Abnormal Karyotype of Pen Shell (Atrina pectinata) During Its Early Embryonic Development in Late Breeding Season
2020-09-27ZHOULiqingWANGXuemeiYANGAiguoWUBiaoSUNXiujunLIUZhihongCHENSiqingandZHAODan
ZHOU Liqing, WANG Xuemei, YANG Aiguo, WU Biao, SUN Xiujun,LIU Zhihong, CHEN Siqing, and ZHAO Dan
Abnormal Karyotype of Pen Shell () During Its Early Embryonic Development in Late Breeding Season
ZHOU Liqing1), 2), 4), WANG Xuemei3), YANG Aiguo1), 2), *, WU Biao1), 2), SUN Xiujun1), 2),LIU Zhihong1), 2), CHEN Siqing1), 2), and ZHAO Dan1), 4)
1),,,266071,2),,266237,3),276826,4),,201306,
The pen shell,, distributes globally. It is one of the most important edible bivalves in east Asian countries. However,there are multiple difficulties in rearing pen shell larvae and juveniles because of their high mortality.To understand the mechanism underlining such high mortality at the early embryonic development stage,we obtained approximately 100 million larvae during later breeding season in June, 2017, andchecked a large amount of mitotic chromosomal plates of the early embryos and post-spawning gonads tissue slices of their parents. The results showed that most diploidembryos have 17 pairs of chromosomes (2=34) as their parents do. The first pair of particularly large chromosomes are heterotypic in some diploid embryoswhile they are homomorphic in others. The primary sex-determination chromosome type is XX/XY. A lot of triploid, pentaploid and aneuploid embryos with different numbers of the largest homomorphic or heteromorphic chromosomes were found due to the degeneration of overmatured parent gonads which hold normal karyotype.These larvae will dieeven though most of them may develop into the trochophore stage with 34 chromosomes. Genetic deficiency of chromosomes will cause a high rate of mortality in early embryos in late breeding season. These findings should enrich the current knowledge of juvenile pen shell aquaculture.
; early embryo; sex chromosome; nuclear ploidy; later breeding season
1 Introduction
The pen shell,, is a large size bivalve that lives naturally in small clusters in the muddy or sandy sediment of tidal flats and subtidal zones. It widely distri- butes in the Indo-West Pacific from southeastern Africa to Melanesia and New Zealand, north to Japan and south to New South Wales.exhibits extensive morpho- logical variations and a rich genetic diversity (Liu., 2011).is one of the important edible bivalves in east Asian countries including China, Japan, Korea among others. However, the standing stock of the species has been dramatically reducing in recent years due to reckless overharvesting (Chung., 2006). Meanwhile, there are multiple difficulties in rearinglarvae and juveniles due to their death by suffocation (Ohashi., 2008).The main sources of pen shell cultures are from na- tural spat collection (Son., 2005). The spawning of this species takes place once a year from June to August whileits spawning concentrates in a short period from June to July when the seawater temperature is around 20℃. Its spawning season sustains from June to August in the Bor- yeong coastal waters of Korea (Chung., 2006); how- ever, in the coastal waters of Rizhao, Shandong, China, June is the later breeding time.
Several previous studies focused on the reproduction, physiology and genetic diversity of. Some re- searchers have tried to understand the mass mortality of pen shell by observing the histology and nutritional conditions (Yurimoto., 2008) and investigating the density-dependent growth and survival rates (Kim., 2008), little is known about its karyotypic variation. The oocyte diameter, early and late apoptosis, embryo development and chromosomal ploidy of blastocysts can be used to assess the parameters of karyotypic variation of prepubertal goat oocytes with different follicle diameters (Romaguera., 2010) and compare the effectiveness of different treatments on the activation and subsequent development of bovine oocytes (Wang., 2008). Therefore, the knowledge of the karyotype of this species will provide information necessary for resolving the high mor- tality at the early embryonic development, juvenile settle- ment and metamorphosis stages, which may help to improve the survival oflarvae and juveniles.
In our earlier studies, we have found that the primary sex-determination type of this species is XX/XY. In the present study, we analyzed the karyotypes ofparents and their early embryos in late breeding season.Our aim was todetermine whether the abnormal karyotype of early embryos caused high mortality.Our findings should enrich the current knowledge of juvenile shellfish aquaculture.
2 Materials and Methods
2.1 Sample Collection, Phenotype Measurement and Pretreatment
All early embryos and their parents were obtained from Rizhao Ocean and Fisheries Research Institute. These pa- rents matured naturally and were induced to spawn with air-drying method.Approximately 100 million larvae were obtained in later breeding season in June, 2017, and seven male and five femalewere selected randomly from parent population. The average adult shell length was 21.77cm±3.72cm and average body weight was 291.46g±53.06g. Early embryos were at blastula stage (5h after insemination) and trochophore stage (20h after insemination). The slides were washed in a strong acid lotion and then immersed in pure ethanol. The post-spawningadult pen shell was placed on corner ground and shaded for 10–30min with their small front of shell leaning against wall.The shell wide back end will actively open. The gill tissue was then clipped quickly to generate a wound which will yield a mass group of proliferating cells at the metaphase stage of mitosis. Thirty minutes later, the animal was placed back into rearing water.
2.2 Gonad Histological Analysis
Five female and five male parents were newly selected for gonad histological analysis.Their gonads were fixed immediately in Bouin’s solution upon spawning. Gonad tissue sections were stained with hematoxylin and eosinto identify the state of gonad development and gametogenesis under a Leica Microsystem (TYPE DM4000B; Leica Microsystems Wetzlar GmbH, Wetzlar, Germany).
2.3 Karyotype Slide Preparation
The gill proliferating cells and early embryos enriched with sieve cloth were immediately placed into 0.04% col- chicine containing 50% sterile seawater for about 35min. All cells and embryos were hypotony treated in 0.075molL−1potassium chloride at room temperature (23℃±3℃) for 45min and transferred to pre-cooled Carnoy’s fixer (3 methanol:1 acetic acid) with the fixer changed every 15min. The fixed tissue was dissociated by 50% acetic acid. Dissociated suspension were spattered onto clean hot slides at approximately 55℃, 3 drops each, to prepare the meta- phase karyotypes. The slides were air-dried, stained with 10% Giemsa solution in phosphate buffer, pH 6.8, for 30min and rinsed with distilled water.
2.4 Nuclear Ploidy Analysis
Photomicrographs of metaphase plates were obtained using a Leica Microsystem (TYPE DM4000B; Leica Microsystems Wetzlar GmbH, Wetzlar, Germany). Each plate, 15–30 metaphases were analyzed to determine the mode number of chromosomes. The normal diploid chromosomal morphometries were obtained using Adobe Photoshop CS5 software. For each chromosome, the lengths of short and long arms, average total length, relative length (RL, the percentage of the length of an chromosome to the sum of the total) and arm ratio (AR, the length ratio of long to short arms) were measured and calculated. The chromosomal pairs were classified following the conventions sug- gested by Levan. (1964) into metacentric (m), submetacentric (sm), subtelocentric (st) and telocentric (t) with AR varied between 1 and 1.7, between 1.7 and 3, between 3 and 7 and between 7 and ∞, respectively. The numbers of sex chromosomes and total chromosomes were used to ploidy analysis.
