberry leaves possess a variety of bioactive compounds and are considered as potential source of functional components[30].The extraordinary content of bioactive constituents seems to be responsible for medicinal effects on human health.In this study,we focused on the anti-adipogenesis effects of fermented mulberry leaves after fermentation of mulberry leaves with C.militaris with silkworm pupa as promotor sources.Silkworm pupa are a good source of several valuable phytonutrients for production of fruit body of C.militaris[20].Fermentation various resources, including mung beans, Radix astragali, chickpea and silkworm pupa, with C.militaris led to increase of the content of some phenolics and flavonoids, such as shikimic acid, chlorogenic acid and biochanin A in fermented chickpeas and improvement of biological activity[18- 20,31].In our study, the contents of cordycepin and pelargonidin, particularly cordycepin, were increased markedly in fermented mulberry leaves extracts, especially in FMLP50 when compared with ML.However, the levels of chlorogenic acid, iso-quercetin and caffeic acid were decreased after fermentation.Pelargonidin is a secondary metabolite in natural organism.Though there is no direct evidence,the biosynthesis of pelargonidin in C.militaris might be regulated by several enzymes, such as transaminase B, N-acetyl-gammaglutamyl-phosphate reductase, anthocyanidin reductase, and anthocyanidin synthase[32].

Figure 5.FMLP50 inhibited expression of PPAR-γ’s target protein in differentiated 3T3-L1 cells.3T3-L1 cells were maintained in culture medium (control)and induced adipogenesis with differentiation medium (DM).After treatment with DMSO or FMLP50 for 8 d, total proteins were extracted using RIPA buffer,and analysis by western blot.The results showed that FMLP50 inhibited expression of PPAR-γ’s target protein at the time point of 8 d.Values present as mean±SD (n=3), *as P<0.05, **as P<0.01.
FMLP50 exhibited much better anti-adipogenesis efficacy than ML and FML, and the efficacy of anti-adipogenic activity was found to response to enrichment of cordycepin and pelargonidin in fermented samples.Cordycepin and pelargonidin have been shown to exert anti-adipogenic activity in 3T3-L1 cells[33,34].The results of extracts on anti-adipogenesis in 3T3-L1 cells were consistent with contents of cordycepin and pelargonidin in fermented products.
Glucose is a nutritional resource in the process of 3T3-L1 cells differentiation, and higher glucose consumption resulted in higher adipogenesis ration in 3T3-L1 cells[35].TG is an important storage form of lipids in adipocytes.In the present work, rosiglitazone was used to induce differentiation in 3T3-L1 cells.Previous studies has been reported that rosiglitazone led to increase glucose consumption and TG content in mice[36,37].FMLP50 significantly inhibited TG accumulation and glucose uptake in a dose-dependent manner after 3T3-L1 cells were incubated with rosiglitazone for 8 d (Figure 3).
The results of HPLC showed that content of cordycepin and pelargonidin in FMLP50 are notable higher than these two in ML.Cordycepin and pelargonidin have been proved to suppress adipogenesis through inhibition of PPAR-γ pathway signaling[33,34].Therefore, we hypothesize FMLP50 potentially inhibited PPAR-γ in 3T3-L1 cells.Quantified real-time PCR and western blot assay proved that expression of PPAR-γ was inhibited by FMLP50 on day 1 and day 8.
Furthermore, total proteins were extracted on day 8 and the expression of PPAR-γ’s target proteins were measured using western blot.A-FABP, which is primarily expressed in adipocytes,regulates lipids storage and lipolysis in adipose tissue[38].FAS plays a critical role in lipids accumulation in the process of lipogenesis[39].Blockage of FAS resulted in inhibition of lipid accumulation[40].Increase expression of LPL, a critical maker protein for preadipocyte differentiation to adipocyte, led to raise of cellular TG content[39].HMGCR plays a vital role in isoprenoid pathway which makes isoprenoids, including cholesterol[41].In this study, we found that expression of them in 3T3-L1 cells was decreased significantly after cells were incubated with FMLP50 for 8 d.Glut4 has an essential role in glucose transportation in the process of adipocytes differentiation[42].In the present study, FMLP50 induced a dosagedependent decrease in the expression of Glut4 (Figure 5).Thus,inhibition of these proteins inhibited TG accumulation and glucose consumption during adipocyte differentiation period, which explains why FMLP50 suppressed adipogenesis in 3T3-L1 cells.
In conclusion, our study illustrated that fermentation of mulberry leaves with C.militaris enhanced efficacy of anti-adipogenesis when compared with ML because of changes in chemicals during fermentation.The results showed that FMLP50 presented better efficacy of anti-adipogenesis than ML.Further studies revealed that FMLP50 inhibited triglycerides accumulation and glucose consumption in the progression of differentiation in 3T3-L1 cells.Finally, western blot analysis revealed that FMLP50 inhibited the expression of PPAR-γ and its target proteins.Thus, the 3T3-L1 cells might be inhibited differentiation by FMLP50 through inhibition of PPAR-γ pathway signaling.Based on the results of this study,FMLP50 could be a potential and effective material for anti-obesity research.
Conflicts of interest statement
The authors declare that there is no conflict of interest.
Funding
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through High Value-added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA)(116027032HD020).
Authors’ contributions
LG and YWC conceived the study design, performed the study,analyzed the data, drafted and revised the manuscript.JSK, YHP,BIJ, DYH and WHJ revised the manuscript.All authors discussed the results and approved the final manuscript.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Current status and future prospects of bacilli-based vector control
- Patterns of hepatitis B virus exposure and associated predictors in Vietnam: A crosssectional study
- Morphological and molecular characterization of Acanthamoeba isolated from contact lens paraphernalia in Malaysia: Highlighting the pathogenic potential of T4 genotype
- Multiplex real-time PCR revealed very high prevalence of soil-transmitted helminth infections among aborigines in Peninsular Malaysia
- In vitro efficacy of new synthetic benzimidazole-related compounds against Schistosoma mansoni adult worms
