添加蒙脱石是否促进白花苜蓿的腐殖化进程?*
2020-10-22张一枫田宇欣段宏美
徐 杨,窦 森,张一枫,田宇欣,段宏美,白 月
添加蒙脱石是否促进白花苜蓿的腐殖化进程?*
徐 杨,窦 森†,张一枫,田宇欣,段宏美,白 月
(吉林农业大学资源与环境学院,长春 130118)
植物残体和微生物生物量是腐殖物质(Humic substance,HS)形成的主要母体材料,同时黏土矿物作为土壤的重要组成部分,在HS的形成中也扮演重要角色,然而目前有关黏土矿物对HS形成过程的具体影响还不清楚。选择将黏土矿物-蒙脱石和微生物-土壤浸提液作为控制条件对白花苜蓿进行模拟培养腐殖化,通过表征培养过程中总有机碳(Total organic carbon,TOC)含量、类腐殖质组成、类胡敏酸(Humic-like acid,HLA)的元素组成和红外光谱特征,探究蒙脱石添加对白花苜蓿腐殖化进程的影响。结果表明,模拟培养腐殖化后,蒙脱石添加虽然加速了TOC的分解,促进HLA的生成,但使HLA的缩合度降低,结构简单化;而土壤浸提液添加则使蒙脱石处理加快了结构更加复杂的HLA积累。培养后,不同处理的HLA均向着接近土壤胡敏酸(Humic acid,HA)O/C和H/C值的方向发展,其中未添加蒙脱石处理(AnM)的HLA复杂化程度与真正的HA最接近,更进一步说明蒙脱石不能促进HLA更接近土壤HA。综上,在模拟培养腐殖化条件下,蒙脱石添加可促进白花苜蓿TOC的分解及其HLA的形成,从而加快白花苜蓿的腐殖化进程,但却使形成的HLA结构更加简单化,与真正的HA仍存在较大的差异,该研究结果为土壤HS的形成机理与起源探索提供了一定的科学借鉴。
白花苜蓿;模拟培养;腐殖化;腐殖物质;类胡敏酸
腐殖物质(Humic substances,HS)是土壤有机质(soil organic matter,SOM)的主体[1],执拗性的植物残体和微生物残体是HS的重要来源[2]。然而,HS形成的机制仍有待阐明,这种知识的缺乏阻碍了我们对土壤管理选项的优化或更新[2]。改变各种来源和动态将导致HS的组成、性质、过程和功能不同。因此,需要对HS的形成途径进行更加深入细致的研究[7]。研究者认为被子植物对HS的形成与转化起着至关重要的作用[3-4],其中白车轴草(L.)俗称白花苜蓿研究较多,具有很大的研究价值,因此本实验选用白花苜蓿为培养材料,以探究HS的形成。
有学者采用微生物纯培养方法来研究HS的形成[5],但由于未加黏土矿物催化,形成的类腐殖物质(Humic-like substances,HLS)与土壤HS相差很大[6]。因此,HS与矿物表面之间的复杂相互作用,以及微生物作为参与者的作用需要比过去更详细地阐明[7]。Birkel等[8]为证实黏土矿物催化作用,通过研究酚类(邻苯二酚,连苯三酚和2,6-二甲基戊酚)在蒙脱石颗粒表面上的反应,得出矿物结构中的铁是黏土催化活性的部分原因的结论。Filip等[9]指出,黏土矿物可缩短暗色物质形成的时间并增加碱提取腐殖酸类聚合物的数量。黏土矿物的催化作用主要通过间接影响酚类物质的形成而实现,但所形成的HS结构未见差异。Duarte等[10]认为矿物表面对土壤胡敏酸(Humic acid,HA)分子中非晶形亚甲基结构数量的提升具有显著抑制作用,即提高了HA的芳香度。然而对HS的形成条件仍有诸多不解,黏土矿物对腐殖化进程的影响仍需要被更加准确和详细的阐明。本研究分别将黏土矿物-蒙脱石和微生物-土壤浸提液作为控制条件,以探究蒙脱石处理对白花苜蓿腐殖化进程的影响,结果可为土壤HS的形成机理与起源探索提供了一定的科学借鉴。
1 材料与方法
1.1 供试材料
白花苜蓿:选取被子植物门,车轴草属的白车轴草,俗称白花苜蓿为培养材料。白花苜蓿于2017年4月7日采自长春市净月区吉林农业大学第三试验田。将采集得到的白花苜蓿进行清洗后风干,置于烘箱中90℃下高温杀酶20 min,65℃烘干2 d,至重量不再变化。经剪切、磨碎后过1 mm(20目)和0.3 mm(60目)筛子备用。含碳量为440.8 g·kg–1,C/N为26.85。
黏土矿物:选用蒙脱石,于2017年3月购自上海国药集团化学试剂有限公司,CAS NO.:1318-93-0。
供试土壤及土壤浸提液:供试土壤为草甸黑土,于2017年4月25日取自吉林农业大学玉米连作试验田(43°48′46″ N,125°23′28″ E)0~20 cm耕层,土壤含碳量为9.76 g kg–1,C/N为7.28。于取土当天按水土比1︰2.5提取土壤浸提液。其余土壤风干过1 mm和0.3 mm筛子备用。
1.2 实验设计
培养实验于2017年4月26日开始。处理A在250 ml塑料烧杯内分别加入45 g蒙脱石和15 g白花苜蓿(60目),混合均匀。接种10 mL土壤浸提液并用蒸馏水调节混合物含水量至田间最大持水量的80%左右,用保鲜膜封口,在保鲜膜上均匀设置5个直径为1 mm的通气孔,放置30℃培养箱培养。同时设置了无蒙脱石(AnM)和无土壤浸提液(AnI)处理,培养条件和方法均与A处理相同。分别于培养0 d、90 d、180 d和360 d取样分析,每个取样时间3个重复,每次取走整瓶。
1.3 分析方法
土壤腐殖质与培养产物的类腐殖质组分采用腐殖质组成修改法进行提取[11-12]。其中水溶性物质(Water soluble substance,WSS)含碳量采用TOC 分析仪(日本岛津 TOC-VCPH)测定;富里酸(Fulvic acid,FA)/类富里酸(Fulvic-like acid,FLA)和胡敏酸(HA)/类胡敏酸(Humic-like acid,HLA)采用重铬酸钾容量法;总有机碳(Total organic carbon,TOC)和胡敏素(Humin,Hu)/类胡敏素(Humin-like,HLu)采用德国碳硫分析仪(Elementar Analysensysteme GmbH)测定。用PQ=HA/(FA+HA)值表示材料的腐殖化程度。