3 Results
3.1 Degeneration of Pparent Gonads in Later Breeding Season
We met success in artificially promoting maturation but failed to inducing spawning in proper breeding season in- door in May, 2017. We fetched the parents matured naturally in sea, induced them to spawn with air-drying me- thod, observed their gonad tissue sections post spawning, and characterized the gonad histology of parents post- spawning in later breeding season (Figs.1 and 2). The orange female gonads looked plump; however, there were still a large number of unspawned eggs in the follicles (Fig.1A). Many oocytes appeared to have a heteronucleus (HN) outside cell nucleus (CN) (Figs.1B, D). Lots of oocytes showed degeneration clues: a condensed cell nucleus and an irregular or lack of nucleolus. These degene- rated oocytes (DO) degraded into fragments gradually (Figs.1C, D). White male gonads showed some transparent blocksalthough they still looked plump and their follicles were full of spermatids (Figs.2A, C, E). Degeneration also appeared in the testes. Degenerated spermatids were clustered in some individuals and mitochondria were rarely seen or not present in spermatids (Figs.2A, B). In some individuals, sperm development fully synchronized with mitochondrion development. However, Sperms lost their flagella and evenly dispersed in follicles (Figs.2C, D). In some individuals, sperm development did not synchronized as the normal sperms did. They did not discharge seminal fluid even when spermiation was induced (Figs. 2E, F).
3.2 Karyotype and Sex Chromosomes of Diploid Parental Pen Shell
A total of 200 sets of well-spread mitotic metaphase chro- mosomes were examined to determine the mode diploid chromosome number (Cn) (Table 1). Fifty metaphases were from blastula and trochophore larval cells; 50 were from ma- ternal gill cells; and 50 were from paternal gill cells. The modal diploid number of chromosomes (2) was 34,which varied between 31 and 92 with a mode of 34. The chromo- some length varied in size between 1.69 and 8.70μm.
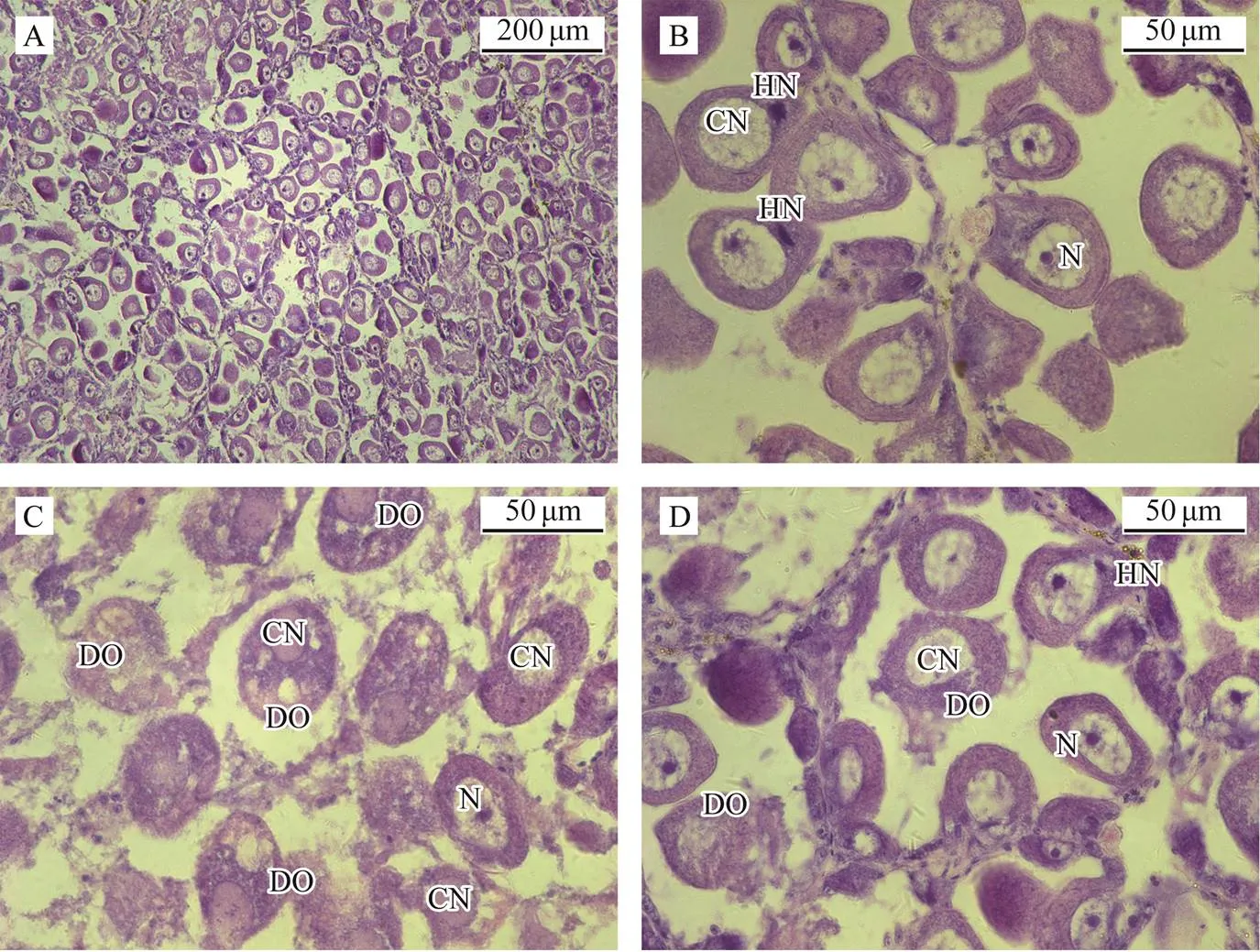
Fig.1 Gonad histological characteristics of maternal clams in the late breeding season. A, A large number of eggs in the gonadfollicles of maternal clams after spawning, ×100; B, Heteronuclear (HN) outside the cell nucleus (CN) in some eggs remaining in follicles, ×400; C, Degenerated oocytes (DO) degraded into fragments gradually, ×400; D, Different egg degradation states in the same follicle, ×400. HN, heteronuclear cell; N, nucleolus; CN, cell nucleus; DO, degenerated oocyte.
