Mechanism Prediction of Monotropein for the Treatment of Colorectal Cancer by Network Pharmacology Analysis
2020-09-25LIChongHOUShoZhenZHOUHu
LI Chong, HOU Sho-Zhen, ZHOU Hu,c*
a.Faculty of Chinese Medicine and State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology,Taipa, Macao 999078, China
b.School of Pharmaceutical Sciences, Guangzhou University of Chinese Medicine, Guangzhou, Guangdong 510006, China
c.Joint Laboratory for Translational Cancer Research of Chinese Medicine of the Ministry of Education of the People’s Republic of China,Macau University of Science and Technology, Taipa, Macao 999078, China
Keywords
Colorectal cancer
Monotropein
Network pharmacology
Target
Protein kinase B (AKT1)
ABSTRACT
Objective To discover the pharmacological mechanisms of monotropein in colorectal cancer by network pharmacology methods.
Methods The main-candidate-target network was constructed by the prediction of targets of monotropein, collection of therapeutic targets of colorectal cancer drugs, and construction of the target network and layers of screening.The data were interpreted by pathway enrichment and target score calculation.
Results This study: (1)Demonstrated the potential of monotropein to be a multi-target drug against colorectal cancer using a computational approach; (2)Discovered 10 candidate targets of monotropein, among which protein kinase B (AKT1)exhibited the highest relevance and importance to colorectal cancer and proto-oncogene tyrosine-protein kinase Src (SRC),Bruton’s tyrosine kinase (BTK), and heat shock protein HSP 90-alpha (HSP90AA1)also exhibited high relevance; (3)Observed 32 possible pathways related to the effects of monotropein on colorectal cancer, which might explain the mechanism of its action; and (4)Established a method to assess the importance of targets in the network.
Conclusions This study offered clues for the mechanism of the bioactivities of monotropein against colorectal cancer by network analysis.Monotropein has the potential to be a multi-target drug against colorectal cancer, which lays the foundation for its clinical applications and further study.
1 Introduction
Colorectal cancer is a common malignant tumour of the gastrointestinal tract.According to the data of the World Health Organization International Agency for Research on Cancer, colorectal cancer seriously threatens human health; its incidence and mortality are among the highest of malignant tumours[1].In China, the incidence and mortality of colorectal cancer continue to increase[2], which may be related to changes in the environment and diet.The pathogenesis of colorectal cancer is complex and still unclear.Possible influencing factors include dietary factors, such as high fat, high protein, and low dietary fibre intake and vitamin deficiency[3]; intestinal flora imbalance[4]; and disease factors, such as ulcerative colitis, colorectal polyps and adenomas, age, and genetic factors[5].Currently, surgery is the main treatment for colorectal cancer, followed by radiotherapy, chemotherapy and immunotherapy.The emergence of targeted therapy has brought significant survival benefits to colorectal cancer patients as it has significantly improved clinical efficacy and 5-year survival rate.Most of these drugs are single-target.However, the treatment of colorectal cancer is still a challenge.Multi-target drugs possess advantages over single-target drugs in clinical practice[6].Therefore, a promising direction is to develop multi-target drugs for the treatment of colorectal cancer.
Monotropein is the primary iridoid glycoside extract from a commonly used Chinese medicines,Morinda officinalis(Rubiaceae).M.officinalishas been used for the treatment of infection, arthritis,diabetes, asthma, hypertension and pain for centuries in China.Monotropein has various bioactive effects, such as its antinociceptive and anti-inflammatory effects on paw oedema induced by carrageenan in rats[7]; antiapoptotic and anticatabolic activities in interluekin-1βstimulated osteoarthritic chondrocytes[8]; mineralization, proliferation and differentiation promotion in osteoblastic MC3T3-E1 cells[9]; and angiogenic promotion and oxidative stress-induced autophagy inhibition in endothelial progenitor cells to accelerate wound healing[10].Although studies regarding its pharmacological activities are limited, those that exist indicate that it may possess multifunctional and multi-target characteristics and have anticancer activity.However, its role in the treatment of colorectal cancer and the mechanism of action are not clear.
In this study, a computational strategy was used to study the possible action mechanism of monotropein on colorectal cancer.In terms of target prediction, the potential targets of monotropein were identified using a pharmacophore mapping approach.In terms of information interpretation, the data were interpreted by the construction of a network of drug targets and therapeutic targets, network analysis, pathway enrichment, and target score calculation.This study may increase our understanding of the bioactivities of monotropein against colorectal cancer and lay the foundation for its clinical application and further study.