土壤HA与培养产物提取物(HLA)采用国际腐殖质协会(International Humic Substances Society,IHSS)推荐的方法进行提取纯化[13]。HA和HLA的元素组成和红外光谱分别采用德国Elementar Vario EL Ⅲ型元素分析仪和美国AVATAR 360傅里叶变换红外光谱仪进行测定。
1.4 数据处理
文中数据采用 Excel 2010 与Origin 7.5 软件进行数据分析处理,用SPSS Statistics 20.0软件进行显著性差异分析,<0.05。
2 结 果
2.1 蒙脱石对白花苜蓿培养产物TOC的影响
如图1所示,与未添加蒙脱石(AnM)相比,添加蒙脱石(A)对白花苜蓿腐殖化进程的影响十分显著,促进了TOC的分解。培养90 d后,TOC的分解率为:A-t1(64.88%)>AnM-t1(22.58%);180 d后,总有机碳(TOC)进入“缓慢分解阶段”,A-t2(66.13%)>AnM-t2(27.72%);360 d后,A-t3(75.36%)>AnM-t3(37.34%),蒙脱石处理始终加速TOC的分解。与未加土壤浸提液(AnI)相比,添加土壤浸提液(A)使蒙脱石处理在培养初期促进TOC的分解,随着培养时间的增加,两类处理的TOC基本接近。培养90 d后,TOC的分解率为:A-t1(64.88%)>AnI-t1(47.75%);180 d后,TOC分解缓慢,A-t2(66.13%)>AnI-t2(54.64%);360 d后,两类处理TOC的减少量基本接近(A-t3 75.36%,AnI-t3 73.98%)。
2.2 蒙脱石对白花苜蓿培养产物中类腐殖质组分的影响及其PQ值与土壤的接近程度
从图2可知蒙脱石对类腐殖质组分相对含量的影响。蒙脱石促进TOC的分解主要是通过促进WSS和HLu的减少来实现的,但同时促进了HLA的形成和HLA与FLA的转化。
各处理类腐殖质组分的有机碳含量如表1所示。WSS的相对含碳量为:A-t0(13.22%)>AnM-t0(12.90%),AnM-t1(28.03%)>A-t1(14.43%),AnM-t2(15.50%)>A-t2(10.43%),AnM-t3(46.48%)>A-t3(7.581%),与未添加蒙脱石(AnM)相比,在模拟培养腐殖化过程中添加蒙脱石(A)使WSS的相对含碳量明显减少。从HLA和FLA的变化看,培养0 d时,A类处理的HLA相对含量明显低于AnM类,两类处理的FLA相对含量相近;90 d后,HLA的相对含碳量:A-t1(21.38%)>AnM-t1(4.002%),FLA的相对含碳量:A-t1(5.216%)> AnM-t1(4.419%),蒙脱石促进HLA和FLA的积累;180 d后,HLA为:A-t2(14.19%)>AnM-t2(2.539%),FLA为:A-t2(8.485%)>AnM-t2(6.765%),HLA的积累量减少,FLA逐渐增加;随着培养时间增加至360 d,HLA为:A-t3(21.38%)> AnM-t3(4.002%),FLA为:A-t1(5.216%)>AnM-t1(4.419%),蒙脱石进一步促进HLA的积累。 HLM在培养初期较容易被微生物分解利用,稳定性较低;随着培养时间的增加,A类处理和AnI类处理的HLu的相对含碳量小幅度增加,逐渐变稳定;AnM类处理的HLu则与之相反。

注:A为加入白花苜蓿、蒙脱石和土壤浸提液的处理, AnM和AnI分别为无蒙脱石和无土壤浸提液的处理,AnM-t1、A-t1、AnI-t1均代表培养90 d,AnM-t2、A-t2、AnI-t2均代表培养180 d,AnM-t3、A-t3、AnI-t3均代表培养360 d。有机碳残留率为某一时间段的TOC与培养0 d TOC的比,即有机碳残留率=TOCt/TOCt0。不同小写字母代表同一时间不同处理间类腐殖质组分差异显著(P<0.05)。下同。Note:A stands for treatment of addition of white alfalfa, montmorillonite and soil extract; AnM for treatment of addition of white alfalfa and soil extract; AnI for treatment of addition of white alfalfa and montmorillonite; and -t1, -t2 and -t3 for duration of the incubation for 90, 180 and 360 days, respectively. Organic carbon residual ratio is the ratio of TOC after a certain period of incubation to the initial TOC(i.e., organic carbon residual ratio = TOCt/TOCt0). Different lower-case letters represent significant differences in humus-like components between treatments after the same period of incubation(P <0.05).The same below.
