Relationships Between Community Structure and Environmental Factors in Xixiakou Artificial Reef Area
2020-09-27YUHaolinYANGWenzhaoLIUChangdongTANGYanliSONGXiefaandFANGGuangjie
YU Haolin, YANG Wenzhao, LIU Changdong, TANG Yanli, SONG Xiefa, and FANG Guangjie
Relationships Between Community Structure and Environmental Factors in Xixiakou Artificial Reef Area
YU Haolin, YANG Wenzhao, LIU Changdong*, TANG Yanli, SONG Xiefa, and FANG Guangjie
,,266003,
The construction of artificial reefs has unparallelly developed for a few decades in China. Artificial reefs can be used to manage and conserve commercially exploited fish and crustacea. However, their suitability as ecological niche is poorly characterized. Therefore, in this study, we detected the seasonal variation of community biodiversity and the corresponding driving environmental factors. We also explored the relationships between dominant species and environmental factors to identify appropriate ecological niche areas. Different statistical analysis methods were used to assess species distribution within an artificial reef area in Xixiakou during nine sampling events in four seasons between 2017 and 2018. Non-metric multidimensional scaling (NMDS) and cluster analysis results indicated that the components of community can be divided into two clusters. Complexity of community, which is exhibited by species number, biodiversity, and catch per unit effort (CPUE), was significantly higher in summer than in other seasons. Generalized additive model (GAMs) results revealed the significant effects of temperature and chlorophyllon the community structure.,,andwere the dominant species in four seasons. GAMs results indicated that temperature, dissolved oxygen (DO), pH and chlorophyllaffect theof dominant species significantly. The distinct suitable ecological niche for each dominant species was found in this study. For example,preferred to live in the area with 20.7–22.1℃, dissolved oxygen 7.07–7.15mgL−1and salinity 31.8–31.9. The results of this study are beneficial to resource conservation and fishery management.
artificial reef; community structure; dominant species distribution; ecological niche
1 Introduction
Artificial reef areas which are produced by placing stones, abandoned fishing boats, oil platforms and concrete components into suitable ocean environments have been boom- ing in China in recent years (Lima., 2019). Artificial reefs can enhance the vertical flux of nutrients and pro- mote the exchange between upper and lower water layers (Falcão., 2009). They also limit the fishing with trawl and provide shelter for fish (Hixon and Beets, 1989). The goals of constructing artificial reef areas include conserve- ing and proliferating fishery resources, improving the pri- mary productivity and restoring the damaged ecological system (Stephens and Pondella, 2002).
Artificial reefs improve water exchange and change the type of substrate in original area. Hence, the benthic com- munity structure may change with the deployment of arti- ficial reefs. Species diversity is an important indicator of local ecological system. The assessment of artificial reef community structure is an important part of evaluating the effects of artificial reefs on the improvement of local ecological system. The variation in abundance and bio-mass of important ecological and economical species af- fects the community structure significantly (Chiba and Sai- no, 2002). Dominant species account for a large part of the biomass and abundance of the entire demersal community. Hence, the spatiotemporal variation of the dominant spe- cies can reflect the dynamic changes of community struc- ture.
Most of the studies have primarily involved the evaluation of resource proliferation effects following reef deployed (Dong., 2015; Tang., 2018), and described the differences in algae, benthic organisms and catch re- sources (Liu., 2015; Tang., 2016). They focused on the comparative analysis of resources and environmen- tal factors in reef areas and control plots (Vicente., 2008; Kilfoyle., 2013; Lowry., 2014). In the com- plex interaction between resources and environmental fac- tors, temperature was found to be an important factor to influence species biomass (Lara and González, 1998). There- fore, the seasonal variation of fishery resources is a re- searching hotspot (Sumaila., 2011) while information on the distribution of dominant species in huge tracts of continuous reefs in four seasons remains limited.
Different approaches have been used to assess the impact of seasonal variation on species diversity and environmental factors (Guisan and Zimmermann, 2000). Uni- variate analysis, such as that with generalized additive mo- del (GAM), can effectively reveal the single indicator’s change during four seasons. Multivariate analysis is often used to explore the interrelation between species and envi- ronmental factors. Combining cluster analysis with non- multidimensional scaling (NMDS) is able to explore the dynamics of complicated community (Li., 2004; Ma- nage, 2008; Valdes, 2008). Moreover, redundancy analysis (RDA) and non-linear RDA are more powerful for de- tecting suitable habitat niches of dominant species distribution. Most scientists use RDA to reveal the correlation between species and environmental factors, especially sig- nificant variables (Mcardle and Anderson, 2001; Jing., 2010). But it is usually a unimodal response of the species distribution to the environmental gradient. Non-linear RDA works by adding a second order explanatory variable to a first order function and running forward selection of the variables, which is more suitable to find species ecological niche (Makarenkov and Legendre, 2002).
Xixiakou marine ranching, which locates in Shandong Province, China, has been deployed large amounts of arti- ficial reefs each year from 2006 to 2014 for stock enhance- ment and restoration of inshore marine ecological environment. Due to the low-cost and convenience of quarry, stones and rocks have been the main materials of artificial reef in past few years, and they have mostly formed a cer- tain scale in current local sea ranching. However, the com- munity structure in the artificial reef area and its vari- ation among seasons have been rarely studied. The purpose of this study is: 1) to analyze the variation of community structure and its driving environmental factors among four seasons, and 2) to explore the relationship between dominant species distribution and environmental factors in the artificial reef area.
2 Materials and Methods
2.1 Study Area and Sampling Procedures
Xixiakou artificial reef locates in Weihai City, Shandong Province (37˚23΄56΄΄N–37˚24΄23΄΄N, 122˚32΄40΄΄E–122˚ 34΄30΄΄E). The survey area is 243hm2with water depths of 2–15m (Fig.1). The seabed is mainly composed of silt and is geologically flat. Reefs have been deployed predominantly with crushed stones from 2006 to 2014. This study was performed using a random sampling method in four seasons, May (Spring), July (Summer), October (Au- tumn) and December (Winter), from 2017 to 2018. A total of 9 sample events were conducted at 68 active survey sta- tions, approximately 16 to 19 stations each season (Fig.1).