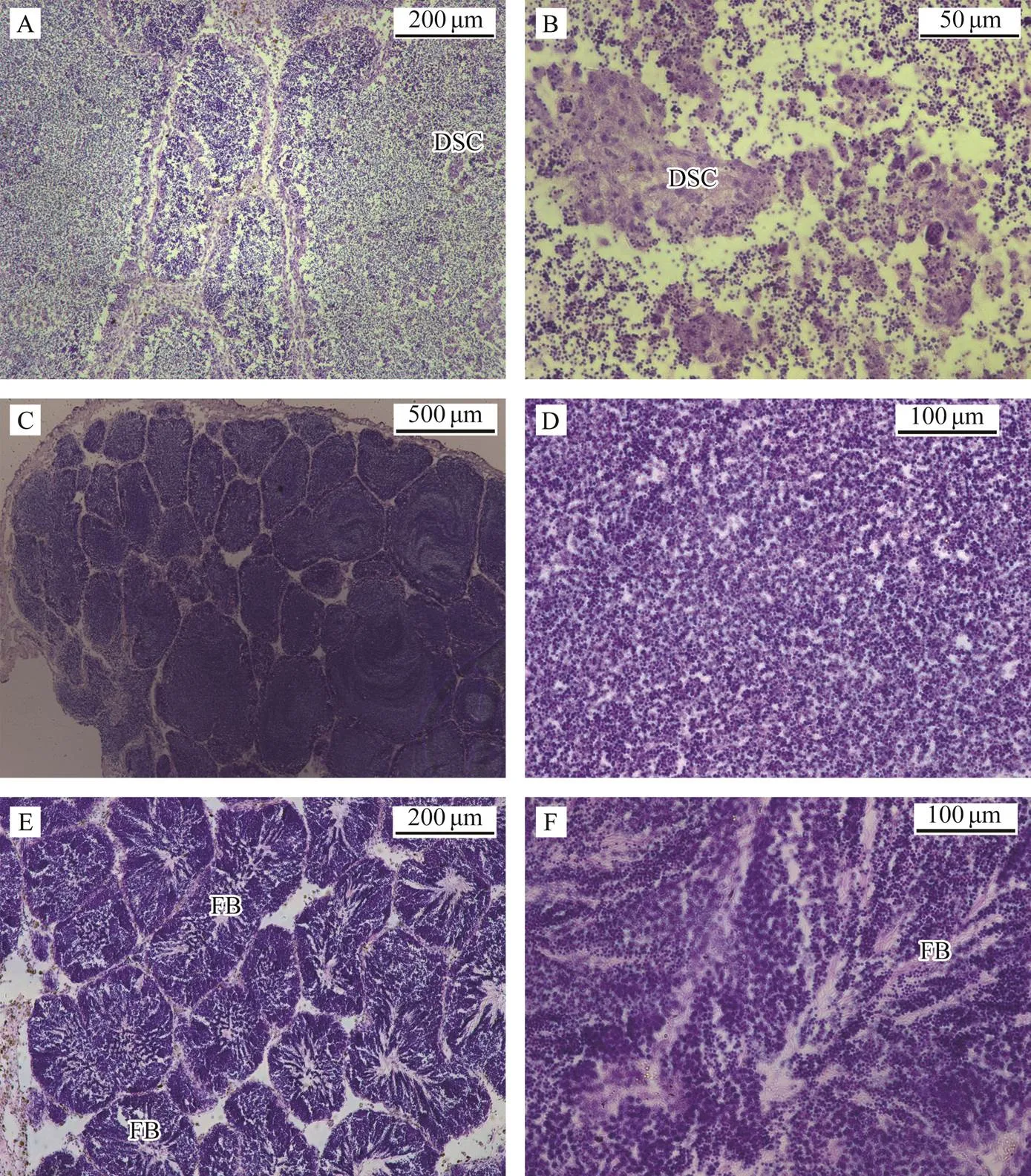
Fig.2 Gonad histological characteristics of paternal clams in the late breeding season. A, Degenerated spermatids were clustered in some follicles, the morphology of mitochondrion and flagellum of sperm is not clear, ×100; B, Degenerated spermatids were clustered in some follicles, the morphology of mitochondrion and flagellum of sperm is not clear, ×400; C, Sperms developed fully-synchronized with mitochondria but without flagella, ×40; D, Sperms developed fully-syn- chronized with mitochondria but without flagella, ×200; E, Sperm development was not synchronized and bundles of flagella of mature sperms was in the center of follicles, ×100; F, Sperm development was not synchronized and bundles of flagella of mature sperms was in the center of follicles, ×200. DSC, degenerated spermatid cluster; FB, flagella bunch.
The well-spread metaphase plate, and the karyogram of the 34 chromosomes from a maternal gill cell with the larg- est pair of homomorphic chromosomes are shown in Figs. 3A and B. The well-spread metaphase plate and the kar- yogram of the 34 chromosomes from a paternal gill cell with the largest pair of heteromorphic chromosomes are shown in Figs.3C and D. The metaphase plate and the kar- yogram from a trochophore-stage cell with the largest pair of homomorphic chromosomes are show in Figs.4A and C. The metaphase plate and the karyogram from another trochophore-stage cell with the largest pair of heteromor- phic chromosomes are shown in Figs.4B and D. There is a primary XX/XY type of sex-determination in, and the largest pair of chromosomes present a lower-level sex differentiation; they were heterotypic in male somatic cells, but the difference between X and Y chromosomes was sometimes not obvious. The 14th pair of chromosomes were metacentric in male but telocentric in female metaphase somatic cells; therefore, the primary sex-deter- mination mechanism manifests as an XX/XY type which we have found before (Zhou., 2018).
Both male and female metaphase chromosomes have almost the same sorting order; the male karyogram consisted of four m, five sm and eight st pairs while the female karyogram consisted of three m, five sm, and nine st pairs. The 14th pair was m in male and st in female me- taphase cells. The result is consistent with our previous results (Zhou., 2018).
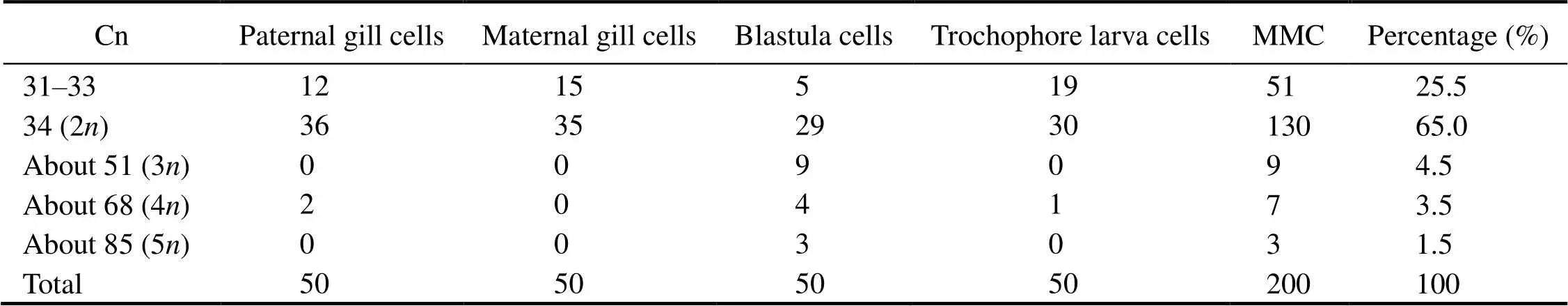
Table 1 Mitotic metaphase cell number, chromosome number and percentageofAtrina pectinata
Notes: MMC, meiotic metaphase cells; Cn, chromosome number. Modal diploid chromosome number (2)=34.
