Response of Phytoneuston Community to Organic Nitrogen and Phosphorus Revealed by HPLC-Pigments Method
2020-09-28WANGZhaohuiZHAOJianggangandXIAOLin
WANG Zhaohui, ZHAO Jianggang, and XIAO Lin
Response of Phytoneuston Community to Organic Nitrogen and Phosphorus Revealed by HPLC-Pigments Method
WANG Zhaohui*, ZHAO Jianggang, and XIAO Lin
College of Life Science and Technology, Jinan University, Guangzhou 510632, China
The sea surface microlayer (SML) is the thin boundary layer between the ocean and the atmosphere. Phytoplankton community in the SML is known as phytoneuston and has a different community structure and biomass from the phytoplankton of the underlying bulk water. In this study, water samples were collected from the SML of Daya Bay in southern China in September, October, and December of 2013. Algal growth potential (AGP) tests were conducted by adding different forms of nitrogen (N), phosphorus (P) and silicate (Si). Phytoneuston grew well with both inorganic and organic nutrients, and showed comparable community structure after nutrient addition. A total of 16 pigments were analyzed in the SML samples collected from Daya Bay in this study. Phytopigments were dominated by the cyanobacteria-diagnostic pigment (zeaxanthin, Zea) in September and October, and by diatom-diagnostic pigment (fucoxanthin, Fuco) in December. The concentrations and proportions of Fuco increased greatly after the nutrient addition, and the concentrations and proportions of Zea decreased accordingly. Additionally, the diatom pigment index (DiatDP) increased after nutrient addition, and the prokaryotic pigment index (ProkDP) conversely decreased. The results suggested that massive occurrences of Zea and cyanobacteria are important characteristics of phytoneuston structure in Daya Bay especially in warm seasons, and nutrient addition especially Si addition promoted the growth of diatoms.
phytoplankton; sea surface microlayer; pigment; nutrient; cyanobacteria; Daya Bay
1 Introduction
The sea surface microlayer (SML), defined here as the upper 50-100μm of the water column, is the thin boundary layer between the ocean and the atmosphere. The SML is an important habitat of the ocean where air-sea exchange and transformation processes take place (Wurl., 2016). With its unique position and properties, the SML is chemically and physically different from the underlying bulk water (UBW) (Cunliffe., 2013). Nutrients, organic matters, trace metals, phytoplankton, and microorganisms are often enriched in the SML (Reinthaler., 2008; Wurl and Holmes, 2008; Engel and Galgani, 2016; Wurl., 2016).
The phytoplankton community in the SML is known as phytoneuston and has a different community structure and biomass from the phytoplankton of the UBW (Montes- Hugo and Alvarez-Borrego, 2007; Yang., 2009). Due to the increasing loading of anthropogenic nitrogen (N) and phosphorus (P) over the past few decades, nutrient enrichment has promoted accelerated rates of primary production (Maar., 2016). The SML is a demanding habitat, and phytoneuston is exposed to a much higher temperature and UV radiation (Cunliffe., 2011;Mohlin., 2012). On the other hand, the enrichment of organic matter and nutrients can render the SML as a favorable habitat for the phytoneuston (Zäncker., 2017). Phytoplankton are generally enriched in the SML (Wang., 2014; Zäncker., 2017), and cyanobacteria have recently been reported to be highly enriched in the SML from worldwide sea areas (Wurl., 2016; Zäncker., 2017). However, the response of phytoneuston to nutrient enrichment has been lightly reported. Our previous study showed that phytoneuston structures shifted from cyanobacteria-dominated to diatom-domina- ted after an inorganic nutrient addition in concert with a decreased temperature and irradiance (Yang, 2015). Responses of phytoneuston to organic nutrients have not been reported up till now.
Daya Bay is located in the northeast part of the South China Sea, which is an important cultural area in southern China. This bay is strongly affected by eutrophication due to the increase in nutrient loading since 1990s (Wang., 2009). It is also the site of two nuclear power stations, the Daya Bay Nuclear Power Station (DNP), the first nuclear power station in China, and the Lingao Nuclear Power Station (LNP). Nutrient enrichment and cooling water discharges from the nuclear power stations have substantially modified the phytoplankton community structure in the area, exhibited by enhanced primary production and altered composition of the phytoplankton community (Wang., 2009). The results from our previous study showed that cyanobacteria were highly enriched in the SML of Daya Bay with respect to the underlying water (Wang., 2014).
Conventional light microscopical observation is the main tool for the identification and enumeration of phytoplankton. However, it has limitations for the identification of the small-sized phytoplankton groups such as pico- to nanosized phytoplankton (Böttjer and Morales, 2007). Some phytopigments are characteristic of the specific phytoplankton groups and can be used as diagnostic markers to classify phytoplankton assemblages (Barlow., 2007; Roy., 2011). For example, fucoxanthin (Fuco), peridinin (Per), 19’-hex-fucoxanthin (Hex), alloxanthin (All), and zeaxanthin (Zea) usually indicate the presence of diatoms, dinoflagellates, prymnesiophytes, cryptophytes, and cyanobacteria, respectively. The key pigments and their corresponding phytoplankton groups are listed in Table 1 (Roy., 2011). Pigment analysis using liquid chromatography is considered as a powerful tool for the characterization and monitoring of phytoplankton abundances and compositions (Wright and Jef- frey, 2006).

Table 1 Phytopigments analyzed in the study and their corresponding phytoplankton community. Major pigments (>10%) are marked in bold font (Roy et al., 2011)
We conducted an annual survey of the phytoplankton community, size-fractionated phytopigments and environmental parameters from the SML and subsurface sea waters (SSW) between 2013 and 2014 to compare phytoplankton community between the SML and SSW of Daya Bay (Xiong, 2014; Jiang., 2016). In this study, natural water samples were collected from the SML of Daya Bay of the South China Sea in September, October, and December 2013. Algal growth potential (AGP) tests were conducted by addition of different forms of organic N, P, and together with silicate (Si) in laboratory conditions. Phytoneuston biomass (Chlorophyll, Chl), phytopigments, and nutrient concentrations were measured. The purpose of this study is 1) to discuss the seasonal differences of the phytoneuston community structure of Daya Bay based on HPLC-derived phytopigment analysis, 2) to understand the response of the phytoneuston community in the SML to organic nutrients, and 3) to reveal the role of nutrient enrichment in concert with the decrease of light intensity on the phytoneuston community structure.
2 Materials and Methods
2.1 Study Area
Daya Bay (22˚30´-22˚50´N, 114˚30´-114˚50´E) is an enclosed embayment in the northwestern part of the South China Sea. It is situated between Shenzhen and Huizhou in Guangdong Province, near the Pearl River Estuary (Fig.1). Its depth ranges from 6m to 15m, and it covers an area of 650km2during the flood tide. Daya Bay is characterized by a subtropical climate. The stronger northeast monsoon prevails from October to April, whe- reas the southwest monsoon lasts from May to September. The bay is dominated by an irregular semidiurnal tide with a narrow tidal range (Wang., 2014). The study area is located at Dapeng Cove (Fig.1), the site of the two nuclear power stations.
