Nutrient Enrichment Regulates the Growth and Physiological Responses of Saccharina japonica to Ocean Acidification
2020-09-28CHUYaoyaoLIUYanLIJingyuWANGQiaohanandGONGQingli
CHU Yaoyao, LIU Yan, LI Jingyu, WANG Qiaohan, and GONG Qingli
Nutrient Enrichment Regulates the Growth and Physiological Responses ofto Ocean Acidification
CHU Yaoyao, LIU Yan*, LI Jingyu, WANG Qiaohan, and GONG Qingli*
,,266003,
Environmental changes, such as ocean acidification and eutrophication, have created threats to kelp mariculture. In this study, the growth, photosynthesis, respiration and nutrient composition ofwere evaluated at different levels of pCO2(400 and 800µLL−1) and nutrients (nutrient-enriched and non-enriched seawater). Elevated pCO2decreased the relative growth rate (RGR), net photosynthetic rate and contents of tissue carbon and tissue nitrogenunder non-enriched nutrient conditions, but it had no significant effect on these parameters under nutrient-enriched conditions. The dark respiration rate was positively affected by elevated pCO2regardless of the nutrient conditions. However, the C:N was unaffected by elevated pCO2at both nutrient levels. These results implied that ocean acidificationcould reduce the production and nutrient contents in the tissuesof, which was associated with nutrient conditions.
eutrophication; growth; nutrient composition; ocean acidification;
1 Introduction
Because of human activities, the atmospheric CO2has continuously risen to a recent level of 400µLL−1(IPCC, 2014) and is predicted to reach 530–1000µLL−1by the end of this century (IPCC, 2013). When excessive CO2is absorbed by the ocean, the seawater pH decreases. This process is called ocean acidification (OA). OA not only alters the fundamental chemistry of the ocean but alsoimposes great effects on marine organisms (Johnson., 2017; Huang., 2018; Jia., 2019). Previous studies have shown that OA could influence the growth of many kind of macroalgae (Schmid, 1996; Mercado., 1999; Olischlaeger.,2012), such as(Zou, 2015),(Xu., 2017),(Chen., 2019),(Chen., 2019) and(Kim., 2016). Additionally, OA could exert various effects on the phy- siological response of different macroalgae. For instance, in terms of photosynthesis and biochemical compositions, a high CO2concentration had a positive effect on(Oh., 2015) and(Celis-Plá., 2017), and a negative effect on(Gutow., 2014) and(Kim., 2016).
In addition to OA, eutrophication is another issue in the coastal seawater environment. Due to anthropogenic ac-tivities, a large amount of wastewater inputs are generated in coastal areas, which affect the coastal ecosystem (Geertz- Hansen.,1993; Smith., 2003). Nutrients are a main factor that influences the growth and physiology of macroalgae. Some previous studies have reported that the photosynthesis and biochemical content of macroalgae are likely to increase under high nutrient availability (Mizuta.,2001; Agatsuma.,2014; Endo.,2017;Kangand Chung, 2018). The maturation ofgametophytes was enhanced at higher nutrient concentrations (Mizuta, 2001), and an increased synthesis of biochemical compositions under a high nutrient availabi- lity was observed in(Boderskov., 2016).
Large kelps constitute an important part of the lower intertidal and subtidal zones and provide a nursery ground and habitat for a variety of marine animals (Graham, 2004; Agatsuma., 2014;Endo., 2017). As an important population of seaweed, kelps also act as ecosystem engineers and carbon sinks in coastal areas (Gao., 1999), which shows the great potential for CO2bioremediation (Gao and McKinley, 1994). In addition to their im- portant ecological significance, kelps are also widely used as food and raw industrial materials (Endo., 2017; Gao., 2017; Xu., 2019). Given their significant commercial values and ecological effects, many studies have been conducted to investigate the interactive effect of OA and eutrophication on them, and the results suggested that the combination of OA and nutrient enrichment could produce synergism or a neutral effect on the growth and physiology of kelp (Russell., 2009; Falkenberg., 2013). For instance, the turf algal percent cover multiplied under higher CO2and nutrient level con- ditions (Russell., 2009). In contrast, the growth and C:N ratio ofwere influenced by nutrients, but CO2and the combination of the two factors did not show significant effects on these parameters (Falkenberg., 2013).
The canopy-forming kelpis an im- portant commercial alga and is widely cultivated in China, Japan and Korea (Selivanova.,2007; Liu., 2009;Hwang., 2018). Some studies have investigated the responses of their early stage and sporophyte to OA (Xu., 2015, 2019). The results of these studies showed that the meiospore germination, fecundity and reproductive success were reduced (Xu., 2015), and the spo- rophyte growth ofwas increased by elevated CO2concentration (Xu., 2019). However, these stud- ies examined the effects of OA individually or the interactions of OA and other environmental factors. Few stud- ies assessed the combined effects of nutrient and OA on the growth and nutrient composition of.
Therefore, in the current study, we investigated the com- bined effects of elevated CO2and NO3−on the growth, photosynthesis, respiration, and nutrient composition of. According to previous studies, we hypothesized that an elevated CO2level would suppress the growth and nutrient composition of this kelp, and a high NO3−level would affect its physiological responses to CO2. The results of this study are expected to provide valuable information on how the future oceanic environment will influence the cultivation and production ofin China and assess the potential effect of this kelp as a carbon sink under the future oceanic conditions.