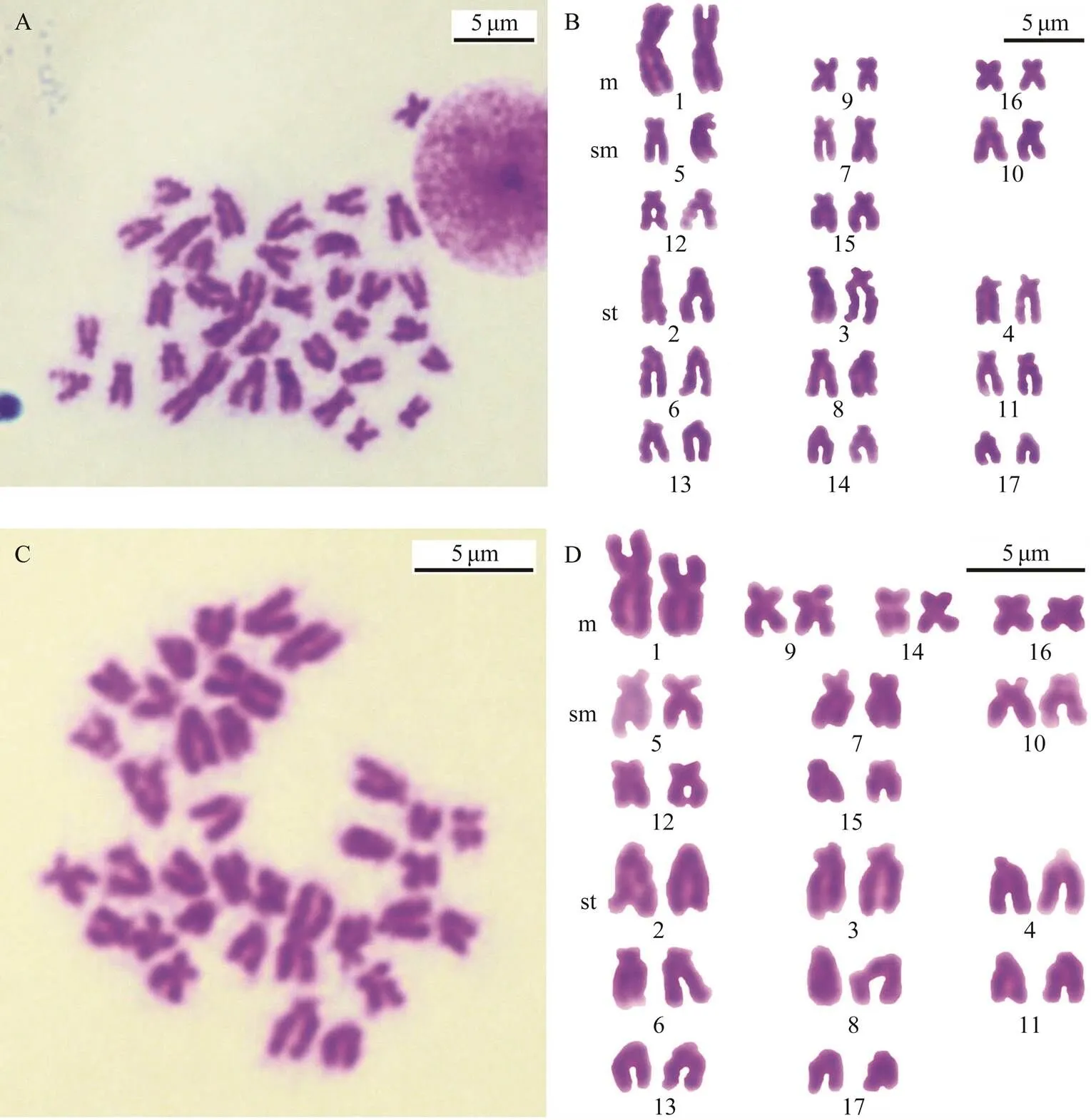
Fig.3 The metaphase chromosome and karyotype of female and male Atrina pectinata. A, B, The metaphase chromosomes and karyotype of female A. pectinata. C, D, Metaphase chromosomes and karyotype of male A. pectinata. Scale indicates 5μm.
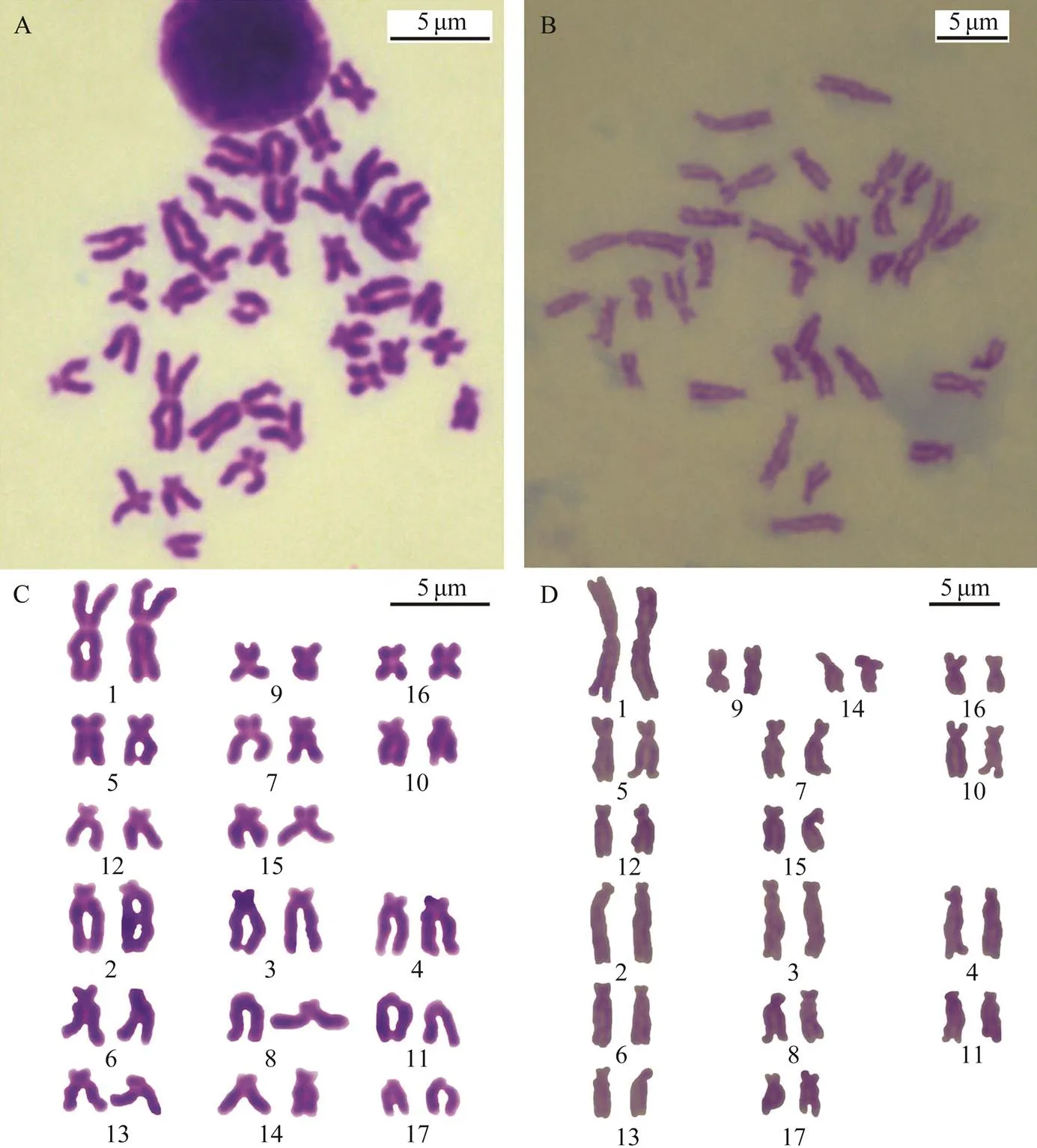
Fig.4 The metaphase chromosome and karyotype of trochophore-stage Atrina pectinata. A, C, Metaphase chromosomes and karyotype of trochophore-stage cells with the largest pairs of homomorphic chromosomes; B, D, Metaphase chromosomes and karyotype of a trochophore-stage cell with the largest pairs of heteromorphic chromosomes. Scale indicates 5μm.