蒙脱石对白花苜蓿培养产物PQ值的影响如图3所示。在培养0 d时,A类PQ值低于AnM类处理;90 d后的PQ值为:A-t1(80.83)>AnM-t1(48.97),添加蒙脱石使PQ值迅速增加;180 d后, 两类处理PQ值接近(A-t2 49.81,AnM-t2 49.36);360 d后,A-t3(62.84)>AnM-t3(26.19),蒙脱石再次使培养产物的腐殖化程度增加,与土壤的PQ值(60.02)十分接近。从AnI类和A类处理看,在蒙脱石条件下,添加土壤浸提液对白花苜蓿碱提取组分的影响较小,360 d后两类处理的PQ值均与土壤十分接近。
2.3 蒙脱石对白花苜蓿培养产物HLA元素组成的影响及HLA与土壤HA的接近程度
培养产物HLA的元素组成见表2,各处理的培养产物HLA中C、H均小于培养物,N和O大体均大于培养物,模拟腐殖化培养促进N和O元素的积累及C和H的消耗。三种不同处理随着培养时间的增加,H/C值逐渐减少,O/C则先增加后减少。通过对比AnM和A处理可知蒙脱石对白花苜蓿腐殖化进程的影响,培养90 d后,A处理的H/C由AnM处理的1.38增加至1.61;180 d后,由1.39变为1.53;360 d后,由1.36变为1.48。前期添加蒙脱石使A处理的H/C大幅度增加,HLA的结构简单化,而随着培养的进行,H/C的增加幅度逐渐减少,两者HLA的复杂化程度的差距缩小。对比AnI和A处理,培养90 d后,A处理的H/C由AnI处理的1.81下降为1.61;180 d后,由1.62变为1.53;360 d后,两者差距减小(AnI 1.50,A 1.48)。在蒙脱石条件下,前期添加土壤浸提液使A处理的H/C大幅度减小,促进HLA结构的复杂化;而随着培养的进行,两类处理的HLA的复杂程度基本接近。
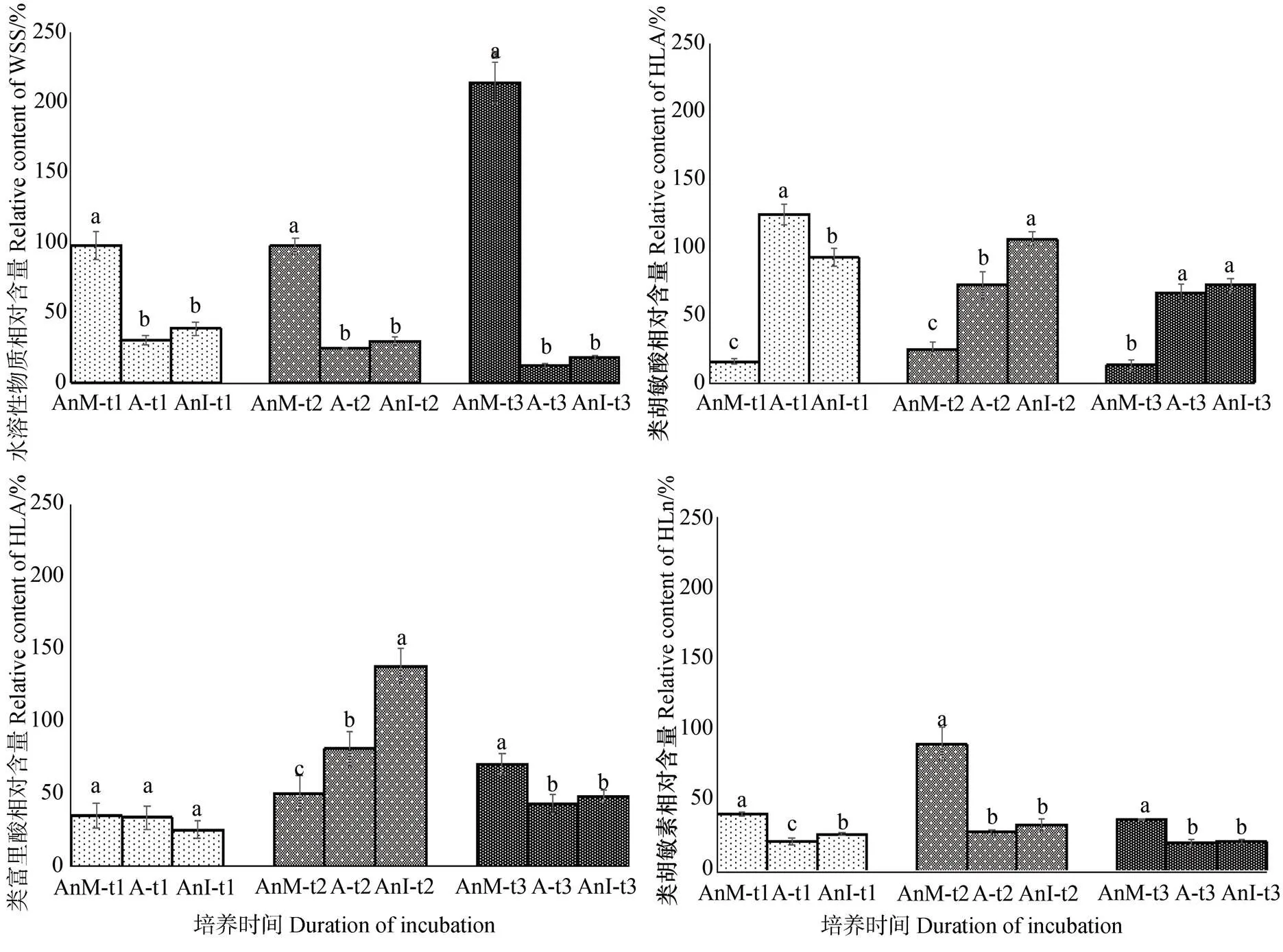
注:类腐殖质组分相对含量为某一时间段类腐殖质组分的含碳量与培养0 d时类腐殖质组分的比,即相对含量=Ct/Ct0。Note:Relative content of humus-like components is the ratio of the carbon content of the humus-like component after a certain period of incubation to the initial value of the humus-like components(i.e., the relative content = Ct/Ct0).

表1 蒙脱石添加对培养产物类腐殖质组分相对含量的影响
