NiS Decorated ZnIn2S4 Photocatalyst for the Selective Oxidation of Aromatic Alcohols with Visible Light
2020-09-25ZHANGQiaoqiaoWUYuchenYEXiangjuCHENShifu
ZHANG Qiaoqiao,WU Yuchen,YE Xiangju,CHEN Shifu,
(1.Key Lab of Clean Energy and Green Circulation,Huaibei Normal University,235000,Huaibei,Anhui,China;2.College of Chemistry and Materials Engineering,Anhui Science and Technology University,233030,Bengbu,Anhui,China)
Abstract:In order to investigate the effect of sulfide co-catalyst on the photocatalytic performance of main photocata⁃lyst,a series of NiS decorated ZnIn2S4 photocatalysts were prepared by refluxing wet chemistry and microwave-assist⁃ed methods.The structure,elemental composition and micromorphology of the resulted materials were characterized by X-ray diffraction,X-ray photoelectron spectroscopy and transmission electron microscopy.The photocatalytic per⁃formance of the as-synthesized samples was evaluated by the selective oxidation of aromatic alcohols to correspond⁃ing aldehydes under visible light irradiation.The results show that,with the content increase of NiS,the photocatalytic activities of NiS/ZnIn2S4 samples for the selective oxidation of aromatic alcohols to aromatic aldehydes increase and then decrease.When the loading amount of NiS is 1.0 wt% (namely 1.0 wt% NiS/ZnIn2S4),the highest photocatalysis activity is obtained.The enhanced photocatalytic performance of ZnIn2S4 sample may be attributed to the efficient spatial separation of the photogenerated electrons and holes after the addition of NiS,thus improving the photocatalyt⁃ic performance.
Key words:NiS/ZnIn2S4;semiconductor;aromatic alcohol;aromatic aldehyde;photocatalysis
0 Introduction
Aromatic aldehydes and their derivatives are important fine chemical intermediates,which have been widely used in the synthesis of spices,alcoholic beverage,drugs etc[1].Recently,developing a green,efficient,sus⁃tainable synthetic method to partially oxidize aromatic alcohols to corresponding aldehydes has attracted wide⁃spread interest.In recent years,narrow band gap semiconductor photocatalysts could directly utilize the visible light region of sunlight and realize the photocatalytic selective oxidation aromatic alcohols to aromatic alde⁃hydes,which have caused more and more chemists′ attention.Many photocatalysts,such as g-C3N4[2],CdS[3],and In2S3[4]etc.,have been used for the selective oxidation of aromatic alcohols to corresponding aldehydes and shown excellent photocatalytic performance under visible light irradiation.
ZnIn2S4,a novel narrow band gap ternary sulfide,has been widely used as a potential photocatalyst in many important fields[5],such as photocatalytic hydrogen evolution[6],photodegradation of organic pollutants[7],and organic photosynthesis[8].According to the previous report,ZnIn2S4photocatalyst synthesized by a hydro⁃thermal method had a good performance toward the selective oxidation of benzyl alcohol to benzaldehyde un⁃der visible light irradiation[9].Very recently,studies on semiconductor photocatalysts have indicated that cocatalysts loaded on the surface of semiconductor materials play important roles in promoting the photocatalyt⁃ic activities[10].Nickel sulfide (NiS),a p-type semiconductor,has received increasing attention owing to its vari⁃ety of specialized applications[11-13].
In this paper,a series of NiS/ZnIn2S4composite photocatalysts were prepared by refluxing wet chemistry and microwave-assisted methods.The as-synthesized NiS/ZnIn2S4composites were applied to evaluate the photocatalytic performance of photocatalytic selective oxidation of aromatic alcohols to corresponding alde⁃hydes in an oxygen atmosphere (0.1 MPa) under visible light illumination.The results indicate that among the NiS/ZnIn2S4composites,1.0 wt.% NiS/ZnIn2S4sample exhibits the best photocatalytic activity toward the selec⁃tive oxidation of aromatic alcohols under visible light for 2 h.The outstanding photocatalytic performance of ZnIn2S4may be ascribed to the efficient separation of the photogenerated electrons and holes after the addi⁃tion of NiS.