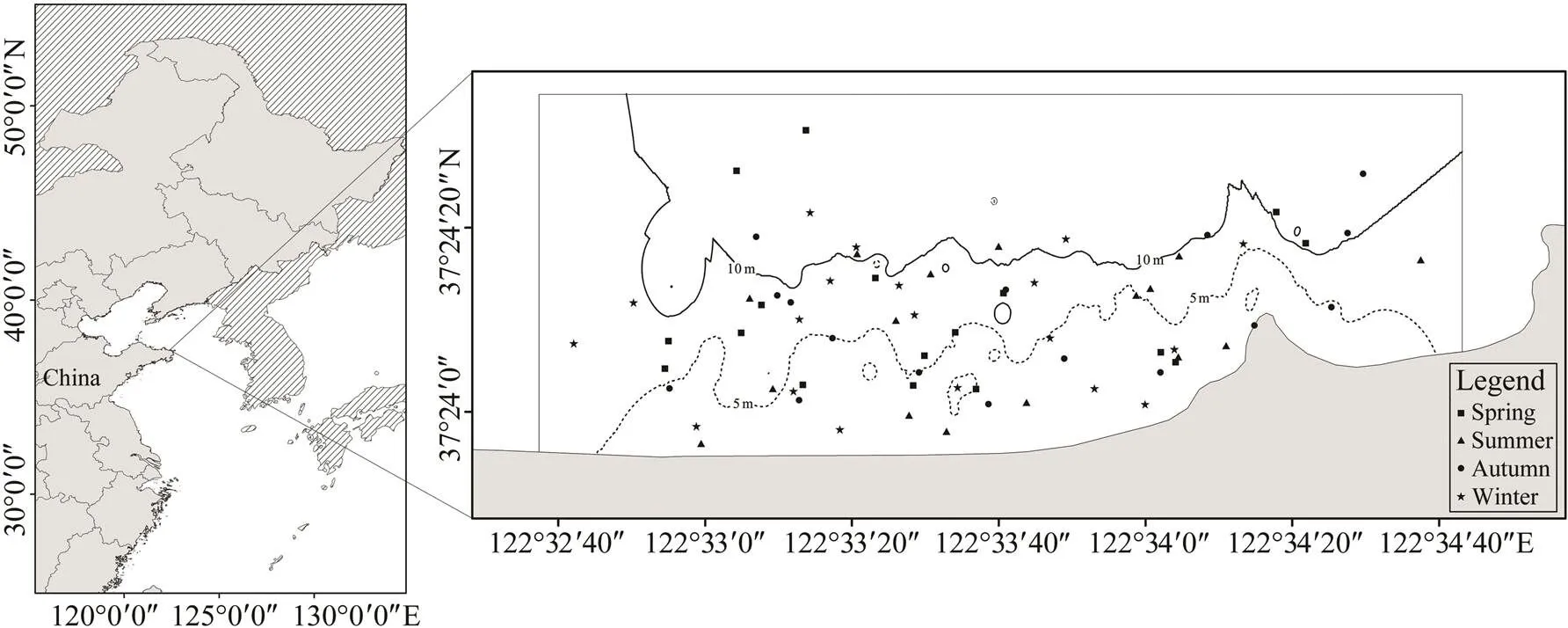
Fig.1 Location of the studying area and surveying stations among four seasons. The area enclosed by shoreline, and grey lines outline the artificial reef zone.
Accordion-shaped traps were used as the catch nets. Each group consists of 3 cages in series in a total length of 30 meters, one group each site. The nets were placed in the area and were surveyed for 48h without any bait in all seasons. All catches were collected and brought back to the laboratory for species identification and biological mea- surements.
The water depth (m) was measured with a sonar lattice fish detector. The temperature (Temp, ℃), salinity, dissolved oxygen concentration (DO, mgL−1) and pH value were measured with a YSI-Pro Plus multi-parameter water quality meter. Water was sampled with a plexiglass water collector and taken back to the laboratory. Chlorophyll(Chl, mgL−1), chemical oxygen demand (COD, mgL−1) were measured. According to our preliminary analysis, the water quality parameters, SiO3-Si (mgL−1), PO4-P (mgL−1) and total inorganic nitrogen, had little effect on species distribution, therefore, these 3 factors were not discussed in this study.
2.2 Data Treatment and Analysis
To describe the local community structure characteristics, Shannon-Weiner diversity index (Shannon) and Pielou’s evenness index () were used in this study (Farré., 2013). They were calculated as follows:


whereis the Shannon-Weiner diversity index,Pdenotes the proportion of speciesindividuals to the total individuals in the community,refers to the number of species, andmaxis the maximum of the diversity index of all sites.
To reduce the interference of low-probability capture spe- cies on community structure, the total number of fish was ranked before analysis, and the species were selected for the analysis when the number exceeded 99.9% of the total and a frequency of occurrence was greater than 5% (Lv., 2011). A total of 10 species were selected for analy- sis. We collected all species at each survey station and cal- culated the catch per unit effort (CPUE) reflected by bio- mass. Then, based on the selected 10 species,re- flected by species individual amount was calculated to avoid some light weight species.
Pinkas index of relative importance () was used to assess the importance of a specific species in an aquifer community (Pinkas., 1971). Species with anabove 1000 were defined as the dominant (Pinkas., 1971). We also calculated theof dominant species.
The differences of environmental factors,and com- munity biodiversity index in four seasons (Ransom, 1974) were analyzed through one-way ANOVA and Turkey HSDtest. The similarity of seasonal variation in community structure was distinguished by cluster analysis and NMDS (Möhlmann., 2017). The relationship between diversity index, dominant speciesand environmental fac- tors was evaluated by generalized additive models (GAMs). GAMs are denoted as

whereis linking function,is the intercept term,is the random error term, andfdenotes a non-parametric function of theth independent variable. Before the processing of GAMs,data were transformed by log(+1) to conform to the normal distribution, and the normal distribution of each response variable was tested by Q-Q plot. Based on the AIC and R-square values, we can find the optimal model (Hastie and Tibshirani, 1987).
Species matrix was transformed by a Hellinger method to normalize the data to reduce the different order of mag- nitudes influence (Batáry., 2010). The catch codes included in the analysis are shown in Table 1, which con- stitute the ‘station×species abundance’ matrix. Canonical correspondence analysis is often used to explore the rela- tionship between environmental factors and the analyzed individual species (Lawesson, 2010). However, redundancy analysis (RDA) was performed in this study because the preliminary detrended correspondence analysis (DCA) in- dicated that the gradient length was less than 3 (Ter Braak and Smilauer, 2002).
The variance inflation factor (VIF) was used to test the collinearity of the significant variables, and the variables with VIF values less than 10 were kept in the next analysis (Lawesson, 2010). The significance of adding new vari- ables to the analysis was determined by stepwise unrestricted Monte Carlo Permutation test (999 iterations). Only significant variables (<0.05) were included. In addition, the nonlinear RDA method was used to detect the optimal ecological field of each dominant species, and the environmental factors that had significant influence on species distribution were defined as the second-order explanatory variables. All analyses were performed using the R programming language (ver 3.5.2, R Foundation for Statistical Computing). Cluster analysis was performed using the ‘cluster’ package. GAMs were performed using the ‘mgcv’package. Multivariate models such as NMDS, RDA and non- linear RDA were performed using the ‘vegan’ package.