注:不同小写字母代表同一时间不同处理间类腐殖质组分差异显著(<0.05=。Note:the annotations are the same with Figure 1. Different lower-case letters represent significant differences in humus-like components between treatments, the same in duration of incubation(<0.05).
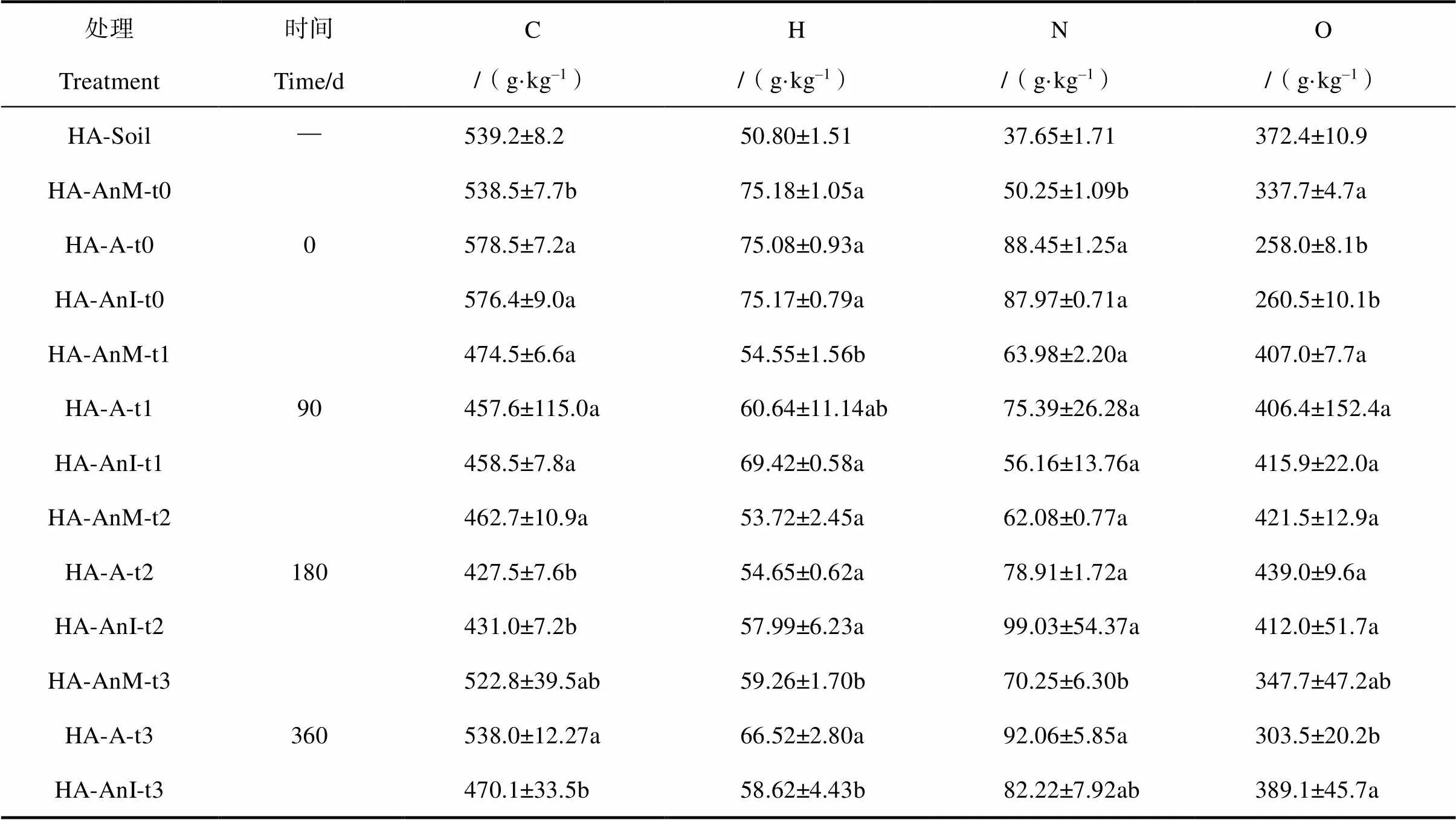
表2 蒙脱石添加对培养产物HLA的元素组成的影响

图3 蒙脱石添加对白花苜蓿培养产物PQ值的影响
由范卡图(图4)可知,3类处理最终均是向着接近土壤HA的O/C和H/C值的方向发展。360 d后HLA与土壤HA的接近程度,AnM>A>AnI。未添加蒙脱石的AnM处理与土壤HA最接近,添加土壤浸提液的A处理其次,添加蒙脱石使形成的HLA结构更加简单,与真正的HA仍存在较大的差异。
2.4 蒙脱石对白花苜蓿培养产物HLA红外光谱的影响及HLA与土壤HA的接近程度
不同处理的白花苜蓿HLA与土壤HA的红外光谱主要吸收峰相对强度见表3。各处理的HLA均显示2 920 cm–1处代表不对称脂族C-H伸缩振动的峰,2 850 cm–1处代表-CH2-对称脂族C-H伸缩振动的峰,1 640 cm–1处代表芳香C=C伸缩振动的吸收峰;培养360 d后,各处理显示出了与土壤HA相同的1 720 cm–1的酮、醛、酸中的羧基 C=O 伸缩振动峰。用2920/1640 比值代表材料脂肪族含量与芳香族含量的比值。
对比HA-AnM处理和HA-A处理的2920/1640比值:HA-A-t0(0.237)>HA-AnM-t0(0.186),HA-A-t1(0.184)>HA-AnM-t1(0.145),HA-A-t2(0.143)>HA-AnM-t2(0.138),HA-A-t3(0.136)> HA-AnM-t3(0.132),加入蒙脱石使生成的HLA脂肪族含量增加,结构简单化,随着培养时间的增加,两类处理HLA的芳构化程度差距缩小。而对比HA-AnI处理和HA-A处理,HA-AnI-t0(0.225)> HA-A-t0(0.186),HA-AnI-t1(0.243)> HA-A-t1(0.184),HA-AnI-t2(0.156)>HA-A-t2(0.143),HA-AnI-t3(0.142)>HA-A-t3(0.136),HA-AnI-t1的突然增加与矿质化有关,添加土壤浸提液使蒙脱石处理有助于HLA的芳构化,随着培养时间的进行,两类处理芳构化程度的差距逐渐缩小。HLA的红外光谱规律与元素组成规律相对应。

图4 白花苜蓿培养产物的HLA与土壤HA的范卡图
3 讨 论
3.1 蒙脱石对培养产物TOC和类腐殖质组分的影响