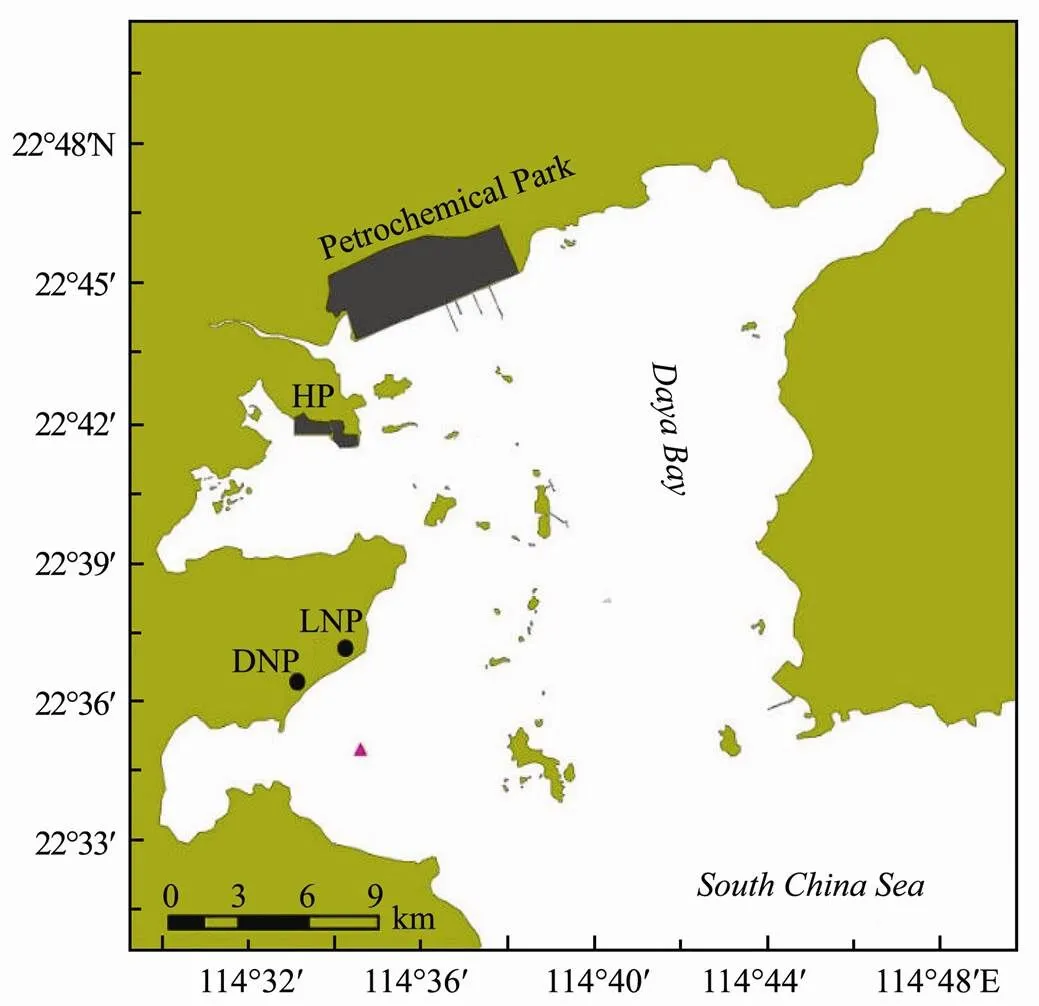
Fig.1 Map of Daya Bay indicating the sampling site. , Sampling site; HP, Huizhou Port; LNP, Lingao Nuclear Power Plant; DNP, Daya Bay Nuclear Power Plant.
2.2 Sample Collection
The SML samples were collected at the offshore site of Dapeng Cove (Fig.1) on September 27, October 24, and December 2, 2013. The SML samples were collected using a glass-plate sampler (40cm wide, 60cm long, 0.5cm thick) at the rear of a small boat. The glass plate was submerged vertically in the water and withdrawn gently at a speed of 10cms−1(as consistently as conditions allowed). The thickness of the microlayer sampled by this technique is 80±10μm after being measured by the volume of the water sample and the area of the sampler. Each adhering SML sample was detached with a neoprene blade and collected in a polyethylene box and then stored in a polyethylene bottle. A total of 20 L SML samples were collected in each sampling, in which 18L water samples were used for the AGP test, and 2L water samples were then used for nutrient and phytopigment analysis.
Water temperature and salinity in the SML samples were measured immediately after collectedby a YSI meter (YSI-556, YSI Incorporated, USA). Samples were kept on ice in the dark during transport (3-5h from sampling). Triplicate 200-mL water samples were filtered through precombusted Whatman GF/F glass-fiber filters (pore size about 0.7μm). Residues in the filters were used for the determination of phytopigments, and the filtrates were used for the determination of dissolved nutrients, including dissolved inorganic nitrogen (DIN, the sum of NO3-N, NO2-N, and NH4-N), dissolved inorganic phosphorus (DIP, PO4-P), dissolved silicate (DSi, SiO3-Si), dissolved total nitrogen (DTN), and dissolved total phosphorus (DTP). The differences between dissolved total nutrients (DTN and DTP) and dissolved inorganic nutrients (DIN and DIP) were defined as dissolved organic nutrients (DON and DOP). Another 200-mL subsample was used to determine the total nitrogen (TN) and total phosphorus (TP). Methods for these analyses are described in Sections 2.4 and 2.5. Water temperature, salinity and nutrient concentrations of water samples are listed in Table 2.

Table 2 Water temperature, irradiance, salinity and nutrient concentrations (Ave±SD) in the SML from Daya Bay in September, October and December of 2013
2.3 Experimental Design
The phytoneuston in the SML samples was acclimated to the new conditions of temperature and light for six hours before the experiment. Growth tests were conducted in batch cultures. An organic N addition test was conducted in September, including three control groups (C, N0P, NP0), and six organic N groups (Uric, Urea, Thr, Ala, Glu, Gly). Organic N and P addition tests were conducted in October and December, including one control group (C), one inorganic nutrient group (NP), three organic N groups (Urea, Glu, Gly), and four organic P groups (GYP, G6P, ATP, AMP). NaSiO3-Si was added in all test groups at the concentration of 35μmolSiL−1. Different forms of N and P were added in the corresponding organic N and P groups. Inorganic P (KH2PO4) was added as the P source in the organic N test groups, and inorganic N (NaNO3) was added as the N source in the organic P test groups. NaNO3-N and KH2PO4-P were nutrient sources in the NP group. Within the three control groups, neither N nor P were added into group C, and only P was added in group N0P and N in NP0. Nutrient forms and concentrations added into each test group of the experiment are listed in Table 3. Nutrient concentrations added in the experiment were according to their maximum concentrations in the SML of Daya Bay (Yang, 2015).