2 Materials and Methods
2.1 Prediction of putative targets for monotropein
To find the relationship between monotropein and colorectal cancer, targets of monotropein were collected by target prediction.The mol2 format structure of monotropein was prepared using Chembio3D Ultru14.0.Then, the mol2 file was uploaded into the PharmMapper server (http://lilab.ecust.edu.cn/pharmmapper/submit_file.php), which is a freely accessible web-server designed to identify potential target candidates for a given probe of small molecules using a pharmacophore mapping approach.The target set was selected as “Human Protein Targets Only (v2010, 2241)”; other parameters were default values.The results were arranged by fit score.The top 50 predicted targets out of 300 were selected for subsequent research.These predicted targets of monotropein were defined as“drug targets”.
2.2 Collection of colorectal cancer drugs and therapeutic targets
To find the relationship between monotropein and colorectal cancer, colorectal cancer drug targets were then collected by retrieval.The colorectal cancer drug target information was collected from the DrugBank database (https://www.drugbank.ca/,version 5.0).“Cancer” “tumour” and “colon” were used as keywords in DrugBank.Drugs approved by the Food and Drug Administration for the treatment of colorectal cancer and drugs used in clinical trials and as experimental medication were collected.Successful and research targets were included in our therapeutic target database.All targets collected from DrugBank were defined as “therapeutic targets”.The result list of colorectal cancer drugs was manually inspected one by one by drug indications to assure the quality of the data.
2.3 Network construction and analysis
The protein-protein interaction (PPI)network among drug targets and therapeutic targets was constructed by MAS 3.0 (http://bioinfo.capitalbio.com/mas3/).According to the method described in ZHANG et al.[11],layers of screening were carried out by calculating network topology parameters to obtain the main candidate nodes.Briefly, the degree of the nodes from the PPI network was calculated to construct the hub network.Four topological features, “degree”“betweenness” “closeness” and “k-core”, were calculated to identify the main candidate nodes in the hub network.Main candidate nodes were defined as those with values of the four features that were larger than the corresponding median values.Statistical analysis of KEGG pathway enrichment of the target profile was performed by MAS 3.0 and visualized by R packetgglot2.Significance of pathway enrichment was reflected byPvalue which was calculated by comparing the observed frequency of an annotation term with the frequency expected by chance.The values of four topological features,“degree” “betweenness” “closeness” and “k-core”were calculated by Pajek 1.0.Networks were visualized by Cytoscape 3.3.0 and Gephi 0.9.2.
2.4 Calculation of comprehensive score (CS)
The CS was calculated to evaluate the relative importance of drug targets.The CS of a target was represented by the module of its vector.A vector for a target had five dimensions, which were related to its degree of fit towards monotropein and their importance in the network.The five dimensions were“fit score” “degree” “closeness” “betweenness” and“k-core”.The values of the five dimensions were standardized before the calculation of the CS.The CS for drug target n CS(n)is defined by:
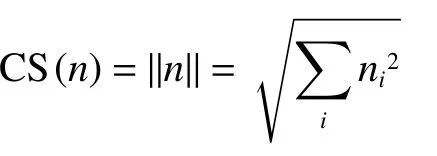
Where:nirepresents parameters in the five dimensions andnrepresents the vector of drug targets.
3 Results
3.1 Prediction of the targets of monotropein by PharmMapper
The molecular formula of monotropein is C16H22O11and the molecular weight is 390.34.The detailed information about the chemical structure of monotropein is shown in Figure 1.To obtain the potential targets of monotropein, the mol2 structure of monotropein was imported into the PharmMapper server for analysis.We obtained the top 50 drug targets out of 300 according to their fit score.
3.2 Collection of colorectal cancer drugs and therapeutic targets in DrugBank
To understand whether and how monotropein affects colorectal cancer, information on disease-related therapeutic targets were collected.The information of colorectal cancer drugs and their targets was gathered from DrugBank database.In total, 178 therapeutic targets for 106 drugs were obtained.The collection included broad-spectrum anticancer and anti-colorectal drugs, such as capecitabine,vandetanib and fluorouracil.