蒙脱石处理和土壤浸提液处理加快了白花苜蓿TOC的分解。由于土壤浸提液中含有与土壤环境相似的微生物群体,对有机质维持和周转的贡献显著[14];而蒙脱石不仅可通过影响微生物群落栖息微环境来影响微生物的代谢[15-17],同时蒙脱石还具有催化作用[18],因此两种处理共同促进了白花苜蓿TOC的分解。蒙脱石促进TOC的分解主要通过促进WSS减少来实现,但同时促进了HLA和FLA的形成与转化。从腐殖质组成的稳定性来看,WSS不稳定,易分解[19],蒙脱石的催化作用使WSS迅速减少。而PQ值的变化主要与前期蒙脱石对HLA的吸附[20]、中期HLA的积累与后期HLA和FLA的相互转化有关,添加蒙脱石使PQ值增加,最终接近土壤HA,促进白花苜蓿的腐殖化进程。Loya等[19]指出腐殖化使水溶性(WS)和酸溶性(AS)组分部分的14C活性降低,但酸不溶组分(AIS)中的14C活性增加,微生物代谢物和降解的化合物结合到SOM的顽固化合物中,本实验结果进一步证实了Loya等的研究。

表3 蒙脱石添加对培养产物HLA的FTIR光谱主要吸收峰相对强度的影响
在白花苜蓿模拟培养腐殖化过程中,蒙脱石加快TOC的分解,促进HLA的积累,结果与Gleixner[21]的研究一致。Gleixner指出当大多数植物有机碳通过土壤微生物时,除一部分用于细胞能量需求外,其余部分用于生物量的积累,微生物生物质主要由细胞碎片形成土壤有机质(SOM)。本实验结果证实了前人的研究,进一步指出蒙脱石促进HLA的生成和FLA与HLA的相互转化,以及对WSS、PQ值的影响,使对腐殖化进程的认识更具体化。
3.2 蒙脱石对培养产物HLA结构特征的影响
研究表明:蒙脱石使HLA的芳构化程度降低,结构趋于简单化。添加土壤浸提液使蒙脱石处理的HLA结构复杂化。培养中期AnI处理的HLA缩合度减少与未添加土壤浸提液前期腐殖化速度较慢有关,培养仍处于矿质化阶段[22],白花苜蓿被微生物分解成简单的有机化合物,使缩合度减少。一些学者认为黏土矿物可提高HA的芳香度,使HA的结构更复杂。Duarte等[10]研究表明,矿物表面对土壤HA分子中非晶形亚甲基结构数量的提升具有显著抑制作用,即提高了HA的芳香度。Filip等[9]表明黏土矿物的催化作用主要通过间接影响酚类物质的形成而实现,但所形成的HS结构未见差异。Fukuchi等[23]研究了邻苯二酚、甘氨酸与葡萄糖在天然沸石催化作用下的缩聚反应,表明沸石有利于类HA中氮含量及分子量的提高,对醌、酮等羰基碳含量亦有提升作用。
本实验的研究结果与前人不同,在模拟培养腐殖化360 d内,添加蒙脱石使HLA的结构简单化,而添加土壤浸提液则可使蒙脱石处理的HLA的芳香度增加。因为在HS形成过程中,生物大分子(例如,蛋白质、多糖和木质素)最初分解为低分子量化合物(例如,氨基酸、糖和酚)。低分子量化合物随后通过缩聚反应进行重组,形成HS。木质素蛋白理论[24]和美拉德反应[25]已被提出作为低分子量化合物重组并最终形成HS的反应途径,这种反应以亲核反应的形式进行。