2 Materials and Methods
2.1 Algal Collection and Maintenance
Adult sporophytes of(approximately 110cm in average length,=20) were collected from the culti- vated populations in Rongcheng, Shandong, China (36˚07΄N,120˚19΄E) in January 2019. The samples were kept in 7℃cool seawater and taken to the laboratory within 5h. Heal- thy sporophytes were selected and rinsed with sterilized seawater to remove any sediments and epiphytic organisms. More than 60 discs (1.4cm in diameter) were cut from the meristem using a cork borer for the subsequent experiments. They were stock-cultured in a 7-L plastic tank containing 6L of filtered seawater, which was acquired from the coastal area of Taipingjiao, Qingdao, with a salinity of approximately30. The light intensity was maintained at 90μmol photonsm−2s−1with a 12h:12h light/ dark cycle, and the temperature was controlled at 7℃, which was close to the seawater temperature at the sampling site, for 3d to recover the cut wounds.
2.2 Culture Experiment and Growth
The culture experiment used a two-way factorial design (2×2 treatments) consisting of two pCO2levels (ambient: 400µLL−1; elevated: 800µLL−1) and two nutrient levels (non-enriched natural seawater and nutrient-enriched sea- water). The cultures were conducted for 6d, and three replicates were prepared for each treatment. During the experiment, alight/dark photoperiod of 12h:12h and a light intensity of 90μmol photonsm−2s−1were held constantly. This experiment used 12 side-arm flasks with each flask containing 500mL of natural seawater or 50% Pro- vasoli’s ES Iodine medium (PESI)-enriched seawater (Tate- waki, 1966). In the 50% PESI-enriched seawater treatment, the culture medium contained either 438μmolL−1NO3−and 28μmolL−1H2PO4−, or 28μmolL−1NO3−and 2μmolL−1H2PO4−in the natural seawater. The elevated nutrient level was based on studies referring to eutrophication (Wu., 2015; Ménesguen., 2018), and nu- trient limitation did not occur at the applied nutrient level during the experiment (based on a preliminary experiment). Four discs were introduced into each flask, which was then gently aerated. The culture medium was regularly renewed every 3d.
For the experimental treatment, two pCO2levels were maintained in two CO2incubators, respectively, 400µLL−1(ambient air) and 800µLL−1(elevated pCO2). The CO2levels were automatically manipulated in two incubators (GXZ-380C-C02, Jiangnan Instruments Factory, Ningbo, China) by regulating the flow of the ambient air and pure CO2gas. Autoclaved natural seawater with the current pCO2level (approximately 400µLL−1) was used as the control. A pH meter (Orion STAR A211; Thermo Scientific) was used to measure the pH value of the medium in each flask. The total alkalinity (TA) was measured using the automatic alkalinity titrator by Gran acidimetric titration (848MPT, Titrino) on 50-mL samples filtered through cellulose acetate membranes (0.22μm). The seawater car- bonate chemistry parameters were estimated from the pH and TA values, salinity, nutrients, temperature, the equilibrium constants K1 and K2 for carbonic acid dissociation (Roy., 1993), and KB for boric acid (Dickson, 1990) using the CO2SYS software programme (Lewis and Wallace, 1998).
After 6 days of culture, the fresh weights of the discs were calculated after being softly blotted with tissue paper. The relative growth rate (RGR) of each culture was calculated using the following formula (Gao., 2017):
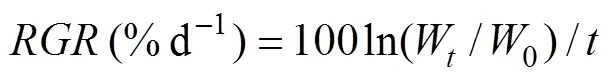
where0andWrepresent the initial and final fresh weights of the 4 discs, respectively, andis the number of days.
2.3 Photosynthesis and Respiration Measurements
After the culture experiment, the net photosynthetic rate (n) and the dark respiration rate (d) of the discs were determined using a FireStingO2II oxygen meter (FirestingO2, Pyro Science). After measuring the fresh weight, four discs were transferred to the oxygen electrode cuvette with 330mL of medium from the culture flask. Then the medium was magnetically stirred during the measure- ment to ensure the even diffusion of oxygen. The tem- perature and light conditions were the same as the mentioned culture experiment above. The value ofnwas ob- tained by increasing the oxygen content in the medium and the value ofdwas obtained by decreasing the oxygen content. Prior to the measurements, the samples were acclimated to the conditions in the cuvette for 5min. The oxygen concentration in the medium was recorded every 1min for 10min. Thenanddvalues were normalized to µmol O2g−1FW h−1.
2.4 Tissue Carbon, Tissue Nitrogen and C:N
For the detection of the tissue carbon (TC) and tissue nitrogen (TN) contents, the samples were dried at 80℃ until the dry weight was constant. Before the further pro- cessing, the dried samples were ground to powder with a mortar and 2–3mg of the powder was selected. The TC and TN contents were analyzed with an elemental analyzer (Vario EL III, Elementar, Germany). Data were represented in percentage of the dry weight. The C:N ratio was expressed as a molar ratio.
2.5 Statistical Analysis
All data in the present study are reported as the mean±SD (=3). Prior to the analysis, tests for the normal dis-tribution (Shapiro-Wilk,>0.05) and homogeneity (Le- vene’s test,>0.05) of variance were conducted. A two- way analysis of variance (ANOVA) was used to test the effects of the nutrient and pCO2levels on the, net photosynthetic rate, dark respiration rate, and the contents of TC, TN and C:N. A Tukey’s HSD test was conducted to determine the significance level of the factors (<0.05).Different letters indicated the significant differences (<0.05) among the different experimental treatments. All statistical analyses were performed using SPSS 22.0 software.