The experiment was conducted in 1000 mL Erlenmeyer flasks containing 600mL SML samples after adding the different forms of nutrients. Other elements besides N, P, and Si were added using the same technique with the f/2 medium (Guillard, 1975). Each test group was set in triplicate. The cultures were incubated at temperature 25± 1℃, salinity 32, with 100μmolphotonm−2s−1of cool- white fluorescent illumination with a dark:light cycle of 12h:12h. The experiment was run for 6-8d until the growth declined.
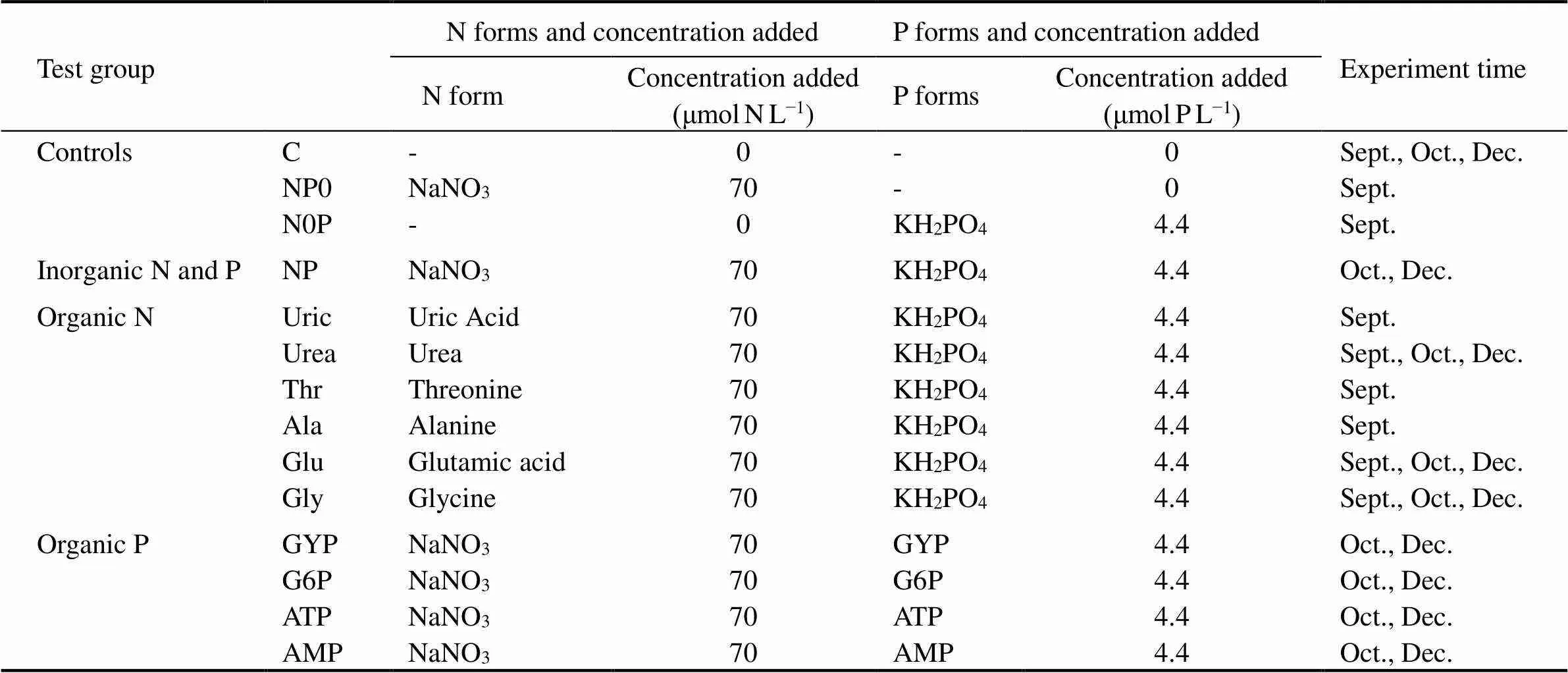
Table 3 Nitrogen and phosphorus forms and concentrations added in test groups (NaSiO3-Si was added in all test groups at the concentration of 35μmol SiL−1)
2.4 Analysis of Chl a and Specific Growth Rate
Chlorophyll(Chl) was measured by a luminoscope (Trilogy Luminoscope, Tumer Designs, USA) daily during the experiment. The concentration of Chlwas determined by the linear relationship of the concentration of Chlof standard samples measured from the HPLC and the fluorescence.
The specific growth rate (, /d, d=day) was calculated using the following equation:
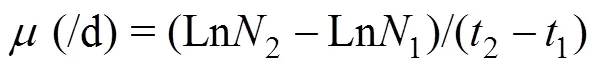
where2and1are Chlconcentrations at times2and1.maxis the specific growth rate.
2.5 Analysis of Nutrients
Nutrients and phytopigments were analyzed before nutrient addition (day 0, d0), the peak of Chl(day 2, d2), and the decline stage (day 4-7, d4-d7). One hundred milliliter cultures were sampled from each flask and were filtered through precombusted Whatman GF/F glass-fiber filters. The filters were used for phytopigment analysis and the filtrates were used for dissolved nutrient analysis. Ten milliliter filtrates were used for determination of NO3-N, NO2-N, NH4-N, PO4-P, and DSi, respectively, and the other 50mL filtrates were for DTN and DTP. TN and TP were measured in the water samples before the experiment (Table 2). Nutrient analysis was performed spectrophotometrically in triplicate following the method of Strickland and Parsons (1972).
2.6 Phytopigment Analysis
Pigment extraction and determination were performed in accordance with the method of Zapata. (2000). The frozen filters were cut into small pieces and transferred into a 15mL polypropylene centrifuge tube, where 3mL 95% HPLC-grade methanol was added. The mixture was ground using a glass rod and subsequently sonicated for 5min in an ice bath under low light. The resulting extracts were separatedcentrifugation (3min, 1500g), and 1.5-2mL of the supernatant was filtered through a 0.45μm PTFE membrane. One milliliter extract was transferred to a 2mL screw-top vial and diluted with 250μL Milli-Q water before injection to improve the separation of pigments in the column.