Table 1 List of analyzed fish species
3 Results
3.1 Overview
A total of 43 species were discovered in this study, including 20 fishes, 12 crustaceans, 7 echinoderms, 2 cepha- lopods and 2 gastropods. The dominant species showed sea- sonal alternations.was the most domi- nant with the largest number and biomass in four seasons.was the dominant species in spring, summer and autumn.was the dominant species in summer and autumn.was the dominant species only in summer. There were four do-minant species in summer, which possessed the most com- plex community structure in this season. There was only one dominant species (in winter, indicating that the community structure was simple in this season.
In this study, 16 environmental variables were measured simultaneously with species abundances, and the re- sult from collinear test showed that the VIF of 6 variables were less than 10 (Table 2), and these 6 variables include temperature (℃), pH, salinity, COD (mgL−1), Chl(mgL−1) and DO (mgL−1).

Table 2 Correlations between environmental factors by ordination axis
Notes: The significance of different variables was conducted by Monte Carlo Permutation test (999 iterations) and VIF less than 10. Axis1, Axis2, correlation between environmental factors and the ordination axes; Significance, ***,<0.001; **,<0.01; *,<0.05.
The variation in environmental factors among seasons is shown in Fig.2 and Table 3. Salinity and pH value did not change much with season, and they tended to increase gradually from spring to winter. The highest concentration of Chlwas in spring (2.22mgL−1), followed by that in winter (1.57mgL−1), autumn (1.16mgL−1) and summer (0.95mgL−1). Only spring was remarkable in difference from other seasons (<0.05). The DO concentration in descend- ing order was winter (12.4mgL−1), spring (9.53mgL−1), autumn (8.03mgL−1) and summer (7.14mgL−1). Except for summer and autumn, the pairwise comparison of DO was statistically significant (<0.05). The highest concentration of COD was found in autumn (1.12mgL−1) and the lowest in winter (0.48mgL−1). ANOVA results show- ed that except for winter, the COD change in three seasons was not significant (>0.05). Water temperature exhibited significant seasonal changes, and its fluctuation range from 4.3℃ in winter to 21.5℃ in summer (<0.05).

Fig.2 Seasonal changes in environmental factors and biodiversity index. Chl a, chlorophyll a; COD, chemical oxygen demand; CPUE, catch per unit effort; DO, dissolved oxygen; J, Pielou’s evenness index; Shannon D, Shannon diversity index.
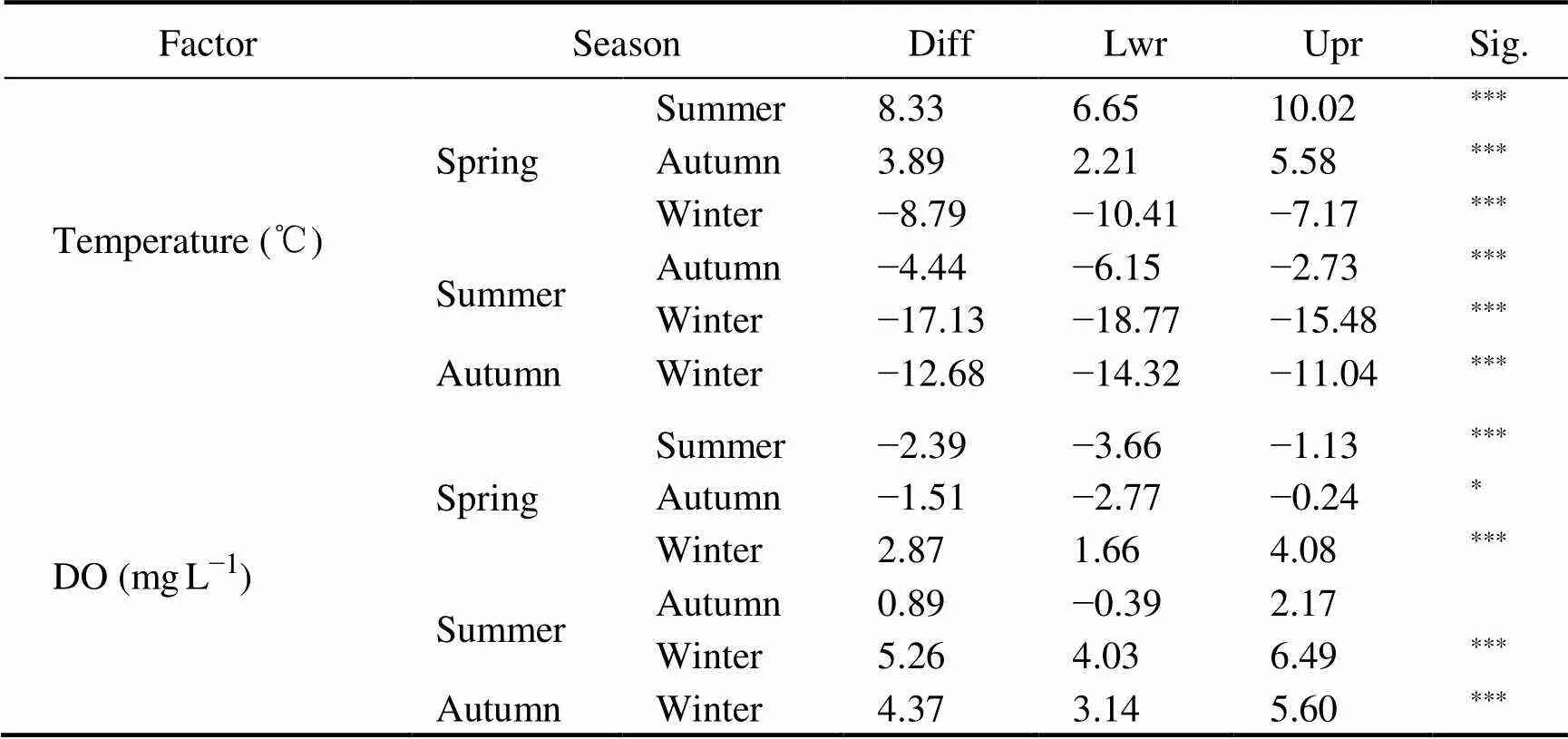
Table 3 Summary of the pairwise comparison