3.3 Ploidy of Early Embryos
By using the largest pair of chromosomes as the classification standard, various multiple-ploidy embryos were found at the blastula stage, which included triploidy with three of the largest homomorphic or heteromorphic chromo- somes (Fig.5), pentaploidy with five of the largest homo- morphic or heteromorphic chromosomes (Fig.6) and aneu- ploidy with different numbers of the largest homomorphic or heteromorphic chromosomes (Fig.7). At the trochophore stage, the 34 chromosomes can be distinguished as female and male groupsthe largest pair of chromosomes length;only few abnormal embryo cells, such as 34 chromosomes together with three of the largest chromosomes, were obser- ved (Fig.8). Ten with the largest pairs of homomorphic chro- mosomes and 10 with the largest pairs of heteromorphic chromosome trochophore-stage metaphase cells were usedfor karyotype analysis. The chromosome set was similar to that of their parents. The two largest chromosomes were also homomorphic X/X and heteromorphic X/Y (Fig.4, Table 2).
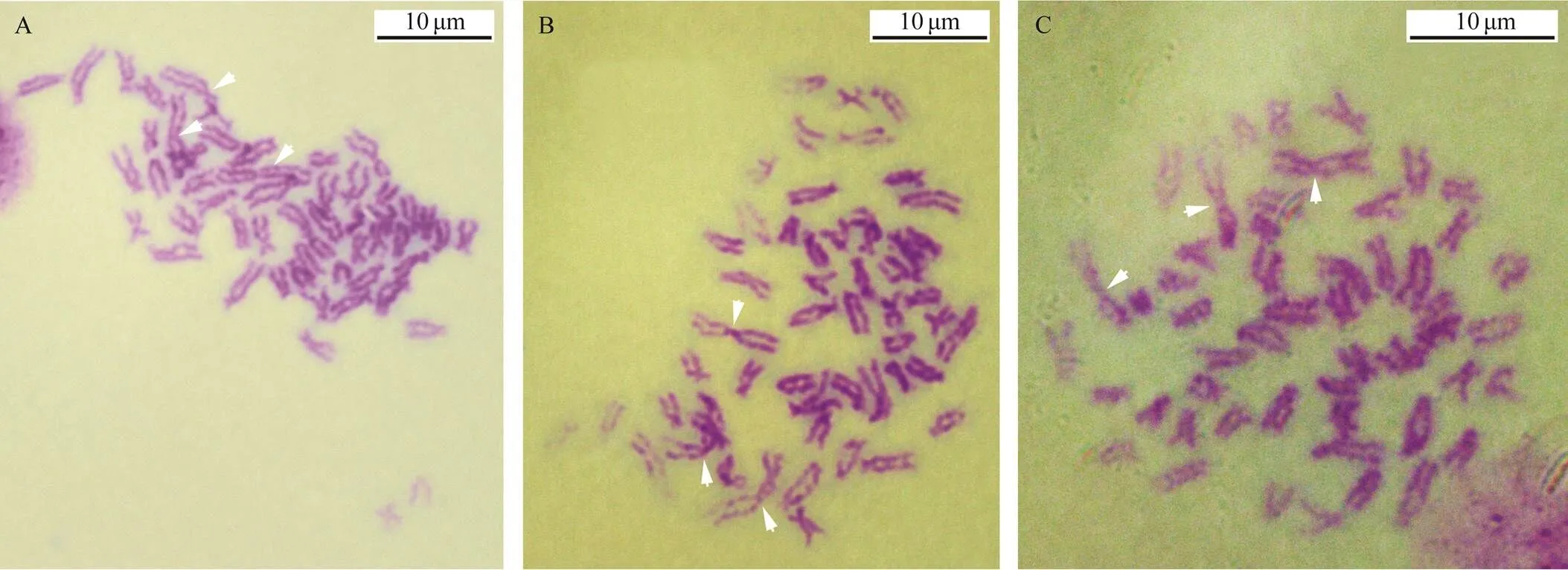
Fig.5 Three triploid metaphase chromosomes at the blastula stage. 3n=51. Arrows show the largest chromosomes. Scale indicates 10μm.
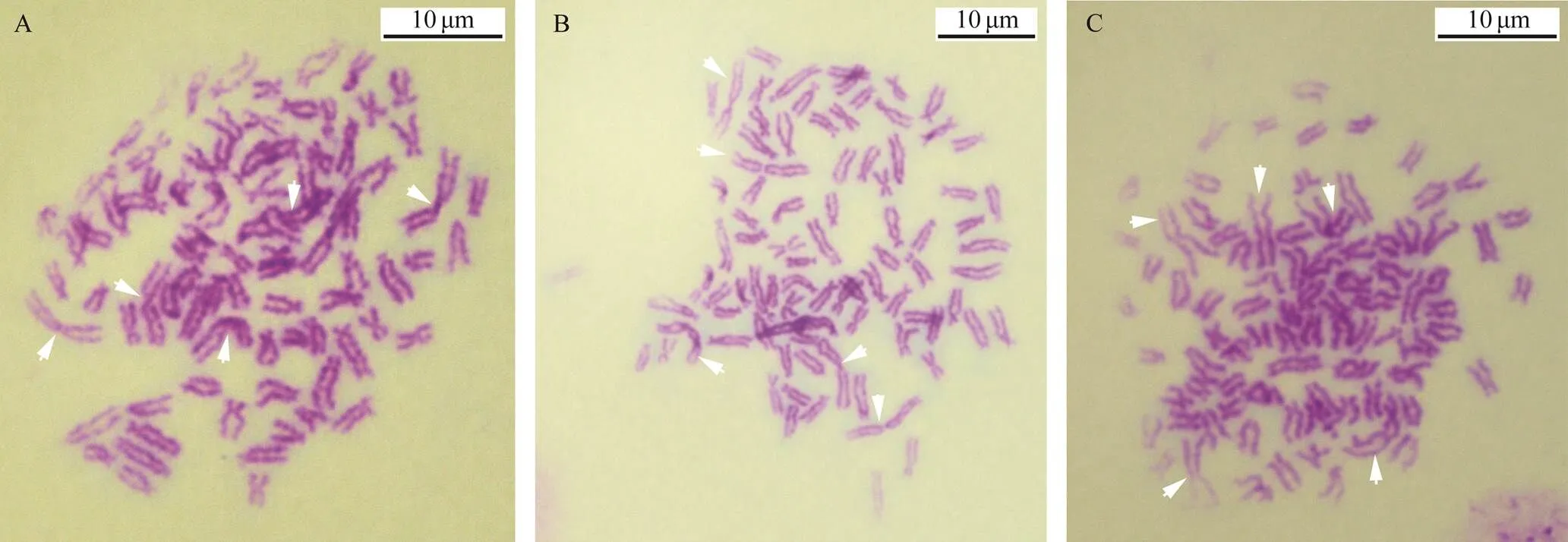
Fig.6 Three triploid metaphase chromosomes at the blastula stage. 5n=85. Arrows show the largest chromosomes. Scale in- dicates 10μm.