通常,在酸性催化剂存在下,亲核反应得到增强[26]。众所周知,黏土矿物以固体酸的形式广泛分布在土壤环境中,所以蒙脱石可加速缩聚反应进行。然而蒙脱石的加入破坏了原本结构相对稳定的HLA,促进低分子量化合物(如氨基酸,糖和酚)之间的缩聚反应,在短时间的培养期内,新缩聚形成的HLA的结构仍然较简单。而在蒙脱石条件下,在同等破坏HLA结构的基础上加入土壤浸提液,更加速了缩聚反应的进行,所以添加土壤浸提液使蒙脱石处理的HLA结构更加复杂。但是随着培养时间的持续增加,蒙脱石的加入是否会使HLA结构复杂化,还需要进行进一步研究。
在白花苜蓿培养产物HLA与土壤HA对比的范卡图中,3类处理最终均是向着接近土壤HA的O/C和H/C值的方向发展,未添加蒙脱石(AnM)的HLA与真正的HA最接近,说明蒙脱石不能促进形成的HLA更接近土壤HA。Ahn等[27]研究表明:自然界中,土壤矿物通过氧化偶联反应催化酚向腐殖物质聚合物转化,采用三元体系法通过长绒毛栓菌所分泌的虫漆酶、水钠锰矿(-MnO2)以及邻苯二酚之间的相互作用来模拟土壤过程,表明酶-土壤矿物-有机质间的相互作用对腐殖质形成过程具有重要意义。本研究证实了蒙脱石的贡献,可加快白花苜蓿的腐殖化进程,但蒙脱石使生成的HLA更加年轻化,与真正的HA仍存在较大的差异。
4 结 论
在模拟培养白花苜蓿腐殖化过程中,蒙脱石促进TOC的分解和HLA的生成,且使HLA的缩合度降低;因此蒙脱石未能像文献报道的那样使HLA的芳构化程度增加,结构复杂化,相反却使其结构更加简单。而土壤浸提液添加,则使蒙脱石处理在促进HLA积累的同时,使HLA的缩合度增加,结构更加复杂化。在白花苜蓿培养产物HLA与土壤HA的范卡图中,3类处理最终均是向着接近土壤HA的方向发展,其中培养后未添加蒙脱石(AnM)处理中HLA与真正的HA最接近,说明蒙脱石不能促进HLA更接近土壤HA。综上,在模拟培养白花苜蓿腐殖化过程中,蒙脱石添加可加快白花苜蓿的腐殖化进程,但却使形成的HLA结构更加简单化,与真正的HA仍存在较大的差异。本研究结果探究了30℃培养条件下,蒙脱石对白花苜蓿模拟培养腐殖化的影响。今后还需要更多的研究来进一步理解黏土矿物对腐殖化过程的具体作用机制。
[ 1 ] Rodríguez F J, Schlenger P, García-Valverde M. A comprehensive structural evaluation of humic substances using several fluorescence techniques before and after ozonation. Part I:Structural characterization of humic substances[J]. Science of the Total Environment, 2014, 476/477:718—730.