The HPLC system,.., the Agilent 1200 series, is equipped with an auto sampler and diode array detector (Model G1315C). The pigments extracted were separated by a C8 column (150mm×4.6mm, 3.5μm particle size, Waters Symmetry) at 25℃. The flow rate was 1.0mLmin−1, and the sample injection volume was 100μL. The pigments were identified based on a comparison of the retention time and diode-array spectroscopy results (wa- velength: 350–750nm, 1.2nm spectral resolution) with the authentic standards. All the pigments were quantified at 440nm except for pheophytin(Phe) and pheoporbide(Pheide) at 430nm. Twenty-four authentic pigment standards (Table 1) were commercially obtained from DHI Inc. (Denmark).
2.7 Diagnostic Pigment Indices
Pigments also provide important chemotaxonomic information concerning community structure, and certain key pigments are signatures for various phytoplankton groups (Roy., 2011, Table 1). Diagnostic pigment (DP) indices were derived to assess the composition of phytoplankton communities (Barlow., 2007; Böttjer and Morales, 2007). DPs were defined as the sum of seven selected biomarker pigments as shown in Table 4. Four major phytoplankton groups,.., diatoms, small flagellates, dinoflagellates, and prokaryotes, were characterized by DiatDP, FlagDP, DinoDP, and ProkDP, respectively.
2.8 Data Analysis
The means and standard deviations (SD) were calculated for each treatment from the three independent replicate cultures. Analysis of variance (ANOVA) was performed to compare the differences between months, test groups, and changes after nutrient addition. Pearson correlation analysis (PCA) was used to examine the correlations among phytopigments. Statistical analysis was performed by SPSS 19.0 for Windows (SPSS Inc., Chicago, Illinois).

Table 4 Diagnostic pigment sums and pigment indices (Barlow et al., 2007)
3 Results
3.1 The Growth of Phytoneuston During the AGP Test
Phytoneuston grew quickly after the addition of both inorganic and organic nutrients. Concentrations of Chlincreased significantly after nutrient addition (<0.05 or 0.01), and peaked at d2, and then decreased sharply (Fig. 2). Concentrations of Chlwere significantly higher (<0.01) in the test groups with all three nutrient elements added than those in the three control groups with only one or two nutrient elements added (C, N0P, and NP0).

Fig.2 Changes in chlorophyll a (Chl a) concentrations in SML samples after the addition of different sources of organic nu- triaents. Each line means different test groups, which are listed in Table 2. A, Experiment in September; B, Experiment in October; C, Experiment in December.

Fig.3 The maximum chlorophyll a (Chl a) concentration (A) and maximum specific growth rate (μmax, B) in each test group of the AGP test. Sept., Oct., and Dec. denote the experiments using the SML samples collected in September, October, and Dece mber, respectively.
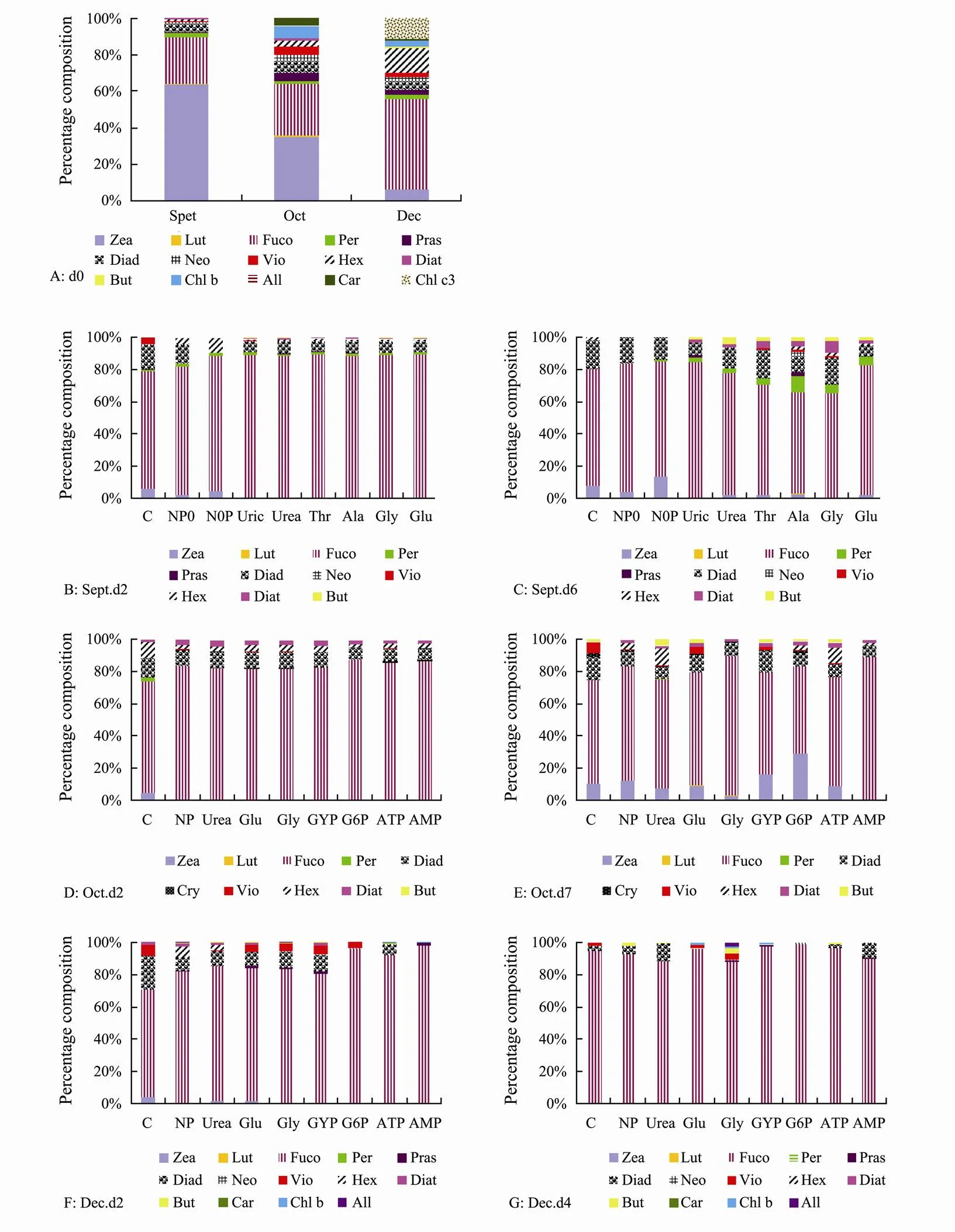
Fig.4 Composition percentages of the total accessory pigments (TAcc) in SML samples before and during the AGP test. A, Pigments in d0, which were the concentrations found in situ before the AGP test; B, C, Pigment profiles in d2 (B) and d6 (C) in the September experiment; D, E, Pigment profiles in d2 (D) and d7 (E) in the October experiment; F, G, Pigment profiles in d2 (F) and d4 (G) in the December experiment.