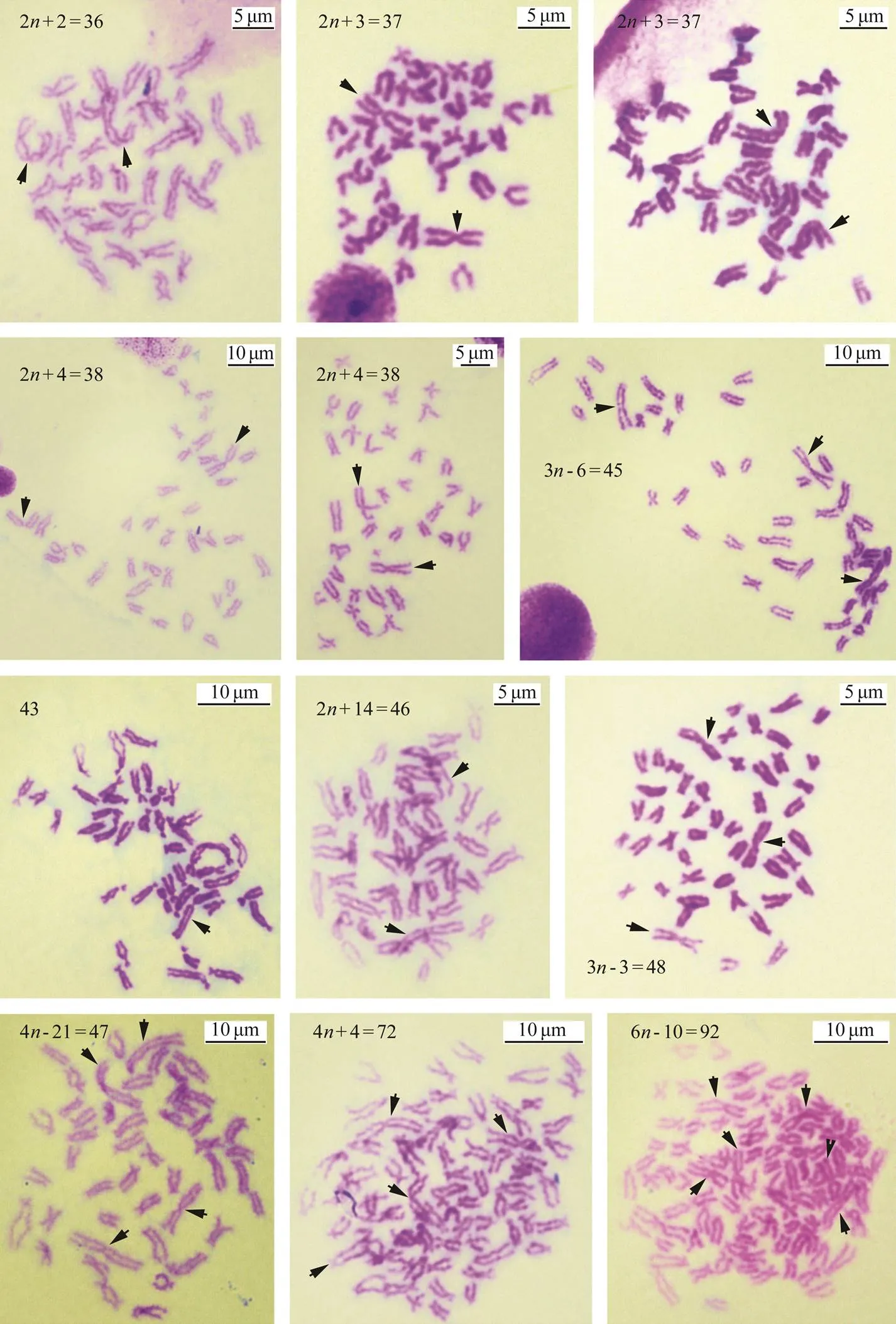
Fig.7 Aneuploid metaphase chromosomes at the blastula stage. Arrows show the largest chromosomes. Scale indicates 5μm or 10μm.
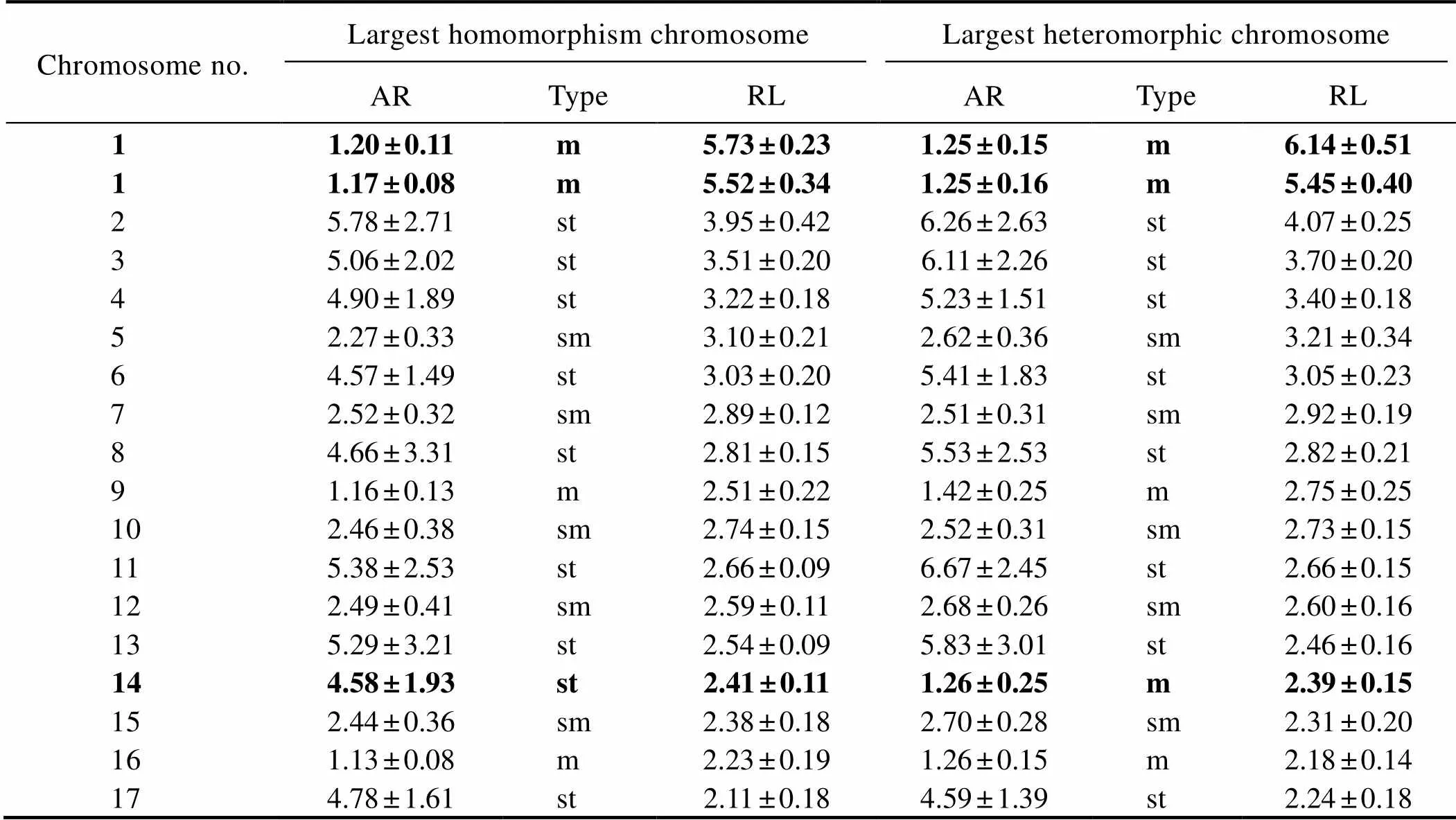
Table 2 Relative length and arm ratio of metaphase chromosomes of trochophore-stage A.pectinata
Notes: m, metacentric; sm, submetacentric; and st, subtelocentric.