1 Experimental
1.1 Materials
Indium chloride tetrahydrate (InCl3· 4H2O),zine sulfate heptahydrate (ZnSO4· 7H2O), thiacetamide(CH3CSNH2),nickel (II) acetate tatrahydeate [Ni(Ac)2·4H2O] and benzotrifluoride (BTF) were purchased from Shanghai Chemical Reagent Co.Ltd.They were of analytical grade and used without further purification.Deion⁃ied water was used in the experiment.
1.2 Synthesis of ZnIn2S4
1.2.1 Synthesis of ZnIn2S4
In a typical procedure,ZnSO4·7H2O (1.0 mmol) and InCl3·4H2O (2.0 mmol) were dissolved in 150 mL of deionized water with a stoichiometric ratio and stirred for 30 min.Afterwards,CH3CSNH2(4.5 mmol) was add⁃ed into the above solution with vigorously stirring for 30 min to obtain a homogeneous mixture.Then the mix⁃ture was transferred into 95 ℃oil bath for 5 h.Finally,the flask was cooled to the room temperature and the final product was collected by centrifugation,washed with deionized water several times,and dried in air at 60 ℃overnight.The as-obtained sample was denoted as ZnIn2S4.
1.2.2 Synthesis ofXwt.% NiS/ZnIn2S4composites.
In a typical procedure,a certain amount of ZnIn2S4were ultrasonically dispersed in 60 mL of water,and then a varying volume of aqueous solution containing Ni(Ac)2·4H2O (1.0 mmol) and CH3CSNH2(1.0 mmol)with a stoichiometric ratio was quickly added into the above solution and then stirred for 5 h.The mass per⁃centages of NiS were selected as 0.5,1.0,1.5,2.0 and 2.5 in the composite samples.Then the solution was transferred into a 100 mL teflon liner and was put in the microwave-assisted synthesis equipment (Micro⁃SYNTH,CEM Co.Ltd,USA),which was maintained at 130 ℃ for 20 min.After naturally cooling to room temper⁃ature,the precipitants were collected by centrifugation and washed with deionized water several times respec⁃tively.The samples were dried at 60 ℃in vacuum to obtain the finalXwt.% NiS/ZnIn2S4(X=0.5,1.0,1.5,2.0 and 2.5) composites with different mass percentages of NiS.
1.3 Characterization
X-ray diffraction (XRD) data of sample were obtained on Bruker D8 advance X-ray powder diffractome⁃ter with Cu Kα radiation (λ=0.154 06 nm).X-ray photoelectron spectroscopy (XPS) was conducted on a Ther⁃mo Scientific K-Alpha at a residual gas pressure of below 1×10-8Pa,and all of the binding energies were cali⁃brated by the C 1 s peak at 284.6 eV.Transmission electron microscopy (TEM) images were performed on JE⁃OL-2010 transmission electron microscopy with an accelerating voltage of 200 kV.
1.4 Photocatalytic activity tests
The photocatalytic selective oxidation of aromatic alcohols to aromatic aldehydes was carried out as fol⁃lows:80 mg catalyst was dispersed in an inert solvent of 15 mL BTF,which contained aromatic alcohol (0.5 mmol).The mixture was transferred into a 100 mL teflon-lined stainless steel autoclave filled with molecular oxygen (0.1 MPa).The reaction was carried out under visible light irradiation by a 300 W Xe lamp (PLS-SXE 300 C,Beijing Perfect light) using a 420 nm cut-off filter as a visible light source at 70 ℃.Prior to the reac⁃tion,the suspension was stirred for 20 min in order to reach adsorption-desorption equilibrium.After the reac⁃tion,the products of the reaction were analyzed by a Gas Chromatograph (FuLi-GC9790) equipped with a SE-30 capillary column (30 m,0.53 mm,Lanzhou Atech Technologies Co.Ltd.).
Conversion of alcohol,yield and selectivity of aldehyde were defined as the follows:

WhereC0is the initial concentration of alcohol,andCalcoholandCaldehydeare the concentrations of the alcohol and aldehyde at a certain time after the photocatalytic reaction,respectively.