3.3 Network construction and analysis
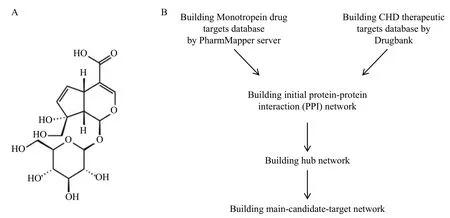
Figure1 The chemical structure of monotropein and research strategy
By connecting 50 drug targets and 178 therapeutic targets, the drug was related to the disease.All drug targets and therapeutic targets were connected to form an initial PPI network that included 2 983 nodes and 6 504 edges (Figure 2A).After the initial PPI network was constructed, layers of screening were carried out by calculating network topology parameters.First, the hub nodes in the initial PPI network were identified by calculating the degree of the nodes.If the degree of a node was greater than twice the median degree of all nodes in the network,the node was defined as a hub.A network of hub nodes that consisted of 766 nodes and 3 722 edges was constructed (Figure 2B).Then, four topological features, “degree” “betweenness” “closeness” and “kcore”, were calculated to identify the main candidate nodes.As a result, 126 main candidate nodes, whose“degree” “betweenness” “closeness” and “k-core”values were all larger than the corresponding median values of all nodes in the hub network, were identified.Then, a network of main candidate nodes that consisted of the 215 nodes was constructed.To focus on the nodes of concern, intermediate proteins generated by MAS 3.0 were deleted from the main candidate node network.Then, the main candidate target network that consisted of 78 nodes was constructed, in which 10 drug targets and 70 therapeutic targets were included (Figure 2C).The 10 main candidate drug targets were Bruton’s tyrosine kinase (BTK), heat shock protein HSP 90-alpha(HSP90AA1), GTPase HRas (HRAS), protein kinase B(AKT1), membrane metalloendopeptidase (MME),deoxyuridine 5’-triphosphate nucleotidohydrolase,tyrosine-protein phosphatase non-receptor type 1(PTPN1), vitamin D3 receptor (VDR), 3-phosphoinositide-dependent protein kinase 1(PDPK1), and proto-oncogene tyrosine-protein kinase Src (SRC).BTK and HSP90AA1 were both drug targets and therapeutic targets.Fifteen of the therapeutic targets were successful targets, whereas the rest were investigational targets.Drug target profiles in the main candidate target network are shown in Table 1.
3.4 Potential role of monotropein in the treatment of colorectal cancer