The maximum Chlconcentration was highest in group Gly out of all the test groups with organic N (Fig. 3A). Within the organic P test groups, maximum Chlconcentrations were higher in AMP and G6P in October and were higher in GYP and AMP in December (Fig.3A). The ANOVA results indicated that there were no significant differences for the maximum Chlconcentration among test groups with the addition of organic and inorganic nutrients within the experiment of the same month (>0.05). The maximum specific growth rates (max) generally occurred at 1-2 d after the nutrient addition and were significantly higher (<0.01) in test groups with all nutrient elements added (1.09-2.67d−1) than those of the three control groups (0.14-0.71d−1, Fig.3B).
3.2 Changes in Phytopigment Structure
Fig.4 illustrates the composition percentages of the total accessory pigments (TAcc) in the SML samples and in cultures after the nutrient addition. A total of 16 pigments (excluding Chl) were analyzed in this study, and 11, 14, and 16 pigments were detected in the samples from September, October, and December, respectively (Fig.4A). Phytopigments were dominated by Zea in September and October, which contributed to 63.6% and 34.8% of the TAcc, respectively (Fig.4A). The relative contribution of Zea to TAcc decreased to 5.9% in December. Fuco, the representative of diatoms, contributed 25.4%-49.6% to TAcc. Diadinoxanthin (Diad), Hex, Per, and prasinoxanthin (Pras) were common pigments as well; however, they mostly contributed to less than 5% of the TAcc. Percentages of Fuco increased significantly after nutrient addition (Fig.4). The maximum proportion of Fuco occurred at d2, with proportions of 62.4%-97.8%. Meanwhile, percentages of Zea decreased greatly after nutrient addition, which accounted for 0-29.3% of the TAcc (Fig.4). Proportions of Diad increased after the nutrient addition, and generally up to 10% with a maximum of 17.9%.
Table 5 lists the pigment indices in the SML samples collected from September, October, and December 2013 before and during the AGP test. Concentrations of total pigments (Tpig), diagnostic pigments (DP), and TAcc in d0 were comparable among the three months. However, the concentration of photoprotective carotenoids (PPC) was approximately four times lower in December than in September and October, while the concentration of photosynthetic carotenoids (PSC) was approximately two times higher in December than in September and October. PPC increased 16.2-65.0 times in d2 after the nutrient additions. PSC increased approximately 139-440 times, and TAcc increased by more than 100 times. The ratio of photoprotective carotenoids to total pigments (PPCTP) showed no obvious increase after the nutrient additions in September and October (>0.05), while they increased by approximately three times in December. The ratio of photosynthetic carotenoids to total pigments (PSCTP) increased sharply, which were approximately 5-16 times higher in d2 than those in d0. The Diatom proportion of the DP index (DiatDP) increased from September to December as the water temperature decreased. DiatDPincreased significantly after the nutrient additions (<0.05). Contrary to DiatDP, the prokaryote proportions of DP (ProkDP) were higher in the warm season. However, ProkDPgenerally decreased to <0.1 after nutrient addition during the massive growth of diatoms and the decrease of light intensity. The dinoflagellate proportion of DP (DinoDP) index was low in d0 and remained low after the nutrient addition. The flagellate proportion of DP (FlagDP) index was relatively higher in October and December, and decreased to less than 0.1 after the nutrient addition.
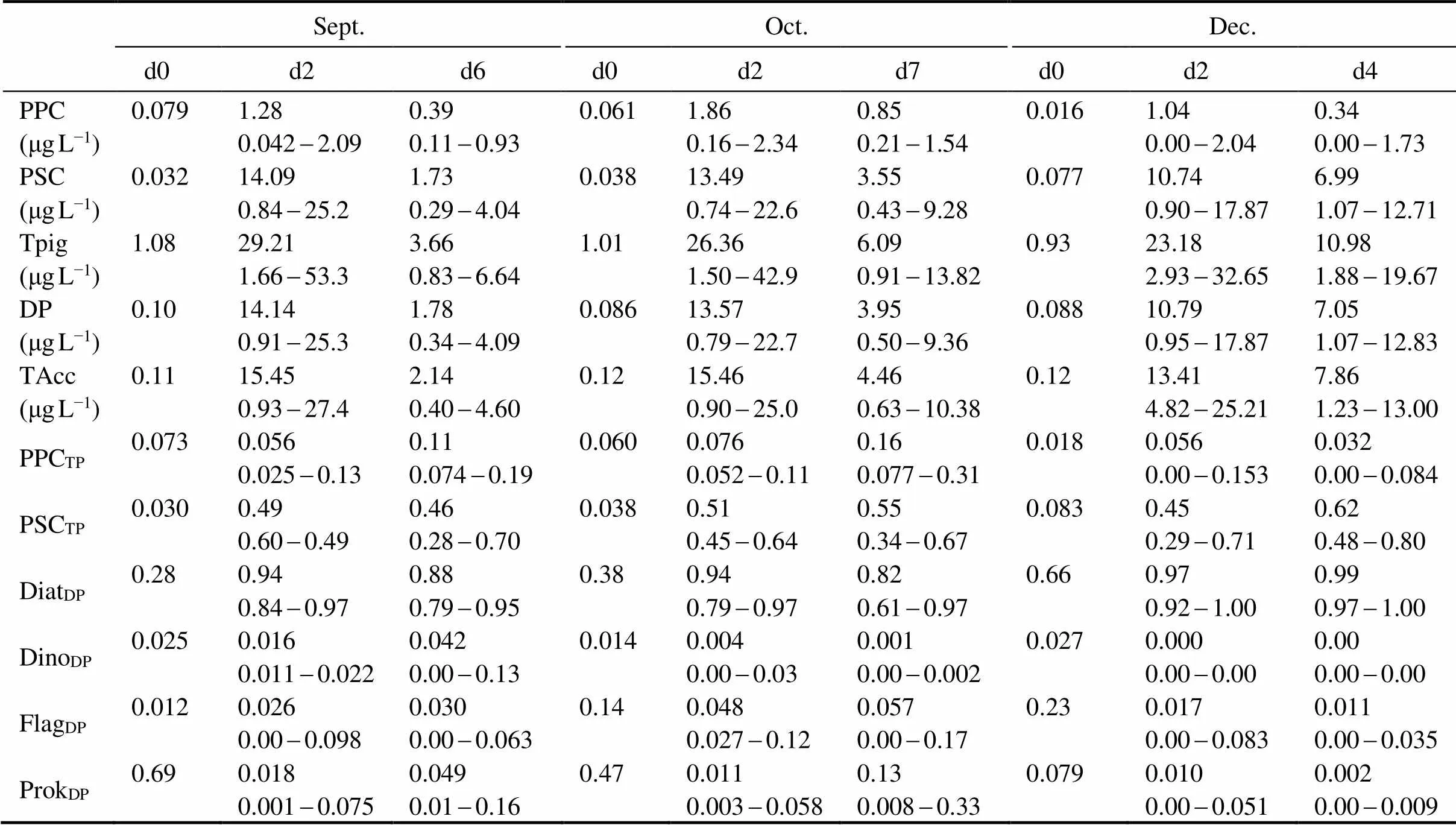
Table 5 Diagnostic pigment indices (averages and ranges) in SML samples before and during the AGP test. (Values in d0 were those of phytoneuston collected in situ before nutrient addition. Averages and ranges in d2-d7 were calculated by pooling all test groups during the same culturing time)
3.3 Variations in Concentrations of Major Phytopigments
All phytopigments showed significant differences (<0.01) between different culturing days based on the results of ANOVA. Zea, Per, Diat, and Hex showed significant differences (<0.05 or 0.01) among the three months, however Fuco and Diad, the main pigments of diatoms, showed no significant differences (>0.05) among the three months. The results from the PCA showed that there were significantly positive correlations among Fuco, Diad, Diat, and Hex, and between these diagnosis pigments and Chl(Table 6,<0.05 or 0.01). However, Zea showed no significant relationships with all other phytopigments (> 0.05).