2 Results and discussion
2.1 Characterization of NiS/ZnIn2S4 photocatalyst
2.1.1 XRD analysis
Fig.1 shows the X-ray diffraction of blank ZnIn2S4,pure NiS and NiS/ZnIn2S4composites with different mass percentages of NiS.According to the standard cards,the crystal phase of blank ZnIn2S4can be assigned to the hexagonal phase (JCPDS No.65-2023) and the crystal phase of pure NiS belongs to the hexagonal phases (JCPDS No.02-1280)[9-10].Notably,it can be found that the XRD patterns of the NiS/ZnIn2S4are similar with the blank ZnIn2S4.Moreover,the characteristic diffraction peaks of NiS can not be clearly observed in all composite samples,possibly because of its low content and high dispersion of NiS in the composites.In addi⁃tion,no other impurities related to reactants,indicating that the NiS/ZnIn2S4composite samples have been suc⁃cessfully synthesized.
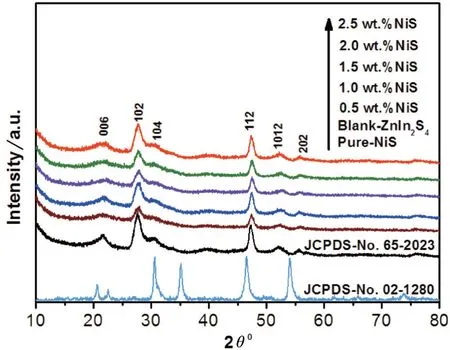
Fig.1 X-ray diffraction patterns of blank-ZnIn2S4,pure-NiS,X wt.% NiS/ZnIn2S4 samples
2.1.2 XPS analysis
XPS was used to study the surface compositions and chemical states of the elements in 1.0 wt.% NiS/ZnIn2S4sample.As shown in Fig.2A,besides the adventitious C and the adsorbed O[14],Ni,Zn,In and S elements can be monitored in the survey spectrum,indicating that 1.0 wt.% NiS/ZnIn2S4sample is composed of Ni,Zn,In and S elements.Two peaks centered at the binding energy (BE) of 1 044.8 eV (Zn 2p1/2) and 1 021.8 eV (Zn 2p3/2) can be found in the high resolution spectrum of Zn 2p,the binding energy located at 451.4 eV and 443.8 eV belongs to the characteristic two peaks of In 3d,and the spectrum S 2p indicates the peak can be overlap into two peaks,one locates at 161.9 eV and the other at 161.2 eV (Fig.2B-D)[15-19].The regional spectrum of Ni 2p shows weak doublet peaks centered at 871.8 and 854.3 eV,the BE values are close to the reported ones (Fig.2E)[20-22].This suggests that the chemical valence states of Zn,In,Ni and S ele⁃ments are +2,+3,+2 and -2,respectively.This further demonstrates that NiS/ZnIn2S4composite sample was suc⁃cessfully synthesized by refluxing wet chemistry and microwave-assisted methods.

Fig.2 XPS spectra of 1.0 wt.% NiS/ZnIn2S4,survey spectum (A)Zn 2p (B)In 3d (C)S 2p (D)Ni 2p (E)respectively
2.1.3 TEM analysis
The microscopic structure of the as-synthesized samples was investigated by TEM (Fig.3).The TEM im⁃age of blank ZnIn2S4shows that it is a layered structure and is irregularly stacked together (Fig.3A).Mean⁃while,the TEM image of 1.0 wt.% NiS/ZnIn2S4composite clearly shows that NiS nanoparticles are loaded even⁃ly on the ZnIn2S4substrate (Fig.3B).The EDS mapping result displays that all elements (such as In,S,Zn and Ni) are detected in the sample and they are dispersed uniformly in the scanning area (Fig.3C),which one again demonstrates that NiS/ZnIn2S4composite is successfully fabricated.