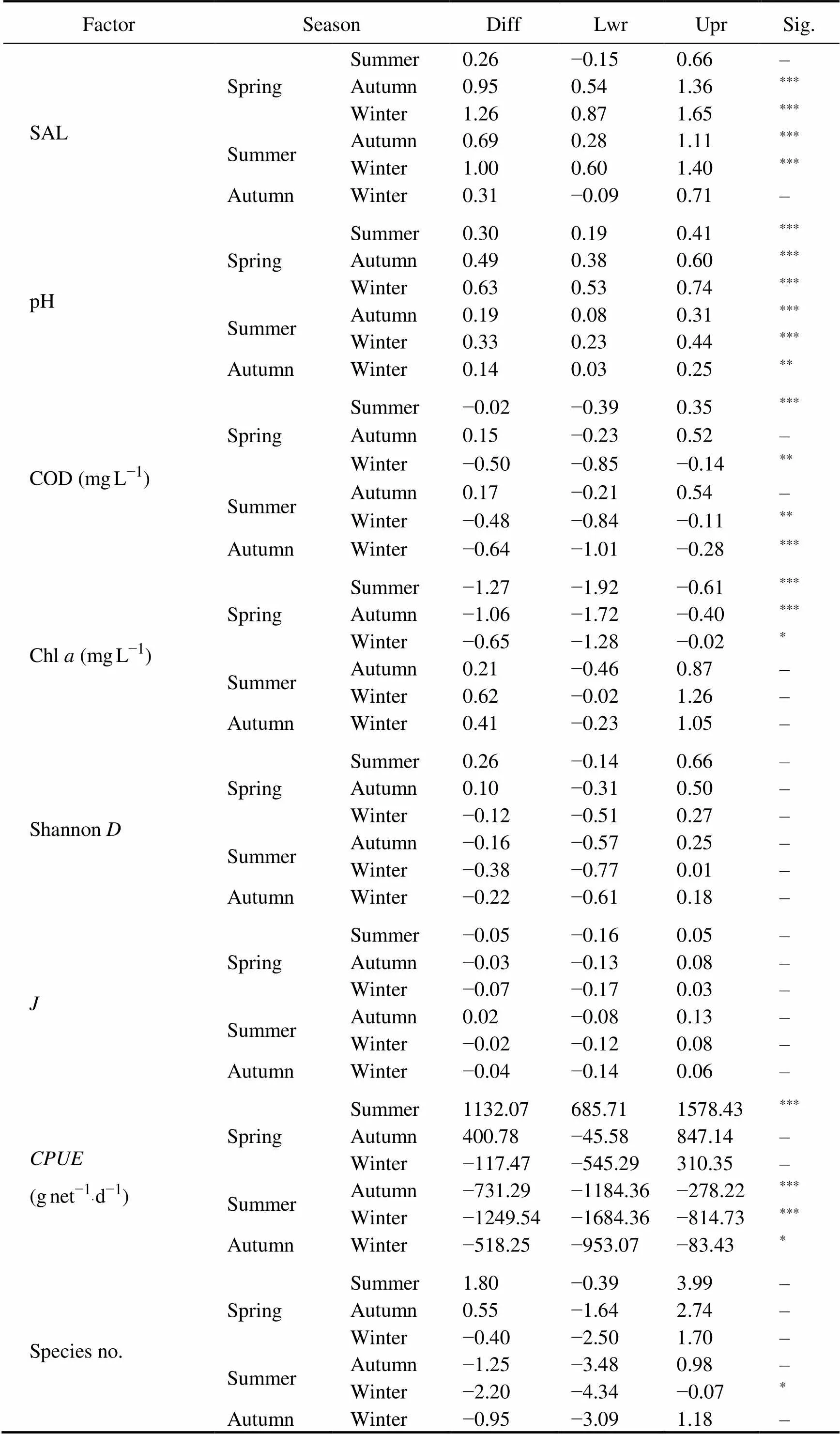
(continued)
Notes: Diff, the difference in the observed means; Lwr, the lower end point of the interval; Upr, the upper end point. ***,<0.001; **,<0.01; *,<0.05; –,>0.05.
3.2 Variation of Community Structure
The average indices of biodiversity (Shannon,, and Species no) are shown in Fig.2 and the corresponding ANOVA results are shown in Table 3. The results indicated that Shannon diversity index (Shannon) had no significant difference among seasons (>0.05) although the value reached the highest 1.48 in summer and in winter returned to 1.1. Pielou’s evenness index () varied little among seasons from 0.78 to 0.86.of all species and the richness of species were significantly higher in summer than in other three seasons (<0.05).
NMDS and cluster results indicated that the community structure showed distinct seasonal differences (Figs.3–4). It could be divided into two clusters, while summer was different from the other seasons. A total of 18 sites were included in the first cluster (Fig.3), and almost all summer survey sites were included. Meanwhile, when the cluster results were considered together with the nMDS results, obvious assembling could be observed while cluster one and cluster two showed significant differences in commu- nity structure.

Fig.3 Cluster analysis of Biomes in different seasons. sp, spring; su, summer; au, autumn; wi, winter.

Fig.4 NMDS of different stations with the result of cluster analysis.
According to the Q-Q plot (Fig.5), all response variables including Shannon Weiner Diversity and transformedof the number of dominant species followed a normal dis- tribution. GAM results indicated that the total variation of diversity index explained by temperature and Chlwas 61.1% (2=0.489) (Table 4). Temperature played an im- portant role in shaping the community structure (<0.05). Fig.6A displays that the community diversity index varied greatly with the temperature, and the general trend was that it increases with temperature, reaching the highest in summer. It can be observed from Fig.6B that the species diversity index changes with the increase of Chlconcentration, reaching 3mgL−1as the maximum.

Fig.5 Q-Q plots of Shannon Weiner Diversity index and CPUE of the number of dominant species included in GAMS. The closer the sample point approaches to the oblique line, the closer it tends to the normal distribution.

Fig.6 Response variable curves of Shannon Weiner Diversity index for temperature and Chl a in GAMs. A, temperature’s response to the diversity; B, Chl a’s response to the diversity. Temp, temperature.