Diatoms are the key contributor of Fuco. The initial concentrations of Fuco were low at d0 and ranged between 0.029 and 0.058μgL−1(Figs.5A-5C). Fuco increased sharply in d2 (<0.01) after the addition of inorganic or organic nutrients. Fuco concentrations in d2 were generally 100-800 times higher than those in d0. Fuco decreased significantly in d6-d7 in September and October compared to d2 (<0.01), however, it did not reduce significantly in d4 in December (>0.05). Fuco was relatively low in test groups C, NP0, and N0P, in which only one or two nutrient elements were added.

Table 6 Pearson’s correlations among phytopigments
Notes:*: Coefficients at 0.05 significance level,<0.05;**: coefficients at 0.01 significance level,<0.01.

Fig.5 Changes in major diagnostic pigment concentrations during the AGP test. A-C, Fucoxanthin (Fuco); D-F, Zeaxanthin (Zea); A, D, Experiment in September; B, E, Experiment in October; C, F, Experiment in December.
Zea is the major pigment of cyanobacteria (Table 1). Zea ranged from 0.0069 to 0.072μgL−1at d0 (Figs.5D-5F). Zea generally decreased significantly after nutrient addition in September (<0.05). Additionally, Zea increased greatly after the nutrient additions in October and December (<0.01). A maximum Zea concentration of 0.91μgL−1was recorded in test group G6P at d7 during the October experiment.
3.4 Changes of Nutrient Concentrations in Cultures
Figs.6-8 illustrate the nutrient concentrations in cultures. Except for NP0 in September, DIN decreased significantly in the d2 in all DIN-incorporated test groups (<0.01), in which over 65% DIN was utilized. However, DIN in groups that added organic N showed no significant decrease (>0.05). DON concentrations were high in the organic N test groups and decreased significantly in d2 in these cultures (<0.01, Figs.6B, 6E, 6H). The initial concentrations of DTN were high except for C and N0P. DTN deceased to less than 50% in d2, and further decreased in d4-d7 (Figs.6C, 6F, 6I).
DIP decreased sharply in groups with inorganic P addition in d2 (<0.01, Figs.7A, 7D, 7G), in which 75%-98% of DIP was utilized by the phytoplankton in these cultures. DIP showed no significant decrease in the organic P groups and the two control groups (C and NP0) during the experiment (>0.05) and increased slightly in some cultures (Figs.7A, 7G). DOP concentrations were high in test groups with organic P additions, and decreased great- ly in d2 (<0.01, Figs.7E, 7H). DTP was added in all test groups except for C and NP0 and deceased significantly in d2 after the nutrient addition (<0.01), which over 70% was utilized by the phytoplankton (Figs.7C, 7F, 7I).
DSi was sufficient in all test groups, and decreased obviously in all test groups (<0.01) except for C, NP0, and N0P (>0.05), and >50% of DSi was utilized in d2, and then further decreased at d4-d7 (Fig.8).

Fig.6 Changes in nitrogen concentration in the cultures. A-C, Experiment in September; D-F, Experiment in October; G-I, Experiment in December; A, D, G, Concentrations of DIN; B, E, H, Concentrations of DON; C, F, I, Concentrations of DTN.
4 Discussion
Zea dominated in the phytoneuston community in this study in September and October of 2013, when water temperatures were over 26℃. However, the proportion of Zea decreased in December, when the water temperature was less than 20℃. Zea is the major pigment of cyano- bacteria and prochlorophytes (Table 1, Roy., 2011). As DV-Chl, another major pigment of prochlorophytes, was undetected in this study, cyanobacteria were the major contributor of Zea. Compared to bulk waters, phytoneuston in the SML were exposed to much higher temperatures and UV radiation (Cunliffe., 2011; Mohlin., 2012; Miranda., 2018). Cyanobacteria are highly resistant to high temperatures and UV radiation (Mohlin., 2012; Paerl and Paul, 2012). Therefore,the SML provides a favorable habitat for the cyanobacterial community, which can withstand the radiation and high temperature. Higher abundances of cyanobacteria, such asspp. andspp., have been detected in the SML from Daya Bay (Jiang., 2016; Yue., 2018) and the worldwide sea areas (Engel and Galgani, 2016; Wurl., 2016, 2018).
Both concentrations and proportions of Fuco increased greatly after nutrient addition (Figs.4-5). Further, the ratios of DiatDPincreased to more than 0.9 after nutrient addition (Table 5). These results suggested that diatoms became the major contributors of phytoneuston after nutrient additions. Silicate was added in all test groups of the experiments, and sufficient Si accelerated the growth of diatoms. Filed surveys and mesocosm experiments have suggested that diatoms usually dominate in nutrient- rich and low-temperature conditions such as upwelling and coastal waters (Loureiro., 2011; Mendes., 2011). By comparison, cyanobacteria are abundant in high temperature and nutrient-deficient waters (Bouman., 2003; Roy., 2006). Therefore, the high nutrient levels, sufficient Si, together with the decreased irradiance resulted in the shift of the phytoneuston community from the cyanobacteria-dominant to the diatom-do- minant during the AGP test in this study. However, Si loading decreased significantly as the construction of dams and many coastal sea areas have been experiencing Si limitation (Conley., 2008; Barroso., 2016). Si limitation cooperating with global warming and high UV radiation will result in changes to phytoplankton community structures, from diatom-dominance to the dominance of nondiatoms such as cyanobacteria (Wang., 2014). Six organic N groups (Uric, Urea, Thr, Ala, Glu, Gly), and four organic P groups (GYP, G6P, ATP, AMP) were used for the experiments. Concentrations of Chlincreased significantly after adding inorganic or organic nutrients (<0.05 or 0.01). The results suggested that phytoneuston in the SML had the ability to utilize wide forms of organic N and P, and nutrient enrichment enhanced the growth of phytoneuston. Furthermore, phytoneuston communities were comparable after nutrient addition, indicating that the organic nutrients have no special character influencing the growth of phytoneuston. The SML is a unique environment in which organic matter and nutrients are generally enriched (Wurl., 2016). The capacity of phytoneuston to utilize organic nutrients accelerates their growth in the SML. It is well known that flagellates such as dinoflagellates and rapidophytes have high ability to use organic nutrients (Wang., 2011). However, diatoms dominated after the nutrient addition in this study, and utilized considerable amounts of organic N and P.

