Factors predisposing to thrombosis after major joint arthroplasty
2020-10-26
Abstract
Key Words:Arthroplasty; Thrombosis; Heparin-induced thrombocytopenia; Coagulation factors; Low-molecular-weight heparin; Fondaparinux
INTRODUCTION
Venous thromboembolism,representing one of the major complications after total joint arthroplasty (TJA) procedures,is related to morbidity and mortality during the perioperative period[1].Deep vein thrombosis (DVT) can easily develop into pulmonary embolism (PE),leading to cardiopulmonary dysfunction and death.The incidence of DVT when no prophylaxis is administered is 42%-57% for total hip arthroplasty (THA) and 41%-85% for total knee arthroplasty (TKA)[2].An estimated baseline risk of symptomatic venous thromboembolism without prophylaxis after major orthopedic surgery is about 4.3%[3].Apart from factors related to surgery and decreased mobility after major TJA,there are many other well defined conditions that are associated with either a prothrombotic state or embolic phenomena,such as mutations or polymorphisms in genes that encode blood coagulation factors,metabolic syndrome,or immune reactions related to pharmacologic agents such as anticoagulants,which could lead to increased risk of venous thromboembolism and its consequences[2,4].
Anticoagulants have been found to reduce the risk of thromboembolic events after major orthopedic surgery by approximately 50%-80% when used prophylactically[5].Heparin and heparin-derived pharmacologic agents,including unfractionated heparin(UFH),low-molecular-weight heparin (LMWH),and occasionally fondaparinux,may stimulate heparin-induced thrombocytopenia (HIT),a prothrombotic adverse drug reaction that is caused by the transient production of platelet-activating antibodies of IgG class that recognize multimolecular complexes of platelet factor 4 (PF4) bound to heparin[6,7,8].The IgG/PF4/heparin complexes may enhance the alteration of endothelial cells,platelets,and monocytes resulting in thrombin generation.Increased thrombin,and not thrombocytopenia,causes several clinical problems such as venous and arterial thrombosis,including the cerebral sinus or splanchnic vessels,pulmonary embolism,stroke,or myocardial or adrenal infraction with a mortality rate of 11.9% to 23.1%[9,10,11].
The risk for venous thromboembolism in patients undergoing major orthopedic surgery is strongly determined by individual factors such as demographics,comorbidities,and medical history.Many studies have highlighted the role of varicose veins,congestive heart failure,female gender,age,hypertension,history of venous thromboembolism,cancer,diabetes,dyslipidemia,obesity,Black race,TJA type(primary or revision),primary disease (osteoarthritis,rheumatoid arthritis),and duration of the procedure in an increased risk of DVT after TJA[2,4,12].Moreover,hypercoagulability,one of the three clinical conditions of the Virchow triad,has been related to several predisposing genetic risk factors and numerous candidate genes.The most prevalent molecular variants,mutations,or polymorphisms causing venous thrombosis in the Caucasian population are factor V Leiden (G1691A),prothrombin(factor II G20210A) and methylenetetrahydrofolate reductase (MTHFR/C677T)[13,14].Factor V Leiden,which results in impaired inactivation of factor Va by activated protein C,is associated with a seven-fold increased risk of thrombosis[15].Additionally,factor II 20210G/A (prothrombin 20210) is a prothrombotic genetic risk factor,particularly in the presence of concomitant risk factors identified in 8%-10% of Caucasians with thrombotic events[16].
The aim of this prospective study is to evaluate the magnitude of individual genetic profiles and adverse pharmacologic (anticoagulant immunologic) reactions activating platelets and causing thrombocytopenia in the development of thrombotic episodes among patients undergoing lower limb TJA.Furthermore,we intended to assess the impact of different types of anticoagulants on the hematologic profile and incidence of HIT and thromboembolism after TJA by selecting a strictly homogeneous patient sample without other well-defined predisposing factors for thrombotic events.