3 Results
3.1 Seawater Carbonate Chemistry
The effects of pCO2and the nutrient level on the seawater carbonate parameters were detected (Table 1). The two-way ANOVA analysis (=0.05) showed that pCO2had a significant effect on all parameters except for TA, while the nutrient level did not influenceany parameter. Elevated pCO2reduced the pH value by 0.3at both the enriched and non-enriched nutrient treatments and CO32−by 48% (non-enriched) and 46% (enriched), but it enhanced the dissolved inorganic carbon (DIC) by 8% (non-enriched) and 7% (enriched), CO2by 147% (non-enrich- ed) and 130% (enriched), and HCO3−by 13% (non-enriched) and 12% (enriched).
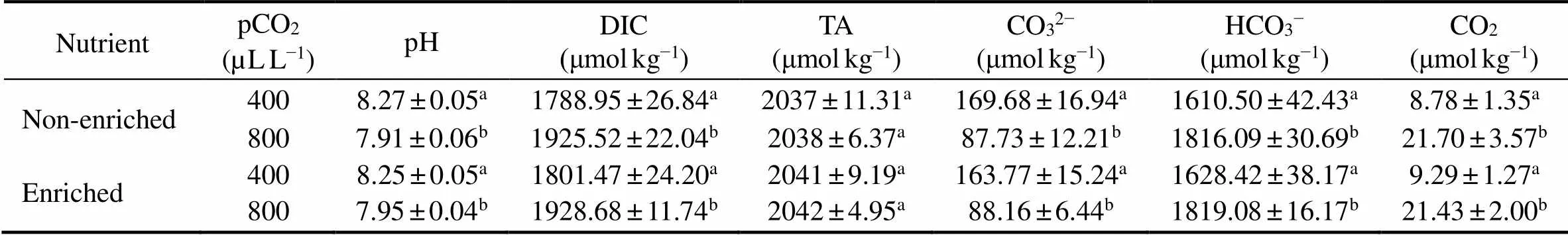
Table 1 The parameters of seawater carbonate system in different treatments
Notes: DIC, dissolved inorganic carbon; TA, total alkalinity. Data are presented as the mean±SD (=3). Different letters indicate significant differences (<0.05) among the different experimental treatments. The units for TA and carbonate chemistry parameters are μmolkg−1. The different letters indicate significant differences (<0.05) between different experimental treatments.
3.2 Growth
Thevalues were significantly affected by pCO2((1,10)=14.498,=0.005) and nutrients ((1,10)=44.779,<0.001), respectively (Fig.1). A significant interaction between pCO2and nutrient was not detected ((1,10)=2.097,=0.186). Under the non-enriched condition, thevalue was significantly higher at 400µLL−1than at 800µLL−1. However, in the nutrient-enriched condition, thevalues had no significant difference between the two pCO2levels. For both pCO2levels, thevalues were higher at nutrient-enriched condition than at the non- enriched condition. Theshowed a maximum of 13.164%d−1at 400µLL−1and the nutrient-enriched conditions.
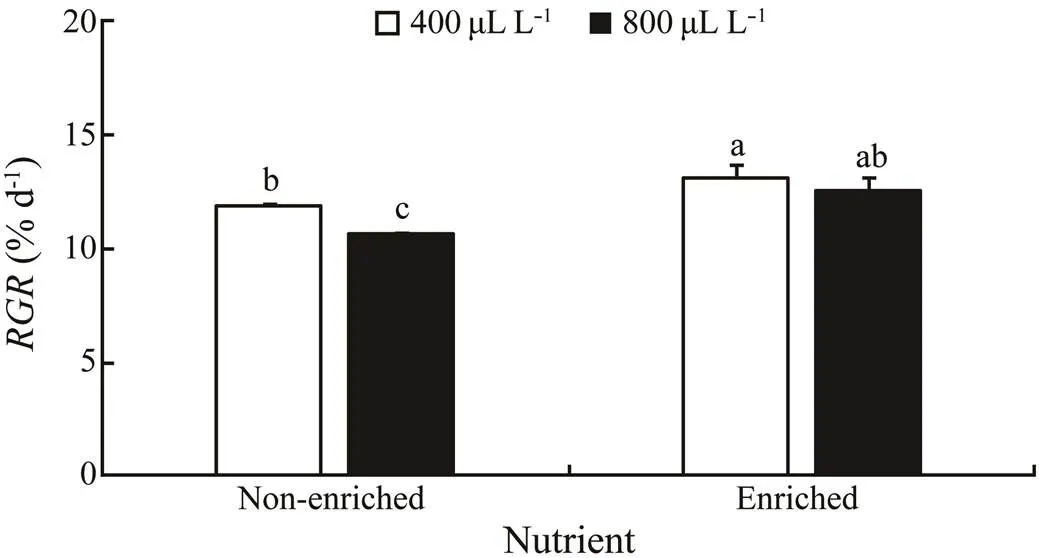
Fig.1 The relative growth rate (RGR) of S. japonica cultured for 6d under two pCO2 levels and two nutrient le- vels. Data represent the mean±SD (n=3). Different letters indicate significant differences (P<0.05) among different experimental treatments.