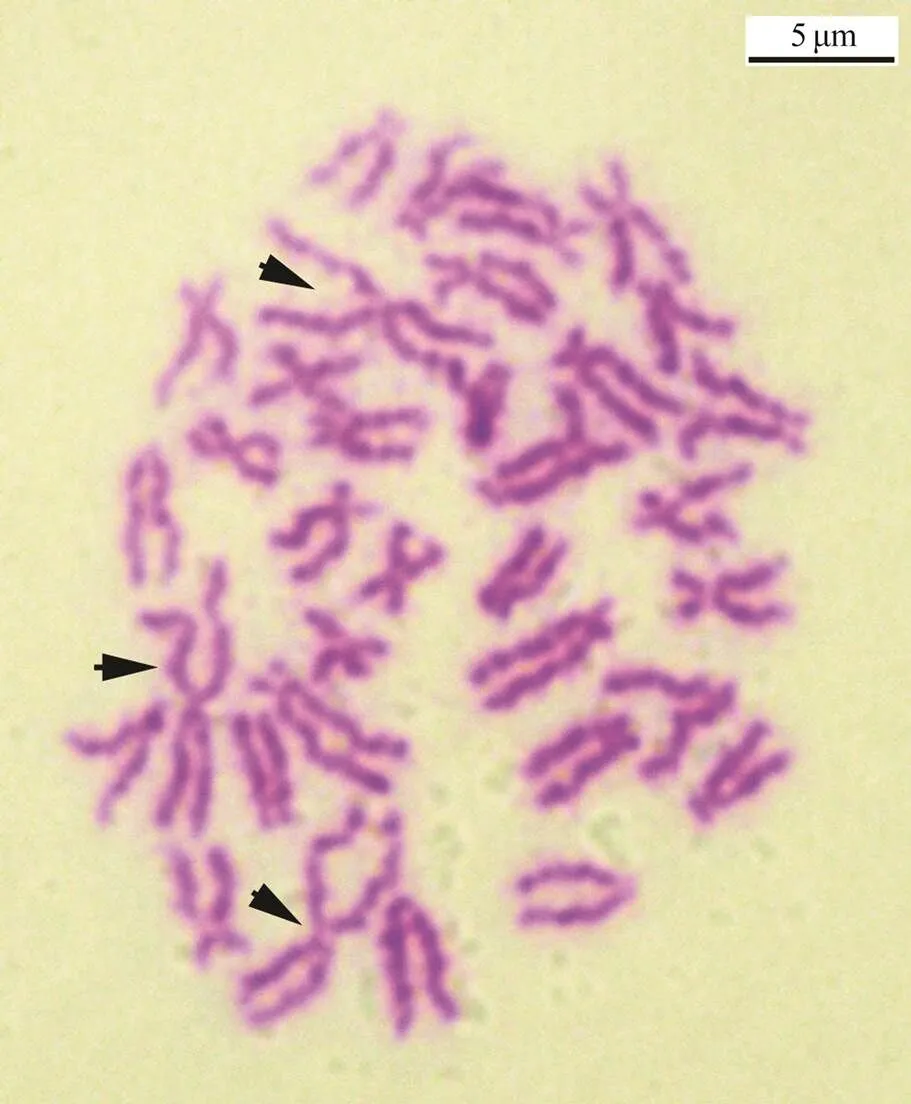
Fig.8 An abnormal chromosome set of trochophore-stage cells with 34 chromosomes but three of the largest chromosomes in one metaphase cell. Arrows show the largest chromosomes. Scale indicates 5μm.
3.4 Death of Early Embryos in the Later Breeding Season
Owing to the degeneration of overmature gonads, the eggs and sperms may have subtle genetic deficient in qual- ity although they fertilized and developed successfully at the beginning time. The embryo development and chromosomal ploidy of the blastula stage was abnormal. A large number of deformative and asynchronous embryos died and degraded on the first hatching day till trocho- phore-stage and all embryos died and degraded at the D- shaped larval stage 2 days after insemination.
4 Discussion
All the observedin this study belong to the same geographical group. Their genetic diversity should be low; however, there were many ploidy and abnormal embryos that eventually died, especially early embryos in their later breeding season. It was presumed that the over- mature gametes underwent degenerationapoptosis or programmed cell death (PCD). The cattle embryo cleavage rates were clearly influenced by the oocyte quality, and the apoptosis happened regardless of the oocyte quality to maintain tissue homeostasis (Bakri., 2016). As an experimental model,has a diapause process during embryo development because of apoptosis, which is induced to resist adverse conditions such as high salinity, low temperature and anoxic conditions (Zhang., 2017). Shellfish species, the calyptraeid gastropodsand,produce viable and nutritive embryos, where nutritive embryos arrest their development after gastrulation by apoptosis and are ingested by their viable developing siblings as a form of intracapsular nutrition. Therefore, the role of MAPK (mitogen-activated protein kinase) signaling and apoptosis were examined as potential players in the early developmental switch between viable and nutritive embryos (Lesoway., 2017). Embryos of the freshwater prawnwere found to be able to activate the cell function to protect against environmental changes such as UVB (ultraviolet radiation b) (Schramm., 2017). Overall, the degeneration of parent gonads and de- formative embryos werepossibly underlined by apoptosis.We presumed thatdid not propagate naturally in an inappropriate season to ensure the fitness and survival of their descendants in the most economical way.
The number of goat embryos developing to term was lower in adolescent than in adult oocytesafter they were transfered to receptor females.It is presumed that those deficiencies could be attributed to cytoplasmic, ultrastruc- tural, metabolic and/or chromosomal abnormalities in oo- cytes and embryos from adolescent females (Romaguera., 2010). According to our results, in the late breeding season, degeneration took place in the gonads of overmature parents as well as in early embryos. Degeneration could not produce a strong generation surviving in worse growth conditions such as colder water temperature and a lack of food; otherwise, the fate of spats is still death in the early development stage, which is a waste of resources. Genetic deficiency may take place in overmature oocytes and earlier embryos in our observation such as condensed cell nuclei, irregular or lack of nucleoli in the oocytes, and rich triploid, pentaploid and aneuploidy embryos with different numbers of the largest homomorphic or heteromorphic chromosomes in earlier embryo cells while the chromosome composition in parents were normal diploid. Though overmature eggs and sperms with degeneration can fertilize successfully, the embryo development and chromosomal ploidy of the blastula stage was abnormal. Most embryos died and degraded, and all embryos died and degraded at the D-shaped larvae stage 2 days after insemination. All of them are called abortion spats. The genetic quality of eggs and sperms is vital. Degeneration in the overmature gonad and abnormal chromosome com- position would cause a high mortality rate in early embryos in the later breeding season. The aneuploids Pacific oysterwere produced by 2♀×3♂ (DT group), 3♀×2♂ (TD group), 3♀×3♂ (TT group). These three groups had more abnormal embryos than nor- mal DD group. Their embryo ploidy mainly was 2.5in 12h and 30h, and became 2in 5d. Mass death of larvae with chromosomes pairing disorder was observed (Gong and Zhang, 2003). The survival rate of D-shaped larvae was only 14.7% when gynogenesis diploids were induced by 6-dimethylaminopurine (60mgL−1; 6-DMAP) which disrupted the spindle at mitosis and inhibited chromosome movement, resulting in the abnormal chromosome composition (Yang., 2009). It is important to raise the parental pen shells in advance, and then cultivate seedlings in an appropriate season to ensure the fitness and survival of their descendants.