MATERIALS AND METHODS
In total,212 (51 male and 161 female) patients that underwent primary THA (100) or TKA (112) due to osteoarthritis during a period of 1 year were enrolled prospectively in the study that had been approved by the hospital ethics committee.All patients gave informed consent prior to their inclusion in the study.Patients with other causes of arthritis (rheumatoid arthritis,avascular necrosis,seronegative spondyloarthropathies,crystal deposition disease,etc.) as well as patients with previous thrombotic events or other major risk factors for thrombosis (including malignancy,diabetes,hypertension,BMI > 35,age > 80 years,operation time > 100 min,congestive heart failure,arrhythmia,smoking,varicose veins) were excluded from this study.The mean age of patients was 65.8 years (range,43.0-80.0).None of the patients had any history of previous heparin exposure within the past 90 d.The operation was performed under regional central anesthesia in all cases.All surgeries were performed by a group of four experienced surgeons sharing the same surgical principles.THAs were performed with a cementless femoral stem and an acetabular component,while TKAs were performed with a cemented component under tourniquet control(operation time with the use of tourniquet was less than 100 min for all patients included in this study,as defined above).The excessive use of pulsatile jet-lavage was a standard procedure before cement application in order to remove fat and bone marrow,which could be potential embolic sources.In addition,we ruled out the appearance of bone cement implantation syndrome of any grade throughout the perioperative period.Drainage was used in all cases and patients were encouraged to start flexing the ankle joint,contracting the quadricep muscle as soon as possible,and to begin ambulation with the help of crutches or ambulator on the first postoperative day.
Platelet counts were obtained from all patients preoperatively,daily for the first seven postoperative days,and on the 20thand 60thpostoperative days,while the presence of IgG anti-PF4/heparin antibodies was evaluated with an immunoassay preoperatively,and on the 3rd,7th,20thand 60thpostoperative days [kit of DiaMed (IDPaGIA)].Fasting serum lipids (total cholesterol and triglycerides) were measured preoperatively.Finally,protein C,protein S,von Willebrand factor,lupus anticoagulant,antithrombin III,677C/T mutation of MTHFR gene,factor V Leiden(G/A),and prothrombin gene G20210A mutations were investigated.Genomic DNA was extracted from fresh blood using the QIAamp DNA Blood Midi extraction kit(Qiagen,Hilden,Germany).
All patients received anticoagulation for a total of 6 wk.Anticoagulants used were:tinzaparin 4500 IU (Innohep,Leo Pharma,France),enoxaparine 40 mg (Clexane,Aventis Pharma,Maisons-Alfort,France),dalteparin 5000 IU (Fragmin,Pharmacia &Upjohn,Pfizer Hellas S.A.,Athens,Greece) or fondaparinux 2.5 mg (Arixtra,Sanofi-Synthelabo,Paris,France).Anticoagulants were administered 12 h postoperatively if there was no clinical evidence of bleeding and were administered on a once daily basis.During the postoperative period care was taken to avoid incidental exposure to small amounts of UFH.
All patients were followed for a minimum period of 3 years (36-44 mo).Sixteen patients were lost to follow-up and excluded from the study; thus 196 patients (42 male and 154 female) remained at the final follow-up.The majority of these patients(173) received LMWH for prevention of thrombosis,while 23 patients received fondaparinux.Patients were monitored clinically for the development of arterial or venous thrombotic events,such as DVT,PE,cerebrovascular accident,and myocardial infarction.On suspicion of a thrombotic event the necessary examinations (triplex,pulmonary ventilation/perfusion scan) were performed to rule out or to confirm the thrombotic incident.
Statistical analysis
After the enrollment of 50 patients in the study,sample size estimations showed that the required sample size to have adequate power to detect potential statistically significant differences should be approximately 200 patients.A post hoc power analysis showed that the power of the final sample of the study equaled 0.71.The Gpower v.3.1 was used in both cases.
AllPvalues were based on two-tailed tests,and the level of statistical significance was set atP< 0.05.The tests used to obtainPvalues were the Pearsonχ2and Fisher’s Exact.Logistic regression analysis was used to study the significant correlations.Repeated measurements analysis was applied to test differences in platelet levels between the two anticoagulants.Analysis was conducted using SPSS 14 (SPSS,Inc.,Chicago,IL,United States).
RESULTS
Thrombocytopenia
Thrombocytopenia,defined as ≥ 50% decrease in platelets assessed relative to the preoperative (and preanticoagulant administration) platelet count or to the highest preceding value[7,16],was observed in 30 patients (15.3%) the first 4 postoperative days and in 2 additional patients (1.0%) on postoperative days 5-7.
Thrombocytopenia in the first 4 postoperative days was temporary in all patients,as expected.Platelet counts below 100 × 109/L were observed in 17 patients (15 patients the first 4 postoperative days and 2 additional patients days 5-7).There was no statistically significant correlation between the type of anticoagulant (LMWH or fondaparinux) and decrease in platelet counts (over 50% from preoperative counts) in the first 4 postoperative days (Fisher’s exact test,Pvalue = 0.134) or between anticoagulant type and the decrease of platelet counts (over 50% of the highest preceding value) in days 5-7 (P= 1.000,Fisher’s exact test).