3.3 Photosynthesis and Respiration
Thenvalues were not significantly affected by pCO2((1,10)=2.353,=0.164) and nutrient ((1,10)=1.874,=0.208), respectively (Fig.2A). However, there was a significant combined effect between pCO2and nutrient ((1,10)=15.482,=0.004). At the non-enriched condition, thenvalues were higher at 400µLL−1than at 800µLL−1. However, in the enriched-nutrient treatment, thenvalues did not significantly differ between the two pCO2levels. Additionally, at the 400µLL−1, thenvalues did not show difference between two nutrient levels. However, at 800 µLL−1, thenvalue in the nutrient-enriched treatment washigher than that in the non-enriched treatment. Thenvalues showed a minimum of 0.493µmolO2g−1FWh−1at 800µLL−1and the non-enriched conditions.
Thedvalues were significantly affected by pCO2((1,10)=19.645,=0.002) (Fig.2B). There was no significant effect of nutrient ((1,10)=0.028,=0.872) on thedvalues. A significant interaction between pCO2and nutrient was not detected ((1,10)=1.569,=0.246). For both nutrient levels, thedvalue at 800µLL−1was higher than that at 400 µLL−1. At both pCO2conditions, thedvalues did not show a significant difference between two nutrient levels. Thedshowed a maximum of 0.248µmolO2g−1FWh−1at 800µLL−1under the enriched-nutrient conditions.
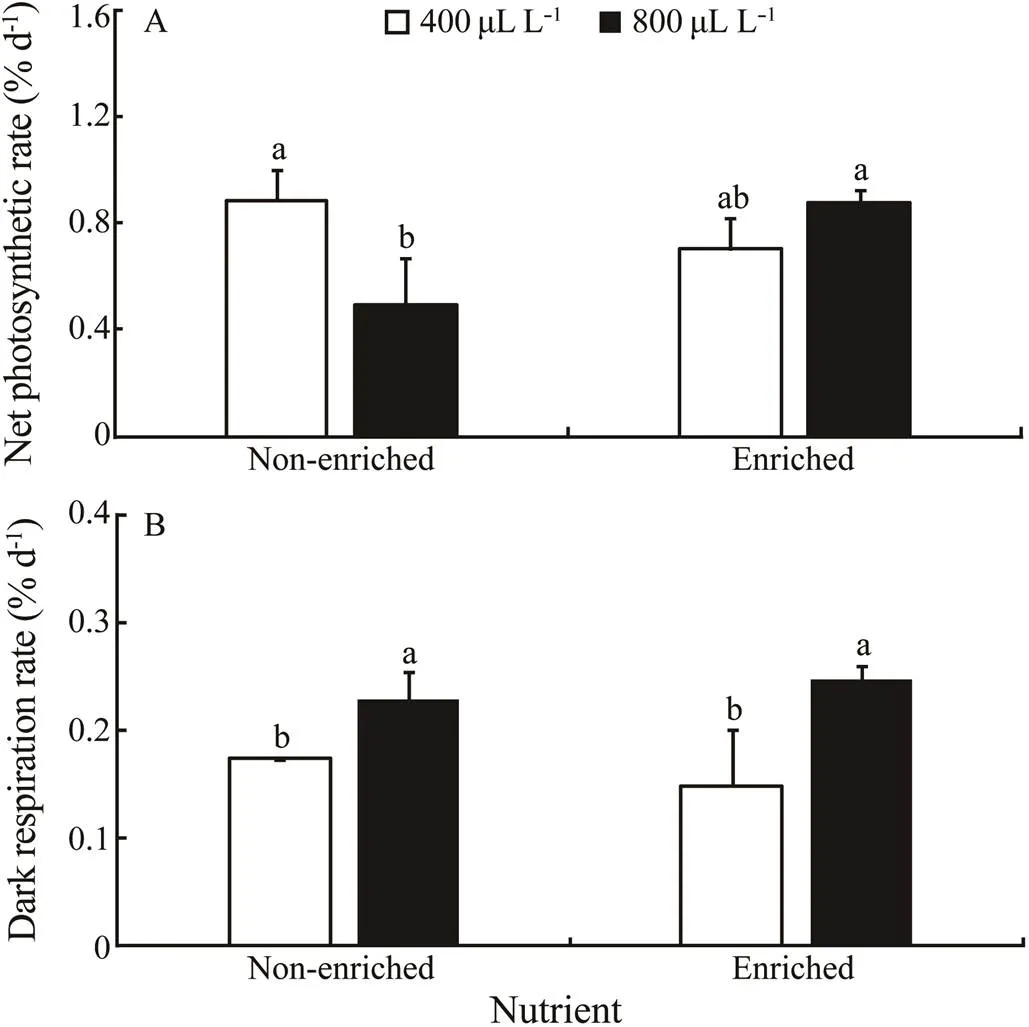
Fig.2 The net photosynthetic rate (A) and dark respiration rate (B) of S. japonica cultured for 6d under two pCO2 levels and two nutrient levels. Data represent the mean±SD (n=3). Different letters indicate significant differences (P<0.05) among different experimental treatments.