It is well known that the growth rate will decrease and even stagnate with increasing age, and the proliferation capacity of somatic cells is limited. By adjusting the water temperature of temporary rearing and using the hyperplasia-healing tissue of the gill for chromosomal investigation, we improved the methods of chromosome preparation, and obtained many well-spread mitotic chromosomal plates of, a large-scale bivalve (Zhou., 2018). Few study on bivalve wound hyperplasia- healing tissue for chromosome preparation was found. In one example, A technique was reported for the preparation of metaphase chromosomes from the mantle tissue of adult pearl oyster, in which they cut off the adductor and removed the shell of one side to expose the mantle and visceral mass, and made shearing cutson the mantle to culture hyperplasia tissue. The experimen- tal animal did not survive, and the researchers suggested that young animals could get better results (Shen., 1993). In the present study, it is simple, practical, and re- peatable to prepare chromosomes of adultBy adjusting the temperature in temporary cultures, and using the hyperplasia-healing tissue of the gill wound, the experimental animal can be kept alive and healthy after the procedure.
In this study, two pairs of chromosomes with sex diffe- rence in adults and larvaewere chosen to studyThe two largest chromosomes were female homomorphic as X and X chromosomes and male heteromorphic as X and Y chromosomes, respectively. The male 14th pair chro-mosomes were metacentric and telocentric in female meta- phase somatic cells, but it was not certain whether they weresex differentiated. In most sexually dimorphic animals, the determination of primary sex characteristics is controlled autonomously by sex chromosomes, secondary sex characteristics may be determined by sex chromosomes or alternatively by hormones released from the developing gonad (Twyman, 2002). The results of the induced gynogenetic and triploid analyses indirectly suggested that the dwarf surf clam may have an XX-female, XY-male sex determination with Y-domination (Guo., 1994). How- ever, no more direct information on chromosomes for sex determination in bivalves was found at present.
5 Conclusions
The gonad tissue slices showed the degeneration of eggs and sperms in later breeding season. The largest chromosomes could be used for sex and ploidy identification of embryos. Abnormal chromosome composition and gene- tic deficiency would cause a high mortality rate in early embryos in the late breeding season. These data will enrich the current knowledge of juvenile pen shell aquaculture.
Acknowledgements
The authors are grateful to Mr. Yuzhong Sun, Director of Rizhao Ocean and Fisheries Research Institute, and engineer Shengnong Zhang at the Yellow Sea Fisheries Institute, Chinese Academy of Fishery Sciences, for their aids in cytogenetic experiments and chromosome preparations. This study was supported in part by the Central Public-Interest Scientific Institution Basal Research Fund, YSFRI, CAFS, China (No. 20603022018004), the National Natural Science Foundation of China (No. 31672637), the National Key R & D Program of China (No. 2018YFD09 00800), and the Key Research and Development Plan of Shandong Province, China (No. 2016GSF115012).
Bakri, N. M., Ibrahim, S. F., Osman, N. A., Hasan, N., Jaffar, F. H. F., Rahman, Z. A., and Osman, K., 2016. Embryo apopto- sis identification: Oocyte grade or cleavage stage?, 23: S50-S55.
Chung, E. Y., Baik, S. H., and Ryu, D. K., 2006. Reproductive biology of the pen shell,()on the Boryeong coastal waters of Korea., 22 (2): 143-150.
Gong, N., and Zhang, G. F., 2003. Embryo development and sur- vival of aneuploid Pacific oysterproduced by mating its triploids and diploids., 10 (1): 5-9.
Guo, X. M., and Allen Jr., S. K., 1994. Sex determination and polyploid gigantism in the dwarf surf clam (Say)., 138 (3): 1199-1206.
Kim, D. H., Yoon, H. S., An, Y. K., Lee, S. D., and Choi, S. D., 2008. Density dependent growth and survival rates ofin Duekryang Bay, Korea., 24 (2): 137-142.
Lesoway, M. P., Collin, R., and Abouheif, E., 2017. Early activation of MAPK and apoptosis in nutritive embryos of Calyptraeid gastropods., 328B: 449-461.
Levan, A., Fredga, K., and Sandberg, A. A., 1964. Nomenclature for centromeric position on chromosomes., 52 (2): 201-220.
Liu, J., Li, Q., Kong, L. F., and Zheng, X. D., 2011. Cryptic diversity in the pen shell(Bivalvia: Pinnidae): High divergence and hybridization revealed by molecular and morphological data., 20 (20): 4332-4345.
Ohashi, S., Fujii, A., Oniki, H., Osako, K., Maeno, Y., and Yoshikoshi, K., 2008. The rearing of the pen shelllarvae and juveniles (Preliminary note)., 56 (2): 181-191.
Romaguera, R., Casanovas, A., Morató, R., Izquierdo, D., Cata- lá, M., Jimenez-Macedo, A. R., Mogas, T., and Paramio, M. T., 2010. Effect of follicle diameter on oocyte apoptosis, embryo development and chromosomal ploidy in prepubertal goats., 74 (3): 364-373.
Schramm, H., Jaramillo, M. L., Quadros, T., Zeni, E. C., Müller, Y. M. R., Ammar, D., and Nazari, E. M., 2017. Effect of UVB radiation exposure in the expression of genes and proteins related to apoptosis in freshwater prawn embryos., 191: 25-33.
Shen, Y. P., Wang, X. J., Ma, W. T., and Zhang, X. Y., 1993. A tech- nique for preparation of metaphase chromosome from mantle tissue of adult pearl oysterDunker.(), 5: 121-122.
Son, P. W., Ha, D. S., Lee, C. H., Jang, D. S., and Kim, D. K., 2005. Study on the natural spat collection of the pen shell,., 21 (2): 113- 120.
Twyman, R. M., 2002.. Bios Scientific Publishers Limited, Norwich, 151-159.
Wang, Z. G., Wang, W., Yu, S. D., and Xu, Z. R., 2008. Effects of different activation protocols on preimplantation development, apoptosis and ploidy of bovine parthenogenetic embryos., 105 (3-4): 292-301.
Yang, Q., Li, Q., Yu, R. H., Kong, L. F., and Zheng, X. D., 2009. Artificial induction of gynogenetic diploids by suppression of the first cleavage in., 33 (10): 63-67.
Yurimoto, T., Yoshida, M., and Maeno, M., 2008. Histology and nutritional condition of the pen shellspro- truded above the sediment surface during summer in the tidal flat of Ariake Bay., 56 (4): 587-594.
Zhang, S., Yao, F., Jing, T., Zhang, M. C., Zhao, W., Zou, X. Y., Sui, L. L., and Hou, L., 2017. Cloning, expression pattern, and potential role of apoptosis inhibitor 5 in the termination of embryonic diapause and early embryo development of., 628: 170-179.
Zhou, L. Q., Wang, X. M., Wu, B., Sun, X. J., Chen, S. Q., Liu, Z. H., Yang, A. G., Zhang, S. N., Zhao, Q., and Zhang, G. W., 2018. Chromosome preparation and karyotypes analysis of bothmale and female., 39 (5): 66-72.
. E-mail:yangag@ysfri.ac.cn
July 6, 2019;
January 8, 2020;
April 16, 2020
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Centurial Evolution of an Offshore Mud Deposition Area in the Changjiang (Yangtze) Estuary and Its Links to Environmental and Anthropogenic Activities
- Characteristics and Origins of Suspended Pyrite in the Mixing Zone of the Yangtze Estuary
- Characterization of Fe(III)-Reducing Enrichment Cultures and Isolation of Enterobacter sp. Nan-1 from the Deep-Sea Sediment, South China Sea
- Evolution of Palaeoenvironment of the South Yellow Sea Since the Last Deglaciation
- Sulfate-Methane Transition Depths and Its Implication for Gas Hydrate
- Comprehensive Investigation and Assessment of Nutrient and Heavy Metal Contamination in the Surface Water of Coastal Bohai Sea in China