Repeated measurements analysis for the comparison of platelet counts preoperatively and postoperatively showed a fluctuation in counts.A sharp decline of platelet counts was found starting from day 1,approaching the lowest level on the 3rdpostoperative day,and then a steady rise to nearly preoperative values on the 5thday.There were no differences in changes of platelet counts between the two anticoagulation groups (Figure 1).

Figure1 Comparison of platelet counts fluctuation between low-molecular-weight heparin and fondaparinux.
Anti-PF4/heparin antibodies
Anti-PF4/heparin antibodies developed in 18 of 196 patients (9.2%).The incidence of these antibodies was 12/173 (6.9%) for LMWH and 6/23 (26.1%) for fondaparinux(Figure 2).There was a statistically significant correlation between the type of anticoagulant (LMWH or fondaparinux) and appearance of anti-PF4/heparin antibodies the 1stpostoperative week (Fisher’s exact test,Pvalue = 0.005).The odds of anti-PF4/heparin antibody emergence the first postoperative week was about 8.2%greater in patients receiving fondaparinux than those receiving LMWH.
There was a peak in the appearance of anti-PF4/heparin antibodies on the 7thpostoperative day (9/18 patients,50.0%).Three patients developed anti-PF4/heparin antibodies on the 3rdpostoperative day,3 on the 20thpostoperative day,2 on the 60thpostoperative day,and 1 patient 3 mo postoperatively.
HIT
HIT,defined as a fall in platelet count > 50% of preoperative values (or the highest value preceding anticoagulant administration) and presence of anti-PF4/heparin IgG antibodies (strongly positive immunoassay,OD > 1) developed in 4 of 196 patients(2.0%) (Figure 3).Two of these patients developed the syndrome in the first postoperative week,while the other 2 patients developed it between the second postoperative week and the 3rdmo.However,there was no significant correlation between the platelet counts of postoperative days 1-4 and the antibodies detected at days 3 or 7,or the antibodies detected across any of the time frames of this study (P=1.000,Fisher’s exact test).Also,there was no significant correlation between the platelet counts of days 5-7 and the antibodies detected on day 7 (P= 1.000,Fisher’s exact test),or the antibodies detected across any of the time frames of this study (P=0.212,Fisher’s exact test).Three of the patients that developed HIT were receiving LMWH for anticoagulation and one was receiving fondaparinux.
Mutations of coagulation factors
The FV1691G/A (factor V Leiden) mutation was detected in 5 patients (2.6%),while heterozygosity and homozygosity for the G20210A mutation in the prothrombin gene was demonstrated in 8 (4.1%) patients and 2 (1%) patients,respectively.Two patients(1.0%) were heterozygotes for both factor V Leiden and G20210A mutation.The 677T MTHFR polymorphism was present in almost 50% of patients (32.7% heterozygous/13.8% homozygous).
Abnormal low protein C and/or S levels were found in 3 of 196 (1.5%) patients,while all patients had normal levels of von Willebrand factor,lupus anticoagulant,and antithrombin III.
Thrombotic events
Symptomatic thrombotic events were observed in 5 of the 196 patients (two incidents of DVT the 7thand 8thpostoperative days,two incidents of PE the 2ndand 14thpostoperative days,and one myocardial infarction in the 5thpostoperative week),while the likelihood of DVT in 10 patients (between the 4thand 56thpostoperative days)or PE in 2 patients (the 4thand 7thpostoperative days) was not confirmed by the triplex or pulmonary ventilation/perfusion scans.

Figure2 Percentage of patients with anti-platelet factor 4/heparin antibodies according to pharmacologic agent (P < 0.05).
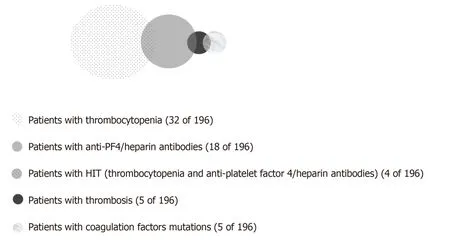
Figure3 Correlation of thrombocytopenia,anti-platelet factor 4/heparin antibodies formation,heparin-induced thrombocytopenia,mutation of coagulation factors,and development of thrombosis.