3.4 TC and TN Contents and C:N Ratio
Both the contents of TC ((1,10)=7.884,=0.023) and TN ((1,10)=26.475,=0.001) were significantly affected by nutrient. pCO2significantly affected the contents of TC ((1,10)=13.824,=0.006), but it had no significant effect on the contents of TN ((1,10)=3.973,=0.081) (Figs.3A and B). Additionally, pCO2and nutrient had an interactive effect on the contents of TC ((1,10)=31.752,<0.001) and TN ((1,10)=18.026,=0.003). In the non- enriched nutrient condition, the contents of both TC and TN contents at 400µLL−1were significantly higher than those at 800µLL−1, but showed no significant difference in the nutrient-enriched condition. Similarly, at 400µLL−1, the TC and TN contents did not show a significantly difference in either nutrient level. However, at 800µLL−1, the TC and TN contents were higher at the nutrient-en-riched condition than at the non-enriched condition. At 800µLL−1and non-enriched nutrient conditions, the TC and TN contents showed a minimum of 26.81% DW and 3.176% DW, respectively.
The C:Nratio wassignificantly affected by nutrients ((1,10)=7.148,=0.028) (Fig.3C). The pCO2did not have a significant effect on the C:N ratio ((1,10)=0.015,=0.905). Moreover, a significant interaction between pCO2and nutrients was not detected ((1,10)=0.680,=0.433). At two pCO2levels, the C:Nratio showed no significant difference between the two nutrient levels. Similarly, the C:N ratio showed no significant difference between the two pCO2levels regardless of the nutrient level.

Fig.3 The TC (A) and TN (B) contents and C:N (C) of S. japonica cultured for 6d under two pCO2 levels and two nutrient levels. Data represent the mean±SD (n=3). Different letters indicate significant differences (P<0.05) among different experimental treatments.
4 Discussion
In the present study, the higher pCO2level decreased the growth ofunder the non-enriched nutrient condition while growth was not affected by OA under the nutrient-enriched condition. It has been reported that elevated pCO2could reduce the growth of kelp (Swanson and Fox, 2007; Gutow., 2014; Gordillo., 2015). For instance, the growth ofwas inhibited when pCO2increased from 380µLL−1to 1000µLL−1(Gor- dillo., 2015). According to previous studies, the ne- gative effect of elevated pCO2on growth may occur because decreased pH could disturb the acid-base balance on the cell surface (Flynn., 2012; Xu., 2017), which can increase the cellular reactive oxygen species (ROS) concentration and affect the normal cellular function such as enzyme activities and nutrient uptake and assimilation (García-Sânchez., 1994; Israel., 1999). However, in this study, elevated pCO2did not affect the growth ofin the nutrient-enriched condition, which indicates that high nutrient availability alleviated the negative effect of the high pCO2level. As observed in, high nutrient level could provide nitrogen enough for the synthesis of functional proteins and enzymes to scavenge ROS and prevent the cell from harm derived from the decreased pH (Gao., 2019). Moreover, becauseacted as an ecosystem engineer and formed great canopies with biomass in the lower intertidal and subtidal rocky zones, the benefits in growth rate of this kelpunder the high nutrient conditionmightboost its great potential and mitigate the effect of OA in future oceanic environment.
Like, photosynthesis was significantly reduced by elevated pCO2in the non-enriched nutrient condition and was not significantly affected by pCO2under the nutrient- enriched condition. The negative effect of pCO2on photosynthesis was also reported in(Iñiguez., 2016) and(Xu and Gao, 2012). According to the previous studies, the carbon concentrating mechanisms (CCMs) of seaweed could be down- regulated by elevated pCO2(Rost., 2003; Wu., 2010) and thus affect the sustainability of the intracellular CO2concentration and availability of CO2around Rubis- co (Xu and Gao, 2012). In addition, an elevated pCO2level could reduce the Rubisco content and activity, which is the primary enzyme of carbon assimilation (García-Sáin- chez., 1994). These findings could explain the lower photosynthesis of the alga grown at the elevated pCO2level. However, elevated pCO2had no significant effect on photosynthesis under nutrient-enriched conditions, which showed that high nutrient availability modified the response of this alga to CO2. It had been reported that sufficient nutrient can stimulate the synthesis of related enzymes (Dawes and Koch, 1990; Crawford, 1995), which can promote photosynthesis. Thus, the negative effect of the elevated pCO2level on photosynthesis was offset by high nutrient availability. Additionally, in this study, the dark respiration rate forwas significantly enhanced by elevated pCO2regardless of the nutrient. Si- milar results have also been observed in(Iñ- iguez., 2016) and(Gao., 2019). It was also showed that the positive effect of elevated pCO2on dark respiration may be associated with the changes of proton gradients across the mitochondrial membrane or pH-dependent changes in the functioning of respiratory enzymes (Amthor, 1991; Iñiguez., 2016). However, increased respiration under the OA condition might play a role in counteracting external pH reduction and provide an additional energy demand for maintaining the internal acid-base stability (Yang and Gao, 2012).
Changes in TC and TN contents were in accordance with the response of the growth rate. The negative effects of elevated pCO2on the contents of TC and TN were also reported in(Iñiguez., 2016) and(Iñiguez., 2016). In contrast, the C:N ratio was not affected by elevated pCO2and nutrient level while similar results have also been observed in(Suárez-Álvarez., 2012),(Kang and Chung, 2017) and(Gordon and Carol, 2017). This finding suggests that elevated pCO2has the ability to alter the chemical composition in tissue. This may be be- cause the uptake and assimilation of carbon and nitrogen were decreased by elevated pCO2, which contributes to decreased intracellular inorganic carbon or nitrogen pools (García-Sânchez., 1994; Israel., 1999; Spijkerman, 2011; Raven., 2012; Qu., 2017). Moreover, a similar result that TC and TN contents were synergistically decreased by OA was reported in(Iñ- iguez., 2016). According to the previous study (Iñ- iguez., 2016), a decrease of TN content could lead to an increase of dissolved organic carbon release and thus maintain the internal C:N ratio.