Pathway enrichment analysis of the main candidate target network of monotropein was performed to explore the potential role of monotropein in the treatment of colorectal cancer (Figure 3).Thirty-two pathways were related.They were classified into four categories, “cancer” (13 pathways)“proliferation,apoptosis and inflammation” (14 pathways)“cell connection and migration” (6 pathways)and“cellular immunity and drug resistance” (7 pathways).The main candidate drug target, AKT1,was involved in the colorectal cancer pathway directly.The main candidate drug targets AKT1,HRAS, HSP90AA1 and PDPK1 were involved in pathways related to cancer.The main candidate drug targets AKT1, HRAS, PDPK1 and SRC were involved in pathways related to proliferation, apoptosis and inflammation.The main candidate drug targets AKT1, HRAS, PDPK1, PTPN1 and SRC were involved in pathways related to cell connection and migration.The main candidate drug targets AKT1, HRAS, BTK and HSP90AA1 were involved in pathways related to cellular immunity and drug resistance.Among the 10 main candidate drug targets, AKT1 exhibited the highest relevance to colorectal cancer.
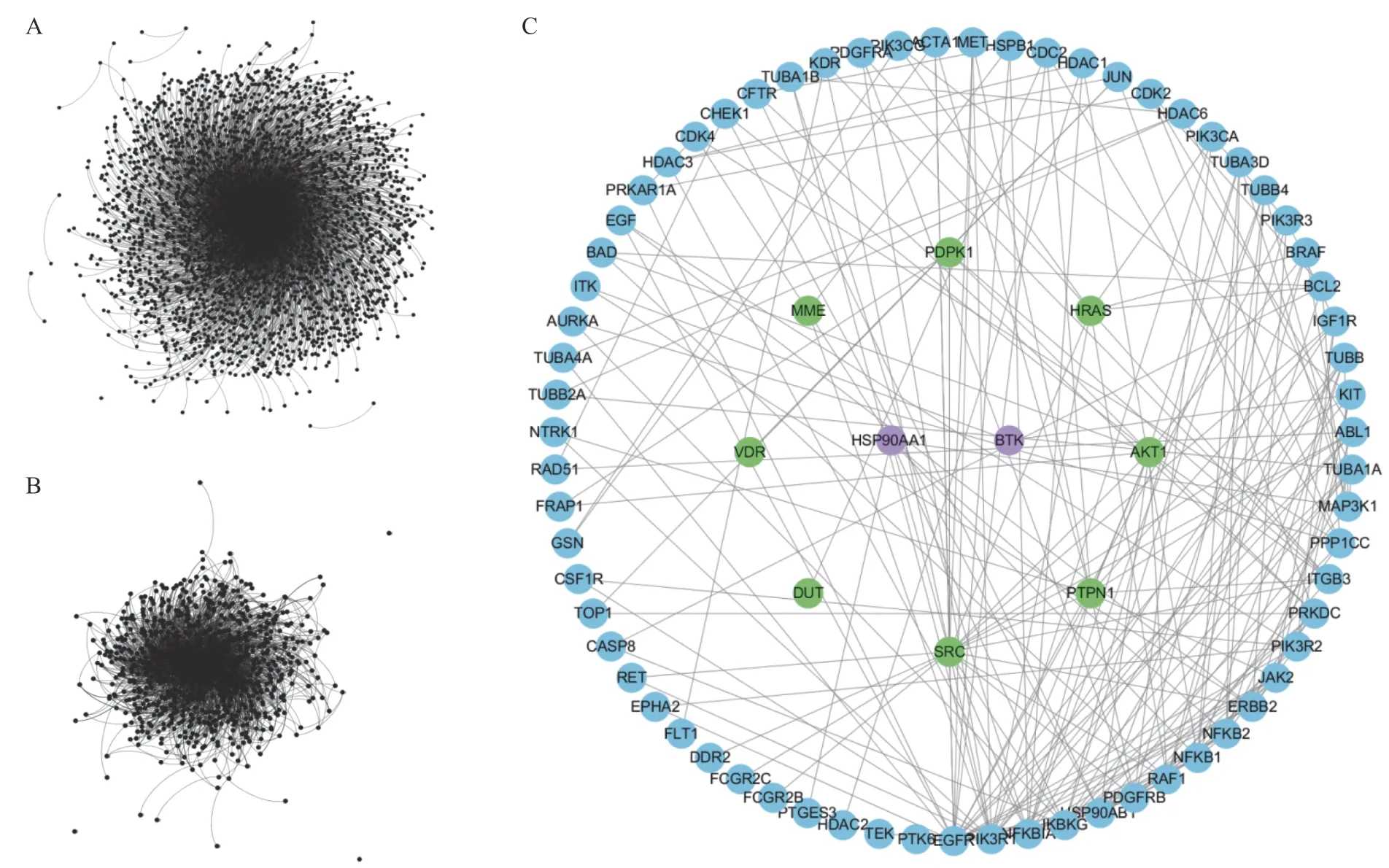
Figure2 Network analysis of monotropein
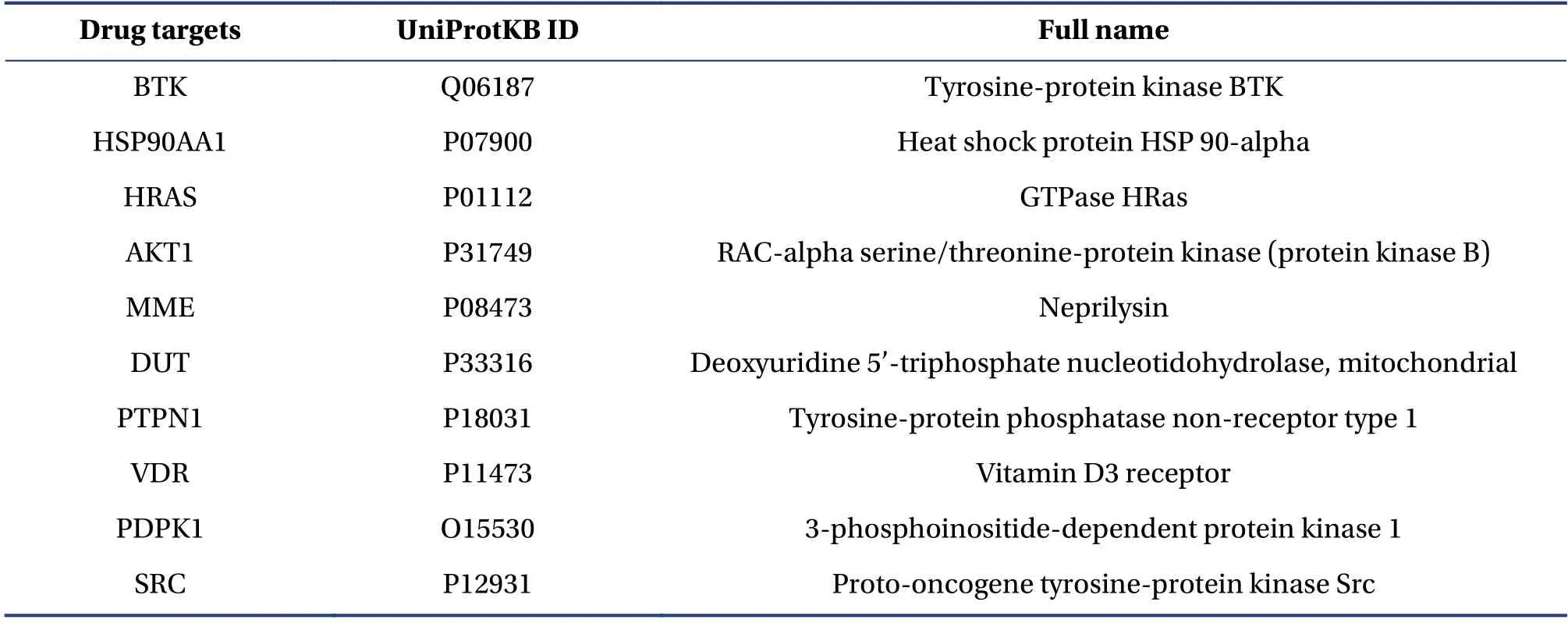
Table1 The main candidate drug target profile of monotropein

Figure3 Pathway enrichment analysis of main candidate target network of monotropein
3.5 Possible targets of monotropein responsible for the treatment of colorectal cancer
The CS of the main candidate drug targets was calculated to rank their possible contribution to the treatment of colorectal cancer (Table 2).CS values ranged from high to low.SRC exhibited the highest CS, followed by AKT1.The values of the five dimensions of SRC were almost the highest compared to the other targets, whereas the values of“degree” and “betweenness” of AKT1 were lower than those of SRC; thus, SRC may have an important role in the drug-disease target network.
3.6 Important targets of monotropein in colorectal cancer-related pathways
To obtain an overall understanding of the role of AKT1, SRC, BTK, HSP90AA1 and VDR on colorectal cancer, they were mapped to colorectal cancerrelated pathways (Figure 4).These five important targets of monotropein were involved in phosphatidylinositol 3-kinase (PI3K)/Akt, nuclear factor-kappa B, VDR and ErbB signalling pathways.They have critical regulatory actions in cell proliferation, differentiation, inflammation, apoptosis, migration and survival in the gut tissue.
4 Discussion
The major results of this study are: (1)Monotropein has the potential to be a multi-target drug against colorectal cancer, as shown by this computational approach; (2)10 candidate targets of monotropein,among which AKT1 exhibited the highest relevance and importance to colorectal cancer, followed by SRC, BTK and HSP90AA1, were identified; (3)32 possible pathways related to the effects of monotropein on colorectal cancer, which might explain the mechanism of its action, were identified;and (4)A method to assess the importance of targets in the network was established.