Fig.7 Changes in phosphorus concentration in the cultures. A-C, Experiment in September; D-F, Experiment in October; G-I, Experiment in December; A, D, G, Concentrations of DIP; B, E, H, Concentrations of DOP; C, F, I, Concentrations of DTP.

Fig.8 Changes in silica concentration in the cultures. A, Experiment in September; B, Experiment in October; C, Experiment in December.
Concentrations of PPC and ratios of PPCTPwere higher in September and October than in December (Table 5) due to the decrease of water temperature and irradiance. However, the PSC and PSCTPratios increased sharply after nutrient additions, which suggests that the algal cells increase the proportion of photosynthetic pigments to improve the efficiency of photosynthesis with high nutrient concentration and suitable temperature and illuminance conditions. Unlike in the underlying waters, organisms in the SML receive maximal UV radiation, which has the potential to cause direct DNA damage or indirect damagethe formation of destructive intermediates such as reactive oxygen species (Santos., 2014). PPCs protect phytoplankton cell from the stresses of high irradiance and temperature in the SML. However, as the nutrient concentrations increased and irradiance decreas- ed during the AGP test, phytoplankton cells need more PSCs for photosynthesis to enhance the productivity. Various field studies have demonstrated that PPCs predominate in surface waters at tropical areas with high irradiance and temperatures and low chlorophyll, while PSCs are prominent in low temperature and irradiance conditions as well in eutrophic high-productivity waters (Lutz., 2003; Barlow., 2007; Naik., 2011).
The SML samples in this study were collected from three periods with different environmental conditions, such as water temperature, salinity, and light intensity (Table 2). The AGP tests were conducted in batch cultures in the same conditions to avoid the effects of different environmental conditions on the growth of phytoneuston. The temperature (25℃) and salinity (32) designed in this study were the annual average levels in Daya Bay (Wang., 2009); however, the designed light intensity (100 µmol photons m−2s−1) was extremely low in comparison to the light conditions measured in situ (Table 2). Phytoneuston in the SML samples was acclimated to the new conditions for six hours before the experiment in this study. Therefore, the results of this study indicated not only the responses of phytoneuston exposed to changes in nutrients but also its responses to changes in temperature, salinity, and particularly the decreased light intensity. The results suggested that nutrient enrichment together with the decrease of light intensity resulted in the change of the phytoneuston community structure from cyanobacteria-dominance to diatom-dominance.
4 Conclusions
The SML samples were collected over three months from Daya Bay, the South China Sea, and phytopigments were analyzed by HPLC in this study. The high proportions of Zea suggested that the phytoneuston community was dominated by cyanobacteria during the warm season. However, diatoms predominated in December when the water temperature decreased to less than 20℃. Phytoneuston can utilize wide forms of organic N and P, and showed comparable community structure after adding organic and inorganic nutrients. Nutrient enrichment increased the concentration of PSC to improve the efficiency of photosynthesis, and thus enhanced the growth of phytoneuston. The sharp increase of Fuco suggested that the diatoms became the major contributors of phytoneuston after the addition of nutrients. However, contributions of the cyanobacteria diagnostic pigment Zea and ratios of ProkDPdecreased greatly after nutrient addition. Nutrient enrichment, sufficient Si, and the decreased irradiance resulted in the shift of the phytoneuston community from cyanobacteria-dominant to diatom-dominant in this study.
Acknowledgement
This study was supported by the Science & TechnologyBasic Resources Investigation Program of China (No. 2018FY100200).
Barlow, R., Stuart, V., Lutz, V., Sessions, H., Sathyendranath, S., Platt, T., Kyewalyanga, M., Clementson, L., Fukasawa, M., Watanabe, S., and Devred, E., 2007. Seasonal pigment patterns of surface phytoplankton in the subtropical Southern Hemisphere., 54: 1687-1703.
Barroso, H. D., Becker, H., and Melo, V. M. M., 2016. Influence of river discharge on phytoplankton structure and nutrient concentrations in four tropical semiarid estuaries., 64: 37-48.
Böttjer, D., and Morales, C. E., 2007. Nanoplanktonic assemblages in the upwelling area off Concepción (~36˚S), central Chile: Abundance, biomass, and grazing potential during the annual cycle., 75: 415-434.
Bouman, H., Platt, T., Sathyendranath, S., Li, W. K. W., Stuart, V., Fuentes-Yaco, C., Maass, H., Horne, E. P. W., Ulloa, O., Lutz, V., and Kyewalyanga, M., 2003. Temperature as indicator of optical properties and community structure of marine phytoplankton: Implications for remote sensing., 258: 19-30.
Conley, D. J., Humborg, C., and Smedberg, E. 2008. Past, present and future state of the biogeochemical Si cycle in the Baltic Sea., 73: 338-346.
Cunliffe, M., Engel, A., Frka, S., Gašparović, B., Guitart, C., Murrell, J. C., Salter, M., Stolle, C., Upstill-Goddard, R., and Wurl O., 2013. Sea surface microlayers: A unified physicochemical and biological perspective of the air-ocean interface., 109: 104-116.
Cunliffe, M., Upstill-Goddard, R. C., and Murrell, J. C., 2011. Microbiology of aquatic surface microlayers., 35: 233-246.
Engel, A., and Galgani, L., 2016. The organic sea-surface microlayer in the upwelling region off the coast of Peru and potential implications for air-sea exchange processes., 13: 989-1007.
Guillard, R. R. L., 1975. Culture of phytoplankton for feeding marine invertebrates. In:. Smith, W. L., and Chanley, M. H., eds., Plenum Press, New York, 26-60.
Jiang, T., Chen, F., Yu, Z., Lu, L., and Wang, Z., 2016. Size- dependent depletion and community disturbance of phytoplankton under intensive oyster mariculture based on HPLC pigment analysis in Daya Bay, South China Sea., 219: 804-814.
Loureiro, S., Reñé, A., Garcés, E., Camp, J., and Vaqué, D., 2011. Harmful algal blooms (HABs), dissolved organic matter (DOM), and planktonic microbial community dynamics at a near-shore and a harbour station influenced by upwelling (SW Iberian Peninsula)., 65: 401- 413.