Of 5 patients that developed thrombotic complications,2 had positive anti-PF4/heparin antibodies (but no thrombocytopenia),and 2 patients (suffering from DVT and PE) were heterozygote for both factor II and V mutations (Figure 3).There was no significant association between thrombotic events and platelet counts preoperatively (P= 1.000,Fisher’s exact test),days 1-4 (P= 0.574,Fisher’s exact test),or days 5-7 (P= 1.000,Fisher’s exact test).Although not statistically significant,the association between the thrombotic events and anti-PF4/heparin antibodies (detected in all time frames) approached significance (P= 0.076,Fisher’s exact test).Finally,the presence of HIT was not associated with a thrombotic event (Fisher’s exact test,P=1.000).Thrombotic episodes were significantly correlated to mutations of factor II(Fisher’s exact test,Pvalue = 0.043) and factor V (Fisher’s exact test,Pvalue = 0.013).For patients with factor V mutation,the odds for thrombosis were 25.6% greater than in patients without this mutation.MTHFR homozygotes were not found to have increased risk for thrombotic episodes (P= 0.58).With logistic regression analysis it was shown that because of the correlation of the mutations only factor V had a significant influence on thrombotic episodes(P =0.022).
No association was found between elevated serum lipids and thrombosis (P= 0.30 for cholesterol andP= 0.31 for triglycerides).Finally,gender was not related to anti-PF4/heparin antibody formation (P= 0.39) or thrombosis (P= 0.11).
DISCUSSION
In this study we prospectively investigated the influence of blood coagulation factors and various pharmacologic antithrombotic prophylactic agents on the development of thrombotic complications in patients with osteoarthritis undergoing primary hip and knee arthroplasty.
Thrombocytopenia is common during the postoperative period in various types of major surgical procedures.A platelet count decrease is expected within 4 d of surgery normally resulting from the pathophysiological mechanisms of the hemodilution along with accelerated platelet consumption related to surgical hemostasis[17].Approximately 16% of the patients in the present study developed thrombocytopenia without a statistically significant correlation to the type of anticoagulant (LMWH or fondaparinux) but to a greater degree than those reported in the literature[18,19].Platelet counts recovered spontaneously in all cases.We speculate that possible blood loss,routine use of drainage,occult vitamin deficiency (B12,folic acid),high threshold to transfusion,or drug related (antibiotics,nonsteroidal anti-inflammatory drugs) excess platelet destruction may explain the variation of the incidence of thrombocytopenia across different studies.
Anti-PF4/heparin enzyme immunoassays have an excellent negative predictive value (up to 99%) but a low positive predictive value because of the detection of clinically insignificant antibodies[7,19].As anti-PF4/heparin antibodies are always present before the platelet count falls,these tests are important as a confirmatory laboratory evaluation after the initial estimation of high probability of HIT by a scoring system like the 4T score[20].In our series with the use of immunoassay,we found that 9.2% of patients developed anti-PF4/heparin antibodies,a percentage within the findings of other series for patients undergoing TKA or THA (2%-35%)[21].
There was no significant correlation between the platelet counts of postoperative days and antibodies detected during all time frames of this study.In addition,only a small percentage (4/18) of patients with anti-PF4/heparin antibodies had a concomitant decrease of platelets (Figure 2).As platelet activation from anti-PF4/heparin antibodies is a dynamic process and platelet-activating antibodies comprise a subset of anti-PF4/heparin antibodies,thrombocytopenia is not an associated finding in every positive PF4-dependent immunoassay[22,23].This is in agreement with the “iceberg model,” where only a small percentage of patients who form anti-PF4/heparin antibodies will develop thrombocytopenia,and an even smaller number will develop thrombosis[23].
An interesting point,in agreement with other studies,is the high incidence of these antibodies among patients receiving fondaparinux (26.1%),an antithrombindependent,sulfated pentasaccharide with selective activated factor X inhibition,while the incidence among patients receiving LMWH was 6.9%[24].Moreover,the odds of anti-PF4/heparin antibody emergence in the first postoperative week was about 8.2%greater in patients receiving fondaparinux than those receiving LMWH.It is well established that fondaparinux triggers an autoimmune type HIT with antibodies that activate platelets in the absence of heparin.The serum of these patients always contains antibodies with different binding affinities for PF4 and PF4/heparin complexes and usually with an inability to promote platelet activation[9].