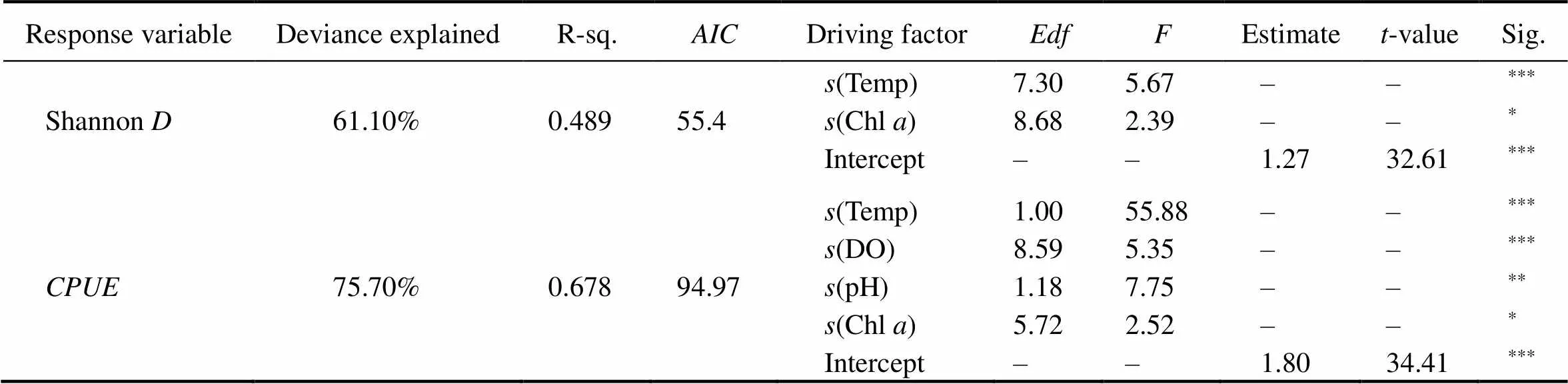
Table 4 The optimal Generalized Additive Model (GAM) describing the response of community Shannon diversity index and CPUE of the number of dominant species according to temperature, DO, pH and Chl a
Notes: We selected the best model by combining the maximum value of, the minimum value ofand the significance of each variable. Data were collected in all sites.: catch per unit effort of dominant species which contained,,and; Shannon: Shannon diversity index;(): represents cubic regression spline;: estimated degree freedom. ***,<0.001; **,<0.01; *,<0.05; –, not available.
3.3 Distribution of the Dominant Species
GAMs were employed to investigate the relationship be- tween driving environmental factors and dominant species. The goodness of fit of GAMs reached 75.7% (2=0.678), implying a high credibility (Table 4). Temperature and DO were the most important driving factors, followed by pH and Chl, and the effects of these four factors were all significant (<0.05).increasedwith the increases of temperature and pH (Figs.7A, C). With the increase of DO concentration,tended to be flatten- ed and reached the maximum at 10mgL−1(Fig.7B). Thewent up to the maximum when Chlwas at 2.9mgL−1although it varied a little beyond the change of Chl(Fig.7D).
RDA results showed that the accumulated constrained proportion explained with environmental factors reached 78%, indicating that the selection of environmental factors was reasonable and reflected the true changes of environmental data in a large part (Table 5). Fig.8 displays the two-dimensional sequence diagram of environmental factors and biological population. The significance from high to low was that of temperature, DO, COD, pH, Chland SAL.andhad positive correlation with temperature and negative correlation with DO and salinity (Fig.8).,as the most dominant species, had negative correlation with COD.was positively correlated with the concentration of Chland pH.

Fig.7 Response variable curves of CPUE of the number of dominant species for temperature, DO, pH and Chl a in GAMs.

Table 5 Summary of RDA ordination
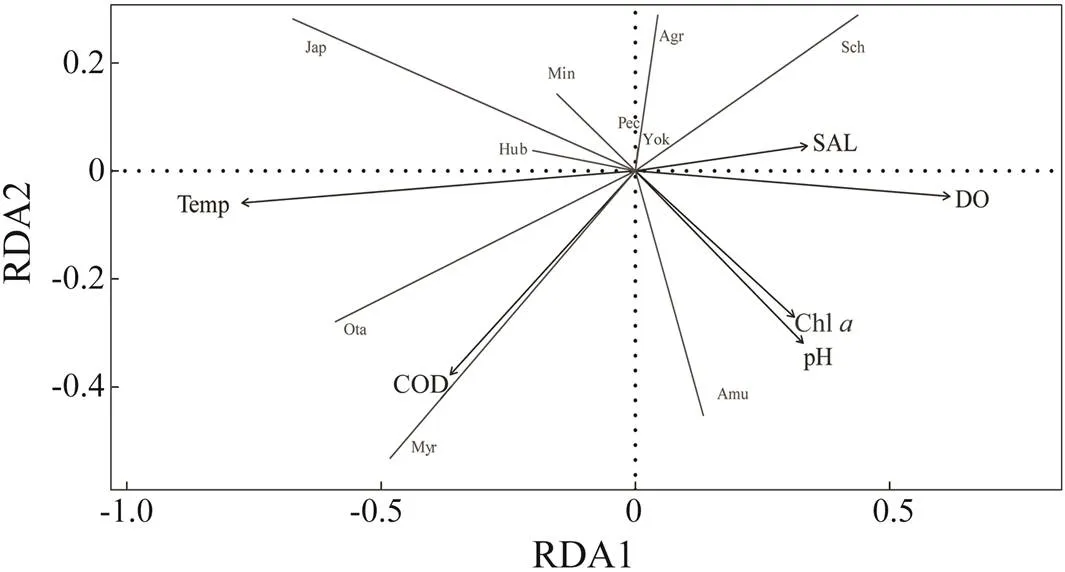
Fig.8 RDA ordination of species distribution and environmental factors. Arrows represent each environmental factor. The length of the line between arrow and origin represents the correlation between population and environmental factors. Straight lines mean projection lines of different species. Species codes can be referred in Table 1.
According to the relationship between species and the environmental factors, non-linear RDA helps to explore the relatively suitable habitat conditions to different species. The results of the nonlinear RDA test are shown in Table 5. This model was established on the fact that thevalue was less than 0.05. The sorting results (Fig.9) showed that the distribution ofwas more inclined to the area of 20.7–22.1℃, DO 7.07–7.15mgL−1, Chl0.75– 0.83mgL−1, pH 7.92–7.94, COD 1.20–1.27mgL−1and sa- linity 31.87–31.9. The subsequenttended to the ecological niche with 20.3–20.6℃, DO 7.23–7.15mgL−1, pH 7.91–7.92, salinity 31.66–31.87 and Chl0.94–0.95mgL−1. Other dominant species, such as, were preferably distributed in the area with Chl0.79–0.85mgL−1, while other factors were not so important.

Fig.9 Non-linear RDA ordination of six significant environmental factors. Line with arrow means the first-order and second-order variable. Temp1, DO1, COD1, pH1, SAL1 and Chl a1, first-order variable; Temp2, DO2, COD2, pH2, SAL2 and Chl a2, second-order variable.
4 Discussion
4.1 Relationship Between Biodiversity and Environmental Factors
The 43 species caught in Xixiakou artificial reef are commonly found in the coastal areas of Shandong Province, and 22 species are economically important. Compared with the study by Wu. (2012) in Rongcheng Island artificial reef area close to ours (about 16km away), the results of this study indicated a higher total biomass and number of species but a lower level of. The diversity index in Xixiakou artificial reef is very low and the values in most stations are close to the minimum, 1.5–3 as was proposed by Magurran (1988). When the fish com- munity is not seriously disturbed by external factors, it will maintain an intrinsic species diversity, which can be considered the baseline (Rice, 2000). Xixiakou has been a famous fishing village before the construction of marine ranches. Long-term over-fishing, poor net selectivity, and environment pollution are the main reasons causing fish diversity a severe decline (Lorenzen., 2010). Marine ranching limits the fishing of most poor selective gears, and environment protection attracts more and more attentions in China recently. Hence, we expect that the ecolo- gical system inXixiakou artificial reef will get better gra- dually.