[ 2 ] Garcia C, Nannipieri P, Hernandez T. The future of soil carbon:Its conservation and formation[M]. London, United Kingdom:Academic Press, 2018.Garcia C, Nannipieri P, Hernandez T. The future of soil carbon//The Future of Soil Carbon. Amsterdam:Elsevier, 2018:239—267.
[ 3 ] Stewart C E. Evaluation of angiosperm and fern contributions to soil organic matter using two methods of pyrolysis-gas chromatography-mass spectrometry[J]. Plant and Soil, 2012, 351(1/2):31—46.
[ 4 ] Ponge J F. Plant-soil feedbacks mediated by humus forms:A review[J]. Soil Biology & Biochemistry, 2013, 57:1048—1060.
[ 5 ] Li Y. Microorganisms use organic materials such as sugars and cyanobacteria to participate in the formation of humus[D]. Changchun:Jilin Agricultural University, 2016. [李艳. 微生物利用糖类和蓝藻等有机物料参与形成腐殖质的研究[D]. 长春:吉林农业大学, 2016.]
[ 6 ] Dou S, Li Y, Guan S, et al. The structural distinctiveness of humic substances and its formation mechanism in simulated incubation[J]. Acta Pedologica Sinica, 2016, 53(4):821—831. [窦森, 李艳, 关松, 等. 腐殖物质特异性及其产生机制[J]. 土壤学报, 2016, 53(4):821—831.]
[ 7 ] Kallenbach C M, Frey S D, Stuart Grandy A. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls[J]. Nature Communications, 2016, 7:13630. https://doi.org/10. 1038/ncomms13630.
[ 8 ] Birkel U, Gerold G, Niemeyer J. Abiotic reactions of organics on clay mineral surfaces[J]. Developments in Soil Science, 2002, 28:437—447.
[ 9 ] Filip Z, Haider K, Martin J P. Influence of clay minerals on growth and metabolic activity ofand[J]. Soil Biology & Biochemistry, 1972, 4(2):135—145.
[ 10 ] Duarte R M B O, Fernández-Getino A P, Duarte A C. Humic acids as proxies for assessing different Mediterranean forest soils signatures using solid-state CPMAS13C NMR spectroscopy[J]. Chemosphere, 2013, 91(11):1556—1565.
[ 11 ] Dou S. Soil organic matter[M]. Beijing:Science Press, 2010. [窦森. 土壤有机质[M]. 北京:科学出版社, 2010.]
[ 12 ] Dong S S, Dou S, Shao M J, et al. Effect of corn stover deep incorporation with different years on composition of soil humus and structural characteristics of humic acid in black soil[J]. Acta Pedologica Sinica, 2017, 54(1):150—159. [董珊珊, 窦森, 邵满娇, 等. 秸秆深还不同年限对黑土腐殖质组成和胡敏酸结构特征的影响[J]. 土壤学报, 2017, 54(1):150—159.]
[ 13 ] Kuwatsuka S, Watanabe A, Itoh K, et al. Comparison of two methods of preparation of humic and fulvic acids, IHSS method and NAGOYA method[J]. Soil Science and Plant Nutrition, 1992, 38(1):23—30.
[ 14 ] Malik A A, Roth V N, Hébert M, et al. Linking molecular size, composition and carbon turnover of extractable soil microbial compounds[J]. Soil Biology & Biochemistry, 2016, 100:66—73.
[ 15 ] Vogel C, Babin D, Pronk G J, et al. Establishment of macro-aggregates and organic matter turnover by microbial communities in long-term incubated artificial soils[J]. Soil Biology & Biochemistry, 2014, 79:57—67.
[ 16 ] Singh M, Sarkar B, Biswas B, et al. Relationship between soil clay mineralogy and carbon protection capacity as influenced by temperature and moisture[J]. Soil Biology & Biochemistry, 2017, 109:95—106.
[ 17 ] Babin D, Ding G C, Pronk G J, et al. Metal oxides, clay minerals and charcoal determine the composition of microbial communities in matured artificial soils and their response to phenanthrene[J]. FEMS Microbiology Ecology, 2013, 86(1):3—14.
[ 18 ] Ertem G, Gan Z H. Role of preparation method on the extent of montmorillonite catalysis for oligomer formation[J]. Applied Clay Science, 2014, 101:90—93.
[ 19 ] Loya W M, Johnson L C, Nadelhoffer K J. Seasonal dynamics of leaf- and root-derived C in arctic tundra mesocosms[J]. Soil Biology & Biochemistry, 2004, 36(4):655—666.
[ 20 ] Chen H F, Koopal L K, Xiong J, et al. Mechanisms of soil humic acid adsorption onto montmorillonite and kaolinite[J]. Journal of Colloid and Interface Science, 2017, 504:457—467.
[ 21 ] Gleixner G. Soil organic matter dynamics:A biological perspective derived from the use of compound-specific isotopes studies[J]. Ecological Research, 2013, 28(5):683—695.
[ 22 ] Kumar M, Kundu D K, Ghorai A K, et al. Carbon and nitrogen mineralization kinetics as influenced by diversified cropping systems and residue incorporation in Inceptisols of eastern Indo-Gangetic Plain[J]. Soil and Tillage Research, 2018, 178:108—117.