In general,heparin and heparin-like anticoagulants differ in their predisposition to trigger HIT due to divergent structural length of their saccharide units (UFH >>LMWH >> fondaparinux).Also,fondaparinux is approximately one third of the length of LMWH,is more homogenous as a synthetic anticoagulant than LMWH and UFH,and thus is characterized by lower immunogenicity andin vivocross reactivity[6,25].These data suggest that the risk of HIT with fondaparinux should be much lower than that observed with LMWH and perhaps should not occur at all.HIT frequency is much lower in patients receiving LMWH than those receiving UFH[9].In a 2005 metaanalysis,it was found that the risk for HIT was 0.2% with LMWH and 2.6% with UFH[23].A small percentage (2.0%) of patients in our series developed HIT(thrombocytopenia in combination with the formation of antibodies,strong positive immunoassay,OD > 1),and all four remained free of thrombotic complications.Although fondaparinux is not believed to cause HIT,one of the four patients of the present series who developed HIT was receiving fondaparinux.After the initial publication of an analogous case in 2007,a review of the literature through May 2013 found eight published cases of fondaparinux-associated HIT and concluded that the risk of fondaparinux-associated HIT,although low,was real.After a decade of extended research,fondaparinux–associated HIT is one of the clinical syndromes that are strongly related to autoimmune heparin induced thrombocytopenia[25,26].
Two of the five patients who developed thrombotic complications had positive anti-PF4/heparin antibodies.The association between the thrombotic events and the anti-PF4/heparin antibodies approached significance in contrast to the presence of HIT that was not associated with a thrombotic event.According to our study,formation of antibodies can lead to thrombosis without thrombocytopenia.The association of antibodies to thrombosis,in the absence of thrombocytopenia,has been recently highlighted in the literature[21,26,27].In a report published in 2008 found 22 instances of patients who developed heparin-dependent antibodies sometimes associated with thrombosis without thrombocytopenia[27].We might also assume that platelet decline and thrombosis are two events that occur in a different sequence in each patient.In a retrospective analysis of 408 patients with HIT,it was shown that in approximately 60% of patients thrombosis was observed either on the same day thrombocytopenia >50% was documented (26.3%) or before thrombocytopenia (33.5%)[28].
Two patients of the present series developed thrombotic complications that were significantly correlated to heterozygotic mutations of factor II and factor V Leiden.Factor V Leiden and prothrombin G20210A revealed a significantly higher risk for DVT (P= 0.013 andP= 0.043,respectively),and the odds for thrombosis were 25.6%greater in patients carrying the factor V Leiden mutation.In contrast,MTHFR C677T polymorphism,as shown by our results,even in a homozygote state was not associated with increased risk of DVT.The factor II,factor V Leiden,and MTHFR C677T mutations are the most common well-recognized conditions predisposing patients to thromboembolism following joint replacement,especially in the Caucasian population[14,29].Individuals who are homozygous for factor V Leiden have been estimated to have a 50-fold increased risk of venous thrombosis,whereas heterozygotes have a 10-fold increased risk[29].Moreover,it has been shown that the copresence of factor V Leiden with prothrombin gene G20210 A heterozygotic mutation multiplies the predicted risk of thrombotic events from about 4.0-5.0 to 20.0[30].The selection of a group of patients with strict input criteria in the study helped to identify with greater clarity the participation of the genetic profile in the occurrence of a thrombotic episode after TJR.Identifying and characterizing genetic risk should help to develop diagnostic and treatment strategies for several etiological mechanisms that cause thrombophilia following major orthopedic surgery.
In conclusion,although the number of patients in our series was relatively small,the rigorous sample selection criteria optimized the process of drawing conclusions and limited the impact of bias.Statistical analysis indicated that symptomatic thrombotic events were not correlated to thrombocytopenia or HIT and also showed that platelet count monitoring does not necessarily uncover cases of formation of anti-PF4/heparin antibodies that may be correlated to venous thromboembolism.Moreover,both LMWH and fondaparinux were found to be responsible for the formation of anti-PF4/heparin antibodies.The correlation of thrombotic events to the formation of anti-PF4/heparin antibodies approached significance whereas mutations of factor II and factor V were significantly correlated to symptomatic thrombosis.Thus,the evaluation of mutations of factor II and factor V preoperatively may reduce thrombotic complications in patients undergoing major joint replacement.Preoperative tests should be based on the clinical benefit and cost effectiveness ratio and should also lead to an individual patient risk assessment or to the most optimal pharmacologic prophylaxis against venous thromboembolism and HIT after elective lower limb arthroplasty.