Fig.3 (A) TEM image of blank ZnIn2S4 (B) TEM image of 1.0 wt.% NiS/ZnIn2S4 (C) elemental mapping of 1.0 wt.% NiS/ZnIn2S4
2.2 Evaluation of photocatalytic activity
The selective oxidation of benzyl alcohol to benzaldehyde under mild conditions (T=70 ℃ and 10.132 5 kPa O2) was selected as a probe reaction to estimate the photocatalytic performance of NiS/ZnIn2S4samples.Fig.4 displays the photocatalytic performance of NiS/ZnIn2S4with different mass percentages of NiS towards the selective oxidation benzyl alcohol to benzaldehyde.It can be seen that,with the content increase of NiS cocatalyst,the photocatalytic activity of NiS/ZnIn2S4for the selective oxidation of benzyl alcohol to benzaldehyde increases and then decreases.Among all of the NiS/ZnIn2S4samples,1.0 wt.% NiS/ZnIn2S4exhib⁃its the best photoactivity,which the conversion of benzyl alcohol reaches 68.1% and the yield of benzalde⁃hyde is 59.0% under visible light irradiation for 2 h.In addition,the control experiment shows that blank NiS has negligible photocatalytic activity.The result shows that the introduction of an appropriate amount of NiS co-catalyst into the bulk ZnIn2S4can efficiently improve its photocatalytic performance.
In order to further research the general applicability of blank ZnIn2S4and 1.0 wt.% NiS/ZnIn2S4samples,the photocatalytic selective oxidation of a series of aromatic alcohols with different substituted groups under the same reaction conditions was carried out and the results were shown in Fig.5.It can be observed that aro⁃matic alcohols show an excellent conversion and high selectivity over blank ZnIn2S4and 1.0 wt.% NiS/ZnIn2S4samples,clearly indicating that the as-prepared samples have wide applicability.In addition,the con⁃versions of aromatic alcohols and yields of aromatic aldehydes over 1.0 wt.% NiS/ZnIn2S4sample are better than that of blank ZnIn2S4sample.Meanwhile,it is noteworthy that the photocatalytic activity sequence of psubstituted benzyl alcohols is as follows:p-methoxybenzyl alcohol> p-methylebenzyl> benzyl alcohol> p-chlo⁃robenzyl alcohol> p-fluorobenzyl alcohol,which reveals that the p-substituted benzyl alcohols with electrondonating group show higher photocatalytic performance than that of the p-substituted benzyl alcohols with electron-withdrawing group[3,23-24]. Meanwhile,the photocatalytic activity sequence of methoxybenzyl alcohol with different substitution positions is as follows:p-methoxybenzyl alcohol> m-methoxybenzyl alcohol> o-me⁃thoxybenzyl alcohol,which implies that there indeed exists an effect of steric hindrance in the reaction[25].
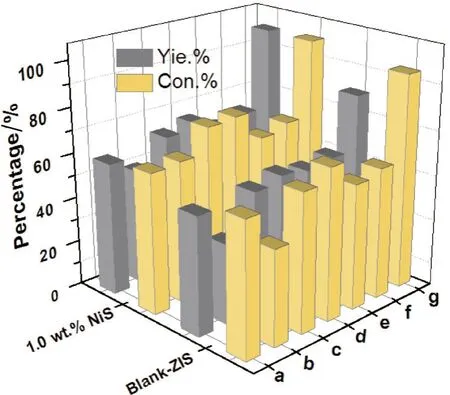
Fig.5 Photocatalytic performance of selective oxidation of aromatic alcohols to corresponding aldehydes with blank ZnIn2S4 and 1.0 wt.% NiS/ZnIn2S4 composite under visible light irradiation.(a) benzyl alcohol;(b) p-fluorobenzyl alcohol;(c) p-chlorobenzyl alcohol;(d) p-methylebenzyl alcohol;(e) o-methoxybenzyl alcohol;(f) m-methoxybenzyl alcohol;(g) p-methoxybenzyl alcohol.Yie.and Con.are short for yield of the product and conversion of the reactant,respectively
3 Conclusions
In this work,NiS/ZnIn2S4composite photocatalysts with different mass percentages of NiS were prepared by refluxing wet chemistry and microwave-assisted methods.Results indicate that the as-obtained NiS/ZnIn2S4composites exhibit excellent photocatalytic performance for the selective oxidation of aromatic alco⁃hols to corresponding aldehydes under visible light irradiation.Furthermore,1.0 wt.% NiS/ZnIn2S4composite shows the best photocatalytic activity among the examined samples.The improved photocatalytic activity may be due to the addition of NiS co-catalyst,which can effectively separate and transfer of the photo-generated electron-hole pairs in the reaction system.