[ 23 ] Fukuchi S, Miura A, Okabe R, et al. Spectroscopic investigations of humic-like acids formed via polycondensation reactions between glycine, catechol and glucose in the presence of natural zeolites[J]. Journal of Molecular Structure, 2010, 982(1/2/3):181—186.
[ 24 ] Tan K H. Humic matter in soil and the environment:Principles and controversies[M]. Boca Raton,United States:CRC Press, 2014.
[ 25 ] Haffenden L J W, Yaylayan V A. Mechanism of formation of redox-active hydroxylated benzenes and pyrazine in13C-labeled glycine/D-glucose model systems[J]. Journal of Agricultural and Food Chemistry, 2005, 53(25):9742—9746.
[ 26 ] Varma R S. Clay and clay-supported reagents in organic synthesis[J]. Tetrahedron, 2002, 58(7):1235—1255.
[ 27 ] Ahn M Y, Martínez C E, Archibald D D, et al. Transformation of catechol in the presence of a laccase and birnessite[J]. Soil Biology & Biochemistry, 2006, 38(5):1015—1020.
Does Amendment of Montmorillonite Promote Humification of White Alfalfa?
XU Yang, DOU Sen†,ZHANG Yifeng, TIAN Yuxin, DUAN Hongmei, BAI Yue
(College of Resource and Environmental Science, Jilin Agricultural University, Changchun 130118, China)
Humic substances (HS), the main component of soil organic matter, are kind of macromolecular compounds, specific and multi-phased in property, formed during humification. However, in the past two decades, doubts have been arising about rationality of the disassociation process of HS. Currently, the research has shifted its focus from on chemical properties of HS to on identification of processes and mechanisms related to HS renewal and stabilization. Plant residues and microbial biomass are the main parent materials for the formation of humic substances, meanwhile, clay minerals, as an important part of the soil, also play an important role in the process. However, still little is known about any specific effects of clay minerals on formation of HS. Some scholars have used microbial pure culture methods to study formation of HS, but because of the absence of clay minerals, the formation of HS studied does not represent that in soil. Therefore, more efforts should be done to further elucidate in detail the complex interactions between HS and the surface of minerals, as well as the role of soil microbes as a participant. In order to explore effects of montmorillonite on humification process, white alfalfa, an angiosperm, was used in an incubation experiment with montmorillonite and microbe-soil extract as control conditions.In this experiment, there were three treatments, i.e. Treatment A, white alfalfa with montmorillonite and soil extract added; Treatment AnM, white alfalfa with soil extract added only; and Treatment AnI, only white alfalfa. Effects of the treatments and duration of the incuabtion on composition of humus-like substances and structure of humic-like acids (HLA) formed as incubation product of white alfalfa were studied. Water-soluble substances (WSS), fulvic-like acid (FLA), humic-like acid (HLA) and humin-like (HLu) were extracted with the modified humus composition method. HLA samples were extracted with the method recommended by the International Humic Substances Society (IHSS) for analysis of structure of HA with the elemental composition- infrared spectroscopy.Results show that the amendment of montmorillonite promoted decomposition of TOC and accumulation of simple structured HLA. After 90 days of incubation, H/C in Treatment A increased from 1.38 to 1.61, after 180 days from 1.39 to 1.53, and after 360 days from 1.36 to 1.48. The sharp increase in H/C in the early stage in Treatment A made the structure of HLA simple. However, with the incubation going on, the increase in H/C gradually dulled in trend. Its ratio of 2920/1640 in the infrared spectrum tended to be in consistence with that in elemental composition. At the end of the incubation, HLA, regardless of treatments, developed eventually quite close to the O/C and H/C of soil HA in value. Among the treatments, the HLA in Treatment (AnM) was the closest to real soil HA in complexity, indicating that montmorillonite cannot help HLA turn into soil HA.Overall, montmorillonite amendment may promote decomposition of TOC and formation of HLA, and accelerate humification process of white alfalfa; however, it does not enhance the aromatization of HLA in degree and the structure of HLA in complexity as speculated or reported in the literature. Instead, it simplifies the structure of HLA, which is still quite different from real soil HA.
White alfalfa; Simulated incubation; Humification; Humic substances; Humic acid
S151.9
A
10.11766/trxb201903060110
徐杨,窦森,张一枫,田宇欣,段宏美,白月. 添加蒙脱石是否促进白花苜蓿的腐殖化进程?[J]. 土壤学报,2020,57(5):1230–1239.
XU Yang, DOU Sen,ZHANG Yifeng, TIAN Yuxin, DUAN Hongmei, BAI Yue. Does Amendment of Montmorillonite Promote Humification of White Alfalfa?[J]. Acta Pedologica Sinica, 2020,57(5):1230–1239.
* 国家自然科学基金项目(41571231)资助 Supported by the National Natural Science Foundation of China(No. 41571231)
,E-mail:dousen1959@126.com
徐 杨(1994—),女,山东青岛人,硕士研究生,从事土壤环境与生物化学研究。E-mail:857472355@qq.com
2019–03–06;
2019–04–21;
优先数字出版日期(www.cnki.net):2019–11–01
(责任编辑:卢 萍)