ARTICLE HIGHLIGHTS
Research background
Numerous studies have emphasized the association of multiple risk factors,including varicose veins,congestive heart failure,female gender,age,hypertension,venous thromboembolism history,cancer,diabetes,dyslipidemia,obesity,black race,total joint arthroplasty (TJA) type (primary or revision),primary disease (osteoarthritis,rheumatoid arthritis),and procedure duration in the increased risk of deep vein thrombosis after TJA.Deep vein thrombosis can easily develop into pulmonary embolism leading to cardiopulmonary dysfunction and death.
Research motivation
Apart from the factors related to surgery,comorbidities,medical history,and decreased mobility after major TJA,there are many other well defined conditions that are associated with either a prothrombotic state or embolic phenomena,such as mutations or polymorphisms in genes that encode blood coagulation factors,metabolic syndrome,or immune reactions related to pharmacologic agents such as anticoagulants,which would possibly lead to increased risk of venous thromboembolism and its consequences.We aimed to assess the impact of different types of anticoagulants on the hematologic profile and the incidence of heparininduced thrombocytopenia (HIT) and thromboembolism after TJA by selecting a strictly homogeneous patient sample uninfluenced by other predisposing factors for thrombotic events.
Research objectives
The aim of this prospective study was to evaluate the influence of individual genetic profiles and adverse pharmacologic (anticoagulant immunologic) reactions activating platelets and causing thrombocytopenia on the development of thrombotic episodes among patients undergoing lower limb TJA.
Research methods
In 212 patients that underwent primary total hip arthroplasty or total knee arthroplasty due to osteoarthritis during a period of 1 year,platelet counts and antiplatelet factor 4 (anti-PF4)/heparin antibodies were evaluated pre/postoperatively and antithrombin III,methylenetetrahydrofolate reductase,factor V,and prothrombin gene mutations were detected.In a minimum follow-up of 3 years patients receiving either low-molecular-weight heparins (LMWH) or fondaparinux were monitored for the development of thrombocytopenia,anti-PF4/heparin antibodies,HIT,and thrombosis.
Research results
Thirty-two patients developed thrombocytopenia (insignificant correlation between anticoagulant type and thrombocytopenia,P= 0.134),and eighteen developed anti-PF4/heparin antibodies (12/173 for LMWH and 6/23 for fondaparinux).There was a significant correlation between anticoagulant type and antibody appearance (P=0.005).Odds of antibody emergence were 8.2% greater in patients receiving fondaparinux than LMWH.Four patients developed HIT (insignificant correlation between thrombocytopenia and antibodies),and five developed thrombosis.Two had positive antibodies and two were heterozygotes for both factor II and factor V mutations.Thrombosis was not significantly correlated to platelet counts or HIT.The correlation of thrombosis to antibodies,factor II,and factor V wasP= 0.076,P= 0.043,andP= 0.013,respectively.
Research conclusions
Screening of coagulation profile,instead of platelet monitoring,is likely the safest way to minimize the risk of post-arthroplasty thrombosis.In addition,fondaparinux can lead to the formation of anti-PF4/heparin antibodies or HIT.
Research perspectives
Although the number of patients of our series was relatively small,the rigorous sample selection criteria optimized the process of drawing conclusions and limited the impact of bias.Statistical analysis indicated that symptomatic thrombotic events were not correlated to thrombocytopenia or HIT and platelet count monitoring does not necessarily uncover cases of formation of anti-PF4/heparin antibodies that may be correlated to venous thromboembolism.Moreover,both LMWH and fondaparinux were found to be responsible for the formation of anti-PF4/heparin antibodies.The correlation of thrombotic events to the formation of anti-PF4/heparin antibodies approached significance,whereas mutations of factors II and V were significantly correlated to symptomatic thrombosis.Thus,the evaluation of mutations of factor II and factor V preoperatively may reduce thrombotic complications in patients undergoing major joint replacement.Preoperative tests should be based on the clinical benefit and cost effectiveness ratio and should also lead to individual patient risk assessment or to the most optimal pharmacologic prophylaxis against venous thromboembolism and HIT after elective lower limb arthroplasties.
ACKNOWLEDGEMENTS
The authors would like to thank George Dimakopoulos for his expert scientific assistance in statistical analysis and Teresa Jane Carr for language evaluation of the manuscript.