In summary, the present study demonstrated the negative effects of OA on the growth, photosynthesis, TC and TN contents ofunder non-enriched nutrient condi- tion in natural seawater. It also indicated that OA showed a positive effect on the dark respiration rate and a neutral effect on the C:N ratio. Moreover, continuous eutrophication seems to alleviate the negative effects of OA. This study provided important information of the effect of OA on the production and nutrient composition of. However, to further understand how OA affects the biomass yield and physiology of this kelp, it is necessary to conduct more experiments to study the responses ofto the interactive effects of OA and other variables, such as salinity, temperature and irradiance levels.
5 Conclusions
In this study, the combined effects of OA and eutrophication on the growth and nutrient composition ofwere investigated. The results showed that OA had a significant negative effect on growth, photosynthesis, tissue carbon and nitrogen of this kelp. Moreover, nu- trient enrichment could alleviate the negative effects of OA. These results indicate that the effect of OA on the production ofwould be regulated by nutrient condition. Furthermore, the beneficial effect of high nutrient supply on the growth rate could enhance the ability of this kelp to mitigate the risks from OA and greenhouse in future oceanic conditions.
Acknowledgements
We would like to thank the reviewers for their helpful comments that improve the manuscript. This research was supported by Ocean University of China in 2018 and the Major Scientific and Technological Innovation Project of Shandong Provincial Key Research and Development Pro- gram (No. 2019JZZY020708).
Agatsuma, Y., Endo, H., Yoshida, S., Ikemori, C., Takeuchi, Y., Fujishima, H., Nakajima, K., Sano, M., Kanezaki, N., Imai, H., Yamamoto, N., Kanahama, H., Matsubara, T., Takahashi, S., Isogai, T., and Taniguchi, K., 2014. Enhancement ofkelp production by nutrient supply in the Sea of Japan off southwestern Hokkaido, Japan., 26 (4): 1845-1852.
Amthor, J. S., 1991. Respiration in a future, higher CO2world., 14: 13-20.
Boderskov, T., Schmedes, P. S., Bruhn, A., Rusmussen, M. B., Nielsen, M. M., and Pedersen, M. F., 2016. The effect of light and nutrient availability on growth, nitrogen, and pigment con- tents of(Phaeophyceae) grown in outdoor tanks, under natural variation of sunlight and temperature, during autumn and early winter in Denmark., 28 (2): 1153-1165.
Celis-Plá, P. S. M., Martínez, B., Korbee, N., Hall-Spencer, J. M., and Figuero, F. L., 2017. Photoprotective responses in a brown macroalgaeto increases in CO2and temperature., 130: 157-165.
Chen, B., Lin, L., Ma, Z., Zhang, T., Chen, W., and Zou, D., 2019. Carbon and nitrogen accumulation and interspecific competition in two algae species,and, under ocean acidification conditions., 27: 721-733.
Crawford, N. M., 1995. Nitrate: Nutrient and signal for plant growth., 7 (7): 859-868.
Dawes, C. J., and Koch, E. W., 1990.Physiological responses of the red algaeandbefore and after nutrient enrichment., 46 (2): 335-344.
Dickson, A. G., 1990.Standard potential of the reaction: AgCl(s)+1/2H2(g)=Ag(s)+HCl(aq), and the standard acidity constant of the ion HSO4−in synthetic seawater from 273.15 to 318.15 K., 22 (2): 113-127.
Endo, H., Okumura, Y., Sato, Y., and Agatsuma, Y., 2017.Interactive effects of nutrient availability, temperature, and irradiance on photosynthetic pigments and color of the brown alga., 29 (3):1683-1693.
Falkenberg, L. J., Russell, B. D., and Connell, S. D., 2013. Contrasting resource limitations of marine primary producers: Implications for competitive interactions under enriched CO2and nutrient regimes., 172 (2): 575-583.
Flynn, K. J., Blackford, J. C., Baird, M. E., Raven, J. A., Clark, D. R., Beardall, J., Brownlee, C., Fabian, H., and Wheeler, G. L., 2012. Changes in pH at the exterior surface of plankton with ocean acidification., 2 (7): 510-513.
Gao, G., Gao, Q., Bao, M., Xu, J., and Li, X., 2019. Nitrogen availability modulates the effects of ocean acidification on biomass yield and food quality of a marine crop,., 271: 623-629.
Gao, K., and McKinley, K., 1994. Use of macroalgae for marine biomass production and CO2remediation: A review., 6: 45-60.
Gao, K., Ji, Y., and Aruga, Y., 1999. Relationship of CO2concentrations to photosynthesis of intertidal macroalgae during emersion., 398: 355-359.
Gao, X., Endo, H., Nagaki, M., and Agatsuma, Y., 2017. Interactive effects of nutrient availability and temperature on growth and survival of different size classes of(Laminariales, Phaeophyceae)., 56 (3): 253-260.