Our study focused on exploring seasonal variation of community structure. The lowest biodiversity index in win- ter may be caused by the lowest temperature in this season. Deep water area is relatively warm at this time, and swimming animals prefer to rest in caves or swim into deep waters in winter (Wu., 2012). However, due to the high cost of research and the restricted accessibility, we cannot conduct the survey in deeper (greater than 15m) waters and this may affect the results. As the temperature rises, the diversity and complexity of community struc- ture in artificial reef area increase too. The cluster results show that it is especially prominent in summer and the result is significantly different from those in other seasons. Nevertheless, the first cluster contains other seasons’ sites in addition to summer sites, which was probably caused by the year-round distribution of the dominant species such as.
4.2 Catchability of Sampling Gear
Due to the restriction of reef, fishhook, gill net and trap are the main fishing gears in the artificial reef (Hawkins., 2007). The catchability of trap is higher than that of other two fishing gears. We selected trap as the sampling gear (Wells., 2008) though its catch rate is lower than that of trawl (Smith Jr.., 1992). Trap without bait was used in this study to decrease the differential lure of bait to different species. Therefore, high species density will present a high probability to be caught by trap (Robichaud., 2000). It is appropriate for us to useof trap to represent the species abundance at the sampling site (Zimmerman and Palo, 2011).
The important ecological and economic species assemblage on the reef can be analyzed based on the fishes caught with trap (Stevenson and Stuart-Sharkey, 1980). However, the selectivity of trap may depend on swimming ability and behavior of species and this will bias our results (Robichaud., 2000). Trap catchability may change amongseasons because of the difference in species behaviors (Guy and Willis, 1991; Tokuhiro., 2019). For example, fish tend to be less active at low temperature, which may reduce the catchability for many species in winter (Wu., 2019). To explore the difference of species abundance among seasons, local fishermen used underwater visual census to conduct qualitative survey in Xi- xiakou artificial reef. The results from underwater videos also indicated the high and low species abundance in summer and winter, respectively (personal communication). Thus, the quantitative analysis of survey data from trap can reflect the change trend of community structure amongseasons though the difference in trap catchability may bias the results.
4.3 Dominant Species Distribution and Optimal Ecological Niche
Most of the species in the artificial reef had similar con-ditional orientation and physiological characteristics, which influence their behavior and spatiotemporal distributions (Leitao., 2008; Zhang., 2018; Ge., 2019). The reason of dominant species being the best delegation is that they account for a large proportion of the total bio- mass and abundance. GAMs presented similar results with RDA in exploring the change of dominant specieswith environmental factors. Moreover, nonlinear RDA made it easier to find species suitable ecological niche.
The distribution of,,andshowed different ecological preferences. GAM results indicated the overall increase trend of do- minant specieswith temperature, dissolved oxygen and pH. However, GAM showed two peaks of Chland the dominat speciesreached the maximum at 3mgL−1of Chl. In spring, a large amount of fresh water with a high concentration of dissolved nutrient inflow into Yel- low Sea (Jin., 2013). Artificial reefs enhance the mix- ing effect from bottom to surface water, which might cause the highat the relative high Chlconcentra- tion (Wang., 2013).
Each domiannt species usually has their own preferable living habits (Bohnsack, 1989) and bait organisms (Bor- tone and Nelson, 1995). This study found specific suit- able ecological niche for each dominant species.was more sensitive with the change of environ- mental factors than the other dominant species. The poor swimming ability, limited sphere of activity (Vazquez Arch- dale., 2003) and feeding on nereid, shellfish and ben- thonic organisms (Zhao., 2012) probably cause the limited distribution area for. Its preferred eco- logical niche was all within the scope of the previous re- search of Zhao. (2012) except for salinity. It was found that the suitable salinity forvaried between 28 and 29, lower than our findings. The upwelling from the artificial reef could bring nutrients from the seabed to the surface, which was beneficial to the growth and re- production of phytoplankton, thus improving the salinity of the water body.
In contrast,.,.and.pre- sented a relatively high environment adaptability.is the year-round dominant species and has a large environment tolerance (Zhang., 2018). Our results showed consistent ecological preference with controlled environmental condition of culture of this species (Kim and Kang, 2016) except for a little lower COD concentration. Xixiakou artificial reef was well regulated and a large amount of organic matter discharging into the water was avoided, which might lead to low COD concentration.andhad similar preferences, especially to temperature, dissolved oxygen and Chl. Smallwere dominant prey forand two species had partly overlapped feeding habits after sexual maturity (Kaifu., 2013; Ji., 2014; Liu., 2018). This may induce the similar environmental preference of these two species. With the comprehensive con- sideration of the distribution density, size and reef arrangement in each water layer and the improvement of survey equipment and method, the more precise ecological niche of the dominant species will be found in the future.
5 Conclusions
This study showed that in Xixiakou artificial reef, the community structure varied among seasons significantly. Summer is significantly different from other seasons no matter in community structure or dominant species distribution. Dominant species including,,, andtend to live in separate suitable ecological niche and this study provides detailed data with dominant distribution pattern. The distribution of dominant species is correlated with temperature, DO, pH and Chlsignificantly. Generally, artificial reefs can assemble and maintain rocky fish and benthic organisms effectively. It is a good tool for the marine ecological re- storation to offer suitable habitat for economically dominant species.
Acknowledgement
This study was supported by the Project of Marine and Fishery Technology Innovation of Shandong (No. 2017 HYCX007).
Batáry, P., Matthiesen, T., and Tscharntke, T., 2010. Landscape-moderated importance of hedges in conserving farmland bird diversity of organic. conventional croplands and grasslands., 143 (9): 2020-2027, DOI: 10.1016/j.biocon.2010.05.005.
Bohnsack, J. A., 1989. Are high densities of fishes at artificial reefs the result of habitat limitation or behavioral preference?, 44 (2): 631-645.
Bortone, S.A., and Nelson, B.D., 1995. Food habits and forage limits of artificial reef fishes in the northern Gulf of Mexico.Japan Inter- national Marine Science and Technology Federation, Tokyo, Japan, 215-218.
Chiba, S., and Saino, T., 2002. Interdecadal change in the upper water column environment and spring diatom community struc- ture in the Japan Sea: An early summer hypothesis.,231(1): 23-35, DOI: 10.3354/meps231023.