Lutz, V. A., Sathyendranath, S., Head, E. J. H., and Li, W. K. W., 2003. Variability in pigment composition and optical characteristics of phytoplankton in the Labrador Sea and the Central North Atlantic.–, 260: 1-18.
Maar, M., Markager, S., Madsen, K. S., Windolf, J., Lyngsgaard, M. M., Andersen, H. E., and Moller, E. F., 2016. The importance of localexternal nutrient loads for Chland primary production in the western Baltic Sea., 320: 258-272.
Mendes, C. R., Sá, C., Vitorino, J., Borges, C., Garcia, V. M. T., and Brotas, V., 2011. Spatial distribution of phytoplankton assemblages in the Nazaré submarine canyon region (Portugal): HPLC-CHEMTAX approach., 87: 90-101.
Miranda, M. L., Mustaffa, N. I. H., Robinson, T. B., Stolle, C., Ribas-Ribas, M., Wurl, O., and Zielinski, O., 2018. Influence of solar radiation on biogeochemical parameters and fluores- cent dissolved organic matter (FDOM) in the sea surface microlayer of the southern coastal North Sea.–,6: 15.
Mohlin, M., Roleda, M. Y., Pattanaik, B., Tenne, S. J., and Wulff, A., 2012. Interspecific resource competition-combined effects of radiation and nutrient limitation on two diazotrophic filamentous Cyanobacteria., 63: 736- 750.
Montes-Hugo, M. A., and Alvarez-Borrego, S., 2007. Differences in photosynthetic pigment signatures between phytoneuston and phytoplankton communities in a coastal lagoon of Baja California., 151: 1225-1236.
Naik, R. K., Anil, A. C., Narale, D. D., Chitari, R. R., and Kulkarni, V. V., 2011. Primary description of surface water phy- toplankton pigment patterns in the Bay of Bengal., 65: 435-441.
Paerl, H. W., and Paul, W. J., 2012. Climate change: Links to global expansion of harmful Cyanobacteria., 46: 1349-1363.
Reinthaler, T., Sintes, E., and Herndl, G. J., 2008. Dissolved organic matter and bacterial production and respiration in the sea-surface microlayer of the open Atlantic and the western Mediterranean Sea., 53: 122- 136.
Roy, S., Llewellyn, C. A., Egeland, E., and Johnsen, G., 2011.. Cambridge University Press, Cambridge, 10-22.
Roy, R., Pratihary, A., Mangesh, G., and Naqvi, S. W. A., 2006. Spatial variation of phytoplankton pigments along the southwest coast of India., 69: 189-195.
Santos, A. L., Baptista, I., Gomes, N. C. M., Henriques, I., Almeida, A., Correia, A., and Cunha, A., 2014. Contribution of chemical water properties to the differential responses of bacterioneuston and bacterioplankton to ultraviolet-B radiation.,87: 517-535.
Strickland, J. D., and Parsons, T. R., 1972.. Fisheries Research Board of Canada, Ottawa, 1-35.
Wang, Z. H., Liang, Y., and Kang, W., 2011. Utilization of dissolved organic phosphorus by different groups of phytoplankton taxa., 12: 113-118.
Wang, Z., Song, S., and Qi, Y., 2014. A comparative study of phytoneuston and the phytoplankton community structure in Daya Bay, South China Sea., 85: 474-482.
Wang, Z., Zhao, J., Zhang, Y., and Cao, Y., 2009. Phytoplank- ton community structure and environmental parameters in
aquaculture areas of Daya Bay, South China Sea.–, 21: 1268-1275.
Wright, S. W., and Jeffrey, S. W., 2006. Pigment markers for phytoplankton production. In:. The Handbook Environmental Chemistry. Volkman, J. K., ed., Springer, Heidelberg, Vol. 2N, 71-104.
Wurl, O., Bird, K., Cunliffe, M., Landing, W. M., Miller, U., Mustaffa, N. I. H., Ribas-Ribas, M., Witte, C., and Zappa, C. J., 2018. Warming and inhibition of salinization at the ocean’s surface by cyanobacteria., 45: 4230-4237.
Wurl, O., and Holmes, M., 2008. The gelatinous nature of the sea- surface microlayer., 110: 89-97.
Wurl, O., Stolle, C., Thuoc, C. V., Thu, P. T., and Mari, X., 2016. Biofilm-like properties of the sea surface and predicted effects on air-sea CO2exchange., 144: 15-24.
Xiong, Y. J., 2014. Comparative studies on phytoplankton com- munity structure between surface microlayer and subsurface water in Daya Bay. Master thesis. Jinan University, Guangzhou.
Yang, G. P., Levasseur, M., Michaud, S., Merzouk, A., Lizotte, M., and Scarratt, M., 2009. Distribution of dimethylsulfide and dimethylsulfoniopropionate and its relation with phyto- neuston in the surface microlayer of the western North Atlantic during summer., 94: 243-254.
Yang, X., 2015. Studies on effects of nitrogen and phosphorus on phytoplankton from the surface microlayer (SML). Master thesis. Jinan University, Guangzhou.
Yue, W. Z., Sun, C. C., Shi, P., Engel, A., Wang, Y. S., and He, W. H., 2018. Effect of temperature on the accumulation of marine biogenic gels in the surface microlayer near the outlet of nuclear power plants and adjacent areas in the Daya Bay, China., 13 (6): e0198735.
Zäncker, B., Bracher, A., Rottgers, R., and Engel, A., 2017. Variations of the organic matter composition in the sea surface microlayer: A comparison between open ocean, coastal, and upwelling sites off the Peruvian coast., 8: 2369.
Zapata, M., Rodríguez, F., and Garrido, J. L., 2000. Separation of chlorophylls and carotenoids from marine phytoplankton, a new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases.–, 195: 29-45.
. E-mail:twzh@jnu.edu.cn
July 30, 2019;
October 14, 2019;
February 2, 2020
(Edited by Ji Dechun)
杂志排行
Journal of Ocean University of China的其它文章
- Dynamic Diurnal Changes in Green Algae Biomass in the Southern Yellow Sea Based on GOCI Images
- Nutrient Enrichment Regulates the Growth and Physiological Responses of Saccharina japonica to Ocean Acidification
- Isolation of Enterococcus faecium with Feeding Attractant Function from Pacific White Shrimp (Litopenaeus vannamei) Intestine
- Taxonomy and Phylogeny of a New Marine Planktonic Ciliate, Strombidium pseudorapulum sp. n.(Protozoa, Ciliophora, Oligotrichia)
- Reducing the Common Environmental Effect on Litopenaeus vannamei Body Weight by Rearing Communally at Early Developmental Stages and Using a Reconstructed Pedigree
- Can Langmuir Circulations Solve the Problem of Insufficient Upper-Ocean Mixing?