García-Sânchez, M. J., Fernândez, J. A., and Niell, F. X., 1994. Effect of inorganic carbon supply on the photosyntetic physiology of., 194 (1): 55-61.
Geertz-Hansen, O., Sand-Jensen, K., Hansen, D. F., and Chris- tiansen, A., 1993. Growth and grazing control of abundance of the marine macroalga,L. in a eutrophic Danish estuary., 46 (2): 101-109.
Gordillo, F. J. L., Aguilera, J., Wiencke, C., and Jiménez, C., 2015. Ocean acidification modulates the response of two Arctic kelps to ultraviolet radiation., 173: 41-50.
Gordon, T. O., and Carol, S. T., 2017. Divergent responses in growth and nutritional quality of coastal macroalgae to the combination of increased pCO2and nutrients., 131: 69-79.
Graham, M. H., 2004. Effects of local deforestation on the diversity and structure of Southern California giant kelp forest food webs., 7 (4): 341-357.
Gutow, L., Rahman, M. M., Bartl, K., Saborowski, R., Bartsch, I., and Wiencke, C., 2014. Ocean acidification affects growth but not nutritional quality of the seaweed(Phaeophyceae, Fucales).,453: 84-90.
Huang, Y., Liu, X., Laws, E. A., Chen, B., Li, Y., Xie, Y., Wu, Y., Gao, K., and Huang, B., 2018. Effects of increasing atmospheric CO2on the marine phytoplankton and bacterial metabolism during a bloom: A coastal mesocosm study., 633: 618-629.
Hwang, E. U., Liu, F., Lee, K. H., Ha, D. S., and Park, C. S., 2018. Comparison of the cultivation performance between Korean (Sugwawon No. 301) and Chinese strains (Huangguan No. 1) of kelpin an aquaculture farm in Korea., 33 (1): 101-108.
Iñiguez, C., Carmona, R., Lorenzo, M. R., Niell, F. X., Wiencke, C., and Gordillo, F. J. L., 2016. Increased CO2modifies the carbon balance and the photosynthetic yield of two common Arctic brown seaweeds:and., 39: 1979-1991.
IPCC, 2013.. Stocker, T. F.,., eds., Cambridge University Press, Cambridge, United Kingdom and New York, 1535pp.
IPCC, 2014...Pachau- ri, R. K., and Meyer, L. A., eds., IPCC, Geneva, 151pp.
Israel, A., Katz, S., Dubinsky, Z., Merrill, J. E., and Friedlander, M., 1999. Photosynthetic inorganic carbon utilization and growth of(Rhorophyta)., 11 (5): 447-453.
Ji, Z., Zou, D., Gong, J., Liu, C., Ye, C., and Chen, Y., 2019. The different responses of growth and photosynthesis to NH4+enrichments betweenand its epiphytic algagrown at elevated atmospheric CO2., 144: 173-180.
Johnson, M. D., Comeau, S., Lantz, C. A., and Smith, J. E., 2017. Complex and interactive effects of ocean acidification and tem- perature on epilithic and endolithic coral-reef turf algal assem- blages., 36: 1059-1070.
Kang, J. W., and Chung, I. K., 2017. The effects of eutrophication and acidification on the ecophysiology ofKjellman., 29: 2675-2683.
Kang, J. W., and Chung, I. K., 2018. The interactive effects of elevated CO2and ammonium enrichment on the physiological performances of(Laminariales, Phaeo- phyta)., 53 (3): 487-497.
Kim, J. H., Kang, E. J., Edwards, M. S., Lee, K., Jeong, H. J., and Kim, K. Y., 2016. Species-specific responses of temperate macroalgae with different photosynthetic strategies to ocean acidification: A mesocosm study., 31 (3): 243-256.
Lewis, E., and Wallace, D., 1998. Program Developed for CO2System Calculations. Carbon Dioxide Information Analysis Cen- ter. Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, Tennessee.
Liu, F., Wang, X., Liu, J., Fu, W., Duan, D., and Yang, Y., 2009. Genetic mapping of the(Laminariales, Phaeophyta) using amplified fragment length polymorphism markers., 45 (5): 1228-1233.
Ménesguen, A., Desmit, X., Dulière, V., Lacroix, G., Thouvenin, B., Thieu, V., and Dussauze, M., 2018. How to avoid eutrophi- cation in coastal seas? A new approach to derive river-specific combined nitrate and phosphate maximum concentrations., 628: 400-414.
Mercado, J., Javier, F., Gordillo, L., Niell, F. X., and Figueroa, F., 1999. Effects of different levels of CO2on photosynthsis and cell components of the red alga., 11 (5): 455-461.
Mizuta, H., Narumi, H., and Yamamoto, H., 2001. Effects of ni- trate and phosphate on the growth and maturation of gametophytes ofMiyabe (Phaeophyceae)., 49: 175-180 (in Japanese with English abstract).
Oh, J. C., Yu, O. H., and Choi, H. G., 2015. Interactive effects of increased temperature and pCO2concentration on the growth of a brown algaein the sporophyte and game- tophyte stages., 37 (3): 201-209.
Olischlaeger, M., Bartsch, I., Gutow, L., and Wiencke, C., 2012. Effects of ocean acidification on different life-cycle stages of the kelp(Phaeophyceae)., 55 (5): 511-525.
Qu, L., Xu, J., Sun, J., Li, X., and Gao, K., 2017. Diurnal pH fluctuations of seawater influence the responses of an economic red macroalgato future CO2- induced seawater acidification., 473: 383-388.