Dong, T. W., Huang, L. Y., Tang, Y. L., Sheng, H. X., and Liu, C. D., 2015. Preliminary evaluation of artificial reef around Rizhao Qiansan Island on the enhancement of fishery resources.,45 (8): 38-45 (in Chi- nese with English abstract).
Falcão, M., Santos, M. N., Drago, T., Serpa, D., and Monteiro, C., 2009. Effect of artificial reefs (southern Portugal) on se- diment–water transport of nutrients: Importance of the hydro- dynamic regime., 83 (4): 451-459, DOI: 10.1016/j.ecss.2009.04.028.
Farré, M., Tuset, V. M., Maynou, F., Recasens, L., and Lombarte, A., 2013. Geometric morphology as an alternative for mea- suring the diversity of fish assemblages., 29: 159-166, DOI: 10.1016/j.ecolind.2012.12.005.
Ge, S. S., Zhao, W. X., Song, J. J., Yu, D. D., Liu, Y., Wang, Q. X., and Zhou, J., 2019. Study on trophic niches ofandin the artificial reef area of Xiaoheishan Island., 39 (18): 6923-6931 (in Chinese with English abstract).
Guisan, A., and Zimmermann, N. E., 2000. Predictive habitat dis- tribution models in ecology., 135 (2): 147-186, DOI: 10.1016/S0304-3800(00)00354-9.
Guy, C. S., and Willis, D. W., 1991. Seasonal variation in catch rate and body condition for four fish species in a South Da- kota natural lake., 6 (3): 281-292, DOI: 10.1080/02705060.1991.9665305.
Hastie, T., and Tibshirani, R., 1987. Generalized additive model: Some applications., 82 (398): 371-386, DOI: 10.1007/978-1-4615-7070-7_8.
Hawkins, J. P., Roberts, C. M., Gell, F. R., and Dytham, C., 2007. Effects of trap fishing on reef fish communities., 17 (2): 111-132, DOI: 10.1002/aqc.784.
Hixon, M. A., and Beets, J. P., 1989. Shelter characteristics and caribbean fish assemblages: Experiments with artificial reefs., 44 (2): 666-680.
Ji, D., Bian, X., Song, N., and Gao, T., 2014. Feeding ecology ofin Lidao Rongcheng., 38 (9): 1399-1409 (in Chinese with English abstract).
Jin, J., Liu, S. M., Ren, J. L., Liu, C. G., Zhang, J., and Zhang, G. L., 2013. Nutrient dynamics and coupling with phytoplankton species composition during the spring blooms in the Yellow Sea., 97: 16-32, DOI: https://doi.org/10.1016/j.dsr2.2013.05.002.
Jing, H., Liu, H., Suzuki, K., Sohrin, R., and Nishioka, J., 2010. Community compositions of bacteria and archaea in the Sea of Okhotsk during summer., 61 (2): 191-204, DOI: 10.3354/ame01450.
Kaifu, K., Miller, M. J., Aoyama, J., Washitani, I., and Tsu- kamoto, K., 2013. Evidence of niche segregation between fresh- water eels and conger eels in Kojima Bay, Japan., 79 (4): 593-603, DOI: 10.1007/s12562-013-0628-3.
Kilfoyle, A. K., Freeman, J., Jordan, L. K., Quinn, T. P., andSpieler, R. E., 2013. Fish assemblages on a mitigation boulder reef and neighboring hardbottom., 75: 53-62, DOI: 10.1016/j.ocecoaman.2013.02.001.
Kim, J.H., and Kang, J. C., 2016. The immune responses in ju- venile rockfish,for the stress by the expo- sure to the dietary lead (II)., 46: 211-216, DOI: 10.1016/j.etap.2016.07.022.
Lara, E. N., and González, E. A., 1998. The relationship be-tween reef fish community structure and environmental vari- ables in the southern Mexican Caribbean., 53: 209-221, DOI: 10.1111/j.1095-8649.1998.tb01028.x.
Lawesson, J. E., 2010. Effects of species partition on explana- tory variables in direct gradient analysis: A case study from Senegal.,8 (3): 409-414, DOI: 10.2307/3237332.
Leitao, F., Santos, M. N., Erzini, K., and Monteiro, C. C., 2008. Fish assemblages and rapid colonization after enlargement of an artificial reef off the Algarve coast (southern Portugal)., 29 (4): 435-448, DOI: 10.1111/j.1439-0485.2008.00253.x.
Li, S. F., Yan, L. P., Li, C. S., and Hu, F., 2004. The analysis of fish composition pattern in the northern East China Sea., 28 (04): 384-392 (in Chinese with English abstract).
Lima, J. S., Zalmon, I. R., and Love, M., 2019. Overview and trends of ecological and socioeconomic research on artificial reefs., 145: 81-96, DOI: 10.1016/j.marenvres.2019.01.010.
Liu, C. D., Guo, X. F., Tang, Y. L., Sheng, H. X., and Huang, L. Y., 2015. Phytoplankton community composition and its re- lationship with environmental factors in the artificial reef area around the Qiansan islets, Haizhou Bay., 22 (3): 545-555 (in Chinese with English abstract).
Lorenzen, K., Leber, K. M., and Blankenship, H. L., 2010. Re- sponsible approach to marine stock enhancement: An update., 18 (2): 189-210, DOI: 10.1080/10641262.2010.491564.
Lowry, M. B., Glasby, T. M., Boys, C. A., Folpp, H., Suthers, I., and Gregson, M., 2014. Response of fish communities to the deployment of estuarine artificial reefs for fisheries enhance- ment., 21 (1): 42-56, DOI: 10.1111/fme.12048.
Lv, Z. B., Li, F., Wang, B., Xu, B. Q., Wei, Z. H., Zhang, H. J.,and Zhang, P. C., 2011. Community structure of fish resources in spring and autumn in the Yellow Sea of Shandong., 35 (5): 692-699 (in Chinese with Eng- lish abstract).
Magurran, A. E., 1988.. Princeton University Press, Princeton, New Jersey, 181pp.
Makarenkov, V., and Legendre, P., 2002. Nonlinear redundancy analysis and canonical correspondence analysis based on poly- nomial regression., 83 (4): 1146-1161, DOI: 10.1890/0012-9658(2002)083[1146:NRAACC]2.0.CO;2.
Manage, A., 2008. Community-level analysis of anthropogenic im- pacts on rocky shore communities in Sri Lanka., 2008: 1-1, DOI: 10.1038/npre.2008.2317.1.
Mcardle, B. H., and Anderson, M. J., 2001. Fitting multivariate models to community data: A comment on distance-based re- dundancy analysis., 82 (1): 290-297, DOI: 10.1890/0012-9658(2001)082[0290:FMMTCD]2.0.CO;2.