Raven, J. A., Giordano, M., Beardall, J., and Maberly, S. C., 2012. Algal evolution in relation to atmospheric CO2: Carboxylases, carbon-concentrating mechanisms and carbon oxidation cycles., 367: 493-507.
Rost, B., Riebesell, U., Burkhardt, S., and Siiltemeyer, D., 2003. Carbon acquisition of bloom-forming marine phytoplankton., 48: 55-67.
Roy, R. N., Roy, L. N., Vogel, K. M., Porter-Moore, C., Pearson, T., Good, C. E., Millero, F. J., and Campbell, D. M., 1993. The dissociation constants of carbonic acid in seawater at salinities 5 to 45 and temperature 0 to 45℃., 44 (2-4): 249-267.
Russell, B. D., Thompson, J. A. I., Falkenberg, L. J., and Connell, S. D., 2009. Synergistic effects of climate change and local stressors: CO2and nutrient-driven change in subtidal rocky habitats., 15: 2153-2162.
Schmid, R., Mills, J., and Dring, M., 1996. Influence of carbon supply on the stimulation of light-saturated photosynthesis by blue light in: Implications for mechanism of carbon acquisition in higher brown algae., 19 (4): 383-391.
Selivanova, O. N., Zhigadlova, G. G., and Hansen, G. I., 2007. Revision of the systematics of algae in the order Laminariales (Phaeophyta) from the Far-Eastern Seas of Russia on the basis of molecular-phylogenetic data.,33 (5): 278-289.
Smith, S. V., Swaney, D. P., Talaue-Mcmanus, L., Bartley, J. D., Sandhei, P. T., McLaughlin, C. J., Dupra, V. C., Crossland, C. J., Buddemeier, R. W., Maxwell, B. A., and Wulff, F., 2003. Humans, hydrology, and the distribution of inorganic nutrient loading to the ocean., 53 (3): 235-245.
Spijkerman, E., 2011. The expression of a carbon concentrating mechanism inunder variable phos- phorus, iron, and CO2concentrations., 109 (1-3): 179-189.
Suárez-Álvarez, S., Gómez-Pinchetti, J. L., and García-Reina, G., 2012. Effects of increased CO2levels on growth, photosynthesis, ammonium uptake and cell composition in the macro- alga(Gigartinales, Rhodophyta)., 24: 815-823.
Swanson, A. K., and Fox, C. H., 2007. Altered kelp (Laminariales) phlorotannins and growth under elevated carbon dioxide and ultraviolet-B treatments can influence associated intertidal food webs., 13 (8): 1696-1709.
Tatewaki, M., 1966. Formation of a crustose sporophyte with unilocular sporangia in, 6: 62-66.
Wu, H., Ding, G., and Xu, Z., 2015. Effects of salt stress on growth and photosynthesis of(Rhodophyta) cultured under different nitrogen conditions., 46: 1210-1217 (in Chinese with English abstract).
Wu, Y., Gao, K., and Riebesell, U., 2010. CO2-induced seawater acidification affects physiological performance of the marine diatom., 7: 2915- 2923.
Xu, D., Brennan, G., Xu, L., Zhang, X. W., Fan, X., Han, W., Mock,T., McMinn, A., Hutchins, D. A., and Ye, N., 2019. Ocean acidi- fication increases iodine accumulation in kelp-based coastal food webs., 25: 629-639.
Xu, D., Wang, D., Li, B., Fan, X., Zhang, X., Ye, N., Wang, Y., Mou, S., and Zhuang, Z., 2015. Effects of CO2and seawater acidification on the early stages ofdevelopment., 49 (6): 3548- 3556.
Xu, J., and Gao, K., 2012. Future CO2-induced ocean acidification mediate the physiological performance of a green tide alga., 160 (4): 1762-1769.
Xu, Z., Gao, G., and Xu, J., 2017. Physiological response of a golden tide alga () to the interaction of ocean acidification and phosphorus enrichment., 14 (3): 671-681.
Yang, G., and Gao, K., 2012. Physiological responses of the marine diatomto increased pCO2and seawater acidity., 79: 142- 151.
Zou, D., 2005. Effects of elevated atmospheric CO2on growth, photosynthesis and nitrogen metabolism in the economic brownseaweed,(Sargassaceae, Phaeophyta)., 250: 726-735.
E-mail: qd_liuyan@ouc.edu.cn
E-mail: qingli@vip.sina.com
October 14, 2019;
December 9, 2019;
March 25, 2020
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Taxonomy and Phylogeny of a New Marine Planktonic Ciliate, Strombidium pseudorapulum sp. n.(Protozoa, Ciliophora, Oligotrichia)
- Can Langmuir Circulations Solve the Problem of Insufficient Upper-Ocean Mixing?
- Dynamic Diurnal Changes in Green Algae Biomass in the Southern Yellow Sea Based on GOCI Images
- Transcriptomic Profiling of the Immune Response to Crowding Stress in Juvenile Turbot (Scophthalmus maximus)
- Reducing the Common Environmental Effect on Litopenaeus vannamei Body Weight by Rearing Communally at Early Developmental Stages and Using a Reconstructed Pedigree
- Isolation of Enterococcus faecium with Feeding Attractant Function from Pacific White Shrimp (Litopenaeus vannamei) Intestine