Möhlmann, T. W., Wennergren, U., Tälle, M., Favia, G., Damiani, C., Bracchetti, L., and Koenraadt, C. J., 2017. Community ana-lysis of the abundance and diversity of mosquito species (Dip- tera: Culicidae) in three European countries at different lati- tudes., 10 (1): 510, DOI: 10.1186/s13071-017-2481-1.
Pinkas, L., Oliphant, M. S., and Iverson, I. L., 1971. Food habits of albacore, bluefin tuna and bonito in California waters., 152: 1-105.
Ransom, J., 1974. Biostatistical analysis J. H. Zar., 36 (5): 316-316.
Rice, J., 2000. Evaluating fishery impacts using metrics of com- munity structure., 57 (3): 682-688, DOI: 10.1006/jmsc.2000.0735.
Robichaud, D., Hunte, W., and Chapman, M. R., 2000. Factors affecting the catchability of reef fishes in Antillean fish traps., 67 (2): 831-844.
Smith Jr., K. L., Kaufmann, R. S., Edelman, J. L., and Baldwin, R. J., 1992. Abyssopelagic fauna in the central North Pacific: Comparison of acoustic detection and trawl and baited trap collections to 5800m., 39 (3-4): 659-685, DOI: 10.1016/0198-0149(92)90094-A.
Stephens Jr., J., and Pondella, D., 2002. Larval productivity of a mature artificial reef: The ichthyoplankton of King Harbor, Cali- fornia, 1974–1997.,59: S51-S58, DOI: 10.1006/jmsc.2002.1189.
Stevenson, D. K., and Stuart-Sharkey, P., 1980. Performance of wire fish traps on the western coast of Puerto Rico..Miami, Florida, 173-193.
Sumaila, U. R., Cheung, W. W., Lam, V. W., Pauly, D., and Her- rick, S., 2011. Climate change impacts on the biophysics and economics of world fisheries., 1 (9): 449-456, DOI: 10.1038/nclimate1301.
Tang, W. Y., Tang, Y. L., Sheng, H. X., and Wan, R., 2018. Eco- system health assessment of artificial reef area in Xigang, Weihai., 48 (3): 55-64 (in Chinese with English abstract).
Tang, Y. L., Sun, X. M., Sheng, H. X., Wang, X. M., and Wan, R., 2016. Community structure of catch and its relationship with environmental factors in Xiaoshidao artificial reef zones of Wei-hai City., 46 (5): 25-34 (in Chinese with English abstract).
Ter Braak, C. J. F., and Smilauer, P., 2002. Canoco reference manual and CanoDraw for Windows user’s guide: Software for canonical community ordination (Version 4.5). Microcom- puter Power, Ithaca, New York, USA.
Tokuhiro, K., Abe, Y., Matsuno, K., Onodera, J., Fujiwara, A., Harada, N., Hirawake, T., and Yamaguchi, A., 2019. Seasonal phenology of four dominant copepods in the Pacific sector of the Arctic Ocean: Insights from statistical analyses of sedi- ment trap data., 19: 94-111, DOI: 10.1016/j.polar.2018.08.006.
Valdes, M. A., 2008. Non-metric multidimensional scaling (NMDS) as a basis for a plant functional group classification and a Ba- yesian belief network formulation for California oak wood- lands. PhD thesis. University of California.
Vazquez Archdale, M., Anraku, K., Yamamoto, T., and Higashi- tani, N., 2003. Behavior of the Japanese rock crab ‘Ishigani’ Charybdis japonica towards two collapsible baited pots: Eva- luation of capture effectiveness., 69 (4): 785-791, DOI: 10.1046/j.1444-2906.2003.00687.x.
Vicente, M., Falcão, M., Santos, M. N., Caetano, M., Serpa, D., Vale, C., and Monteiro, C., 2008. Environmental assessment of two artificial reef systems off southern Portugal (Faro and Olhão): A question of location., 28 (6): 839-847, DOI: 10.1016/j.csr.2007.12.009.
Wang, F., Zhang, S., and Lin, J., 2013. Study of chlorophylldistribution in marine ranching planning area of Xiangshan Bay., 22 (2): 266-273.
Wells, R. D., Boswell, K. M., Cowan Jr., J. H., and Patterson III, W. F., 2008. Size selectivity of sampling gears targeting red snapper in the northern Gulf of Mexico., 89 (3): 294-299, DOI: 10.1016/j.fishres.2007.10.010.
Wu, Z. X., Zhang, L., Zhang, X. M., Zhang, P. D., and Li, W. T., 2012. Nekton community structure and its relationship with main environmental variables in Lidao artificial reef zones of Rongcheng., 32 (21): 6737-6746 (in Chinese with English abstract).
Wu, Z., Tweedley, J. R., Loneragan, N. R., and Zhang, X., 2019. Artificial reefs can mimic natural habitats for fish and macro- invertebrates in temperate coastal waters of the Yellow Sea., 139: 105579, DOI: 10.1016/j.ecoleng.2019.08.009.
Zhang, Y., Xu, Q., Xu, Q., Alós, J., Zhang, H., and Yang, H., 2018. Dietary composition and trophic niche partitioning of spotty-bellied greenlings, fat green- lings, Korean rockfish, and Japa- nese seaperchin the Yellow Sea re- vealed by stomach content analysis and stable isotope analy- sis., 10 (2): 255-268, DOI: 10.1002/mcf2.10019.
Zhao, J., Liu, H., Yuan, Z. Z., Liu, X., Wang, H. W., Jiang, Y. S., Li, X. D., Liu, H. Y., Zheng, Y., and Yao, J. G., 2012. Selec- tive feeding on three bivalves and feeding rhythm in Asian swimming crab., 27 (3): 226-230 (in Chinese with English abstract).
Zimmerman, J. K., and Palo, R. T., 2011. Reliability of catch per unit effort (CPUE) for evaluation of reintroduction programs–A comparison of the mark-recapture method with standar- dized trapping., 401: 07, DOI: 10.1051/kmae/2011016.
. Tel: 0086-532-82031076
E-mail: changdong@ouc.edu.cn
July 31, 2019;
February 12, 2020;
May 10, 2020
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Centurial Evolution of an Offshore Mud Deposition Area in the Changjiang (Yangtze) Estuary and Its Links to Environmental and Anthropogenic Activities
- Characteristics and Origins of Suspended Pyrite in the Mixing Zone of the Yangtze Estuary
- Characterization of Fe(III)-Reducing Enrichment Cultures and Isolation of Enterobacter sp. Nan-1 from the Deep-Sea Sediment, South China Sea
- Evolution of Palaeoenvironment of the South Yellow Sea Since the Last Deglaciation
- Sulfate-Methane Transition Depths and Its Implication for Gas Hydrate
- Comprehensive Investigation and Assessment of Nutrient and Heavy Metal Contamination in the Surface Water of Coastal Bohai Sea in China