Among the 10 candidate targets of monotropein,AKT1, SRC, BTK and HSP90AA1 are of great importance according to the results of this network analysis.AKT1 was directly involved in the colorectal cancer pathway and was involved in all four related categories of monotropein.SRC exhibited the highest importance in the drug-disease target network.BTK and HSP90AA1 were both drug targets and therapeutic targets.
AKT1, also known as RAC-alpha serine/threonine-protein kinase, is one of three closely related serine/threonine-protein kinases (AKT1, AKT2 and AKT3).AKT1 regulates many processes including metabolism, proliferation, survival, growth and angiogenesis.These processes are mediated through the serine and/or threonine phosphorylation of a range of downstream substrates[12].The PI3K/ AKT/mammalian target of rapamycin pathway is one of the most commonly dysregulated signalling pathways related to cancer development and progression[13].AKT participates in the colorectal cancer pathway and regulates processes of cell apoptosis and proliferation[14].
SRC, also known as proto-oncogene tyrosine-protein kinase Src, is a non-receptor protein tyrosine kinase.It participates in signalling pathways that control a diverse spectrum of biological activities, including gene transcription, the immune response,cell adhesion, cell cycle progression, apoptosis,migration and transformation[12].ROCHA et al.[15]showed that Annexin A2 (ANXA2)overexpression plays a pivotal role in colorectal cancer invasiveness through Src/ANXA2/signal transducer and activator of transcription 3 pathway activation.CHEN et al.[16]observed that 3-hydroxy-3-methylglutaryl-CoA synthase 2 enhances invasion and metastasis via direct interaction with peroxisome proliferator-activated receptor-αto activate SRC signalling in colorectal cancer.
The tyrosine-protein kinase BTK is indispensable for B lymphocyte development, differentiation andsignalling.BTK is also a target of an investigational small molecule drug called XL418[17].XL418 inhibits the activity of AKT and S6 kinase, which act downstream of PI3K.The activation of these kinases is a frequent event in human tumours as this promotes cell growth, survival, and resistance to chemotherapy and radiotherapy.However, the action of BTK in colorectal cancer is currently unknown.

Table2 CS of main candidate drug targets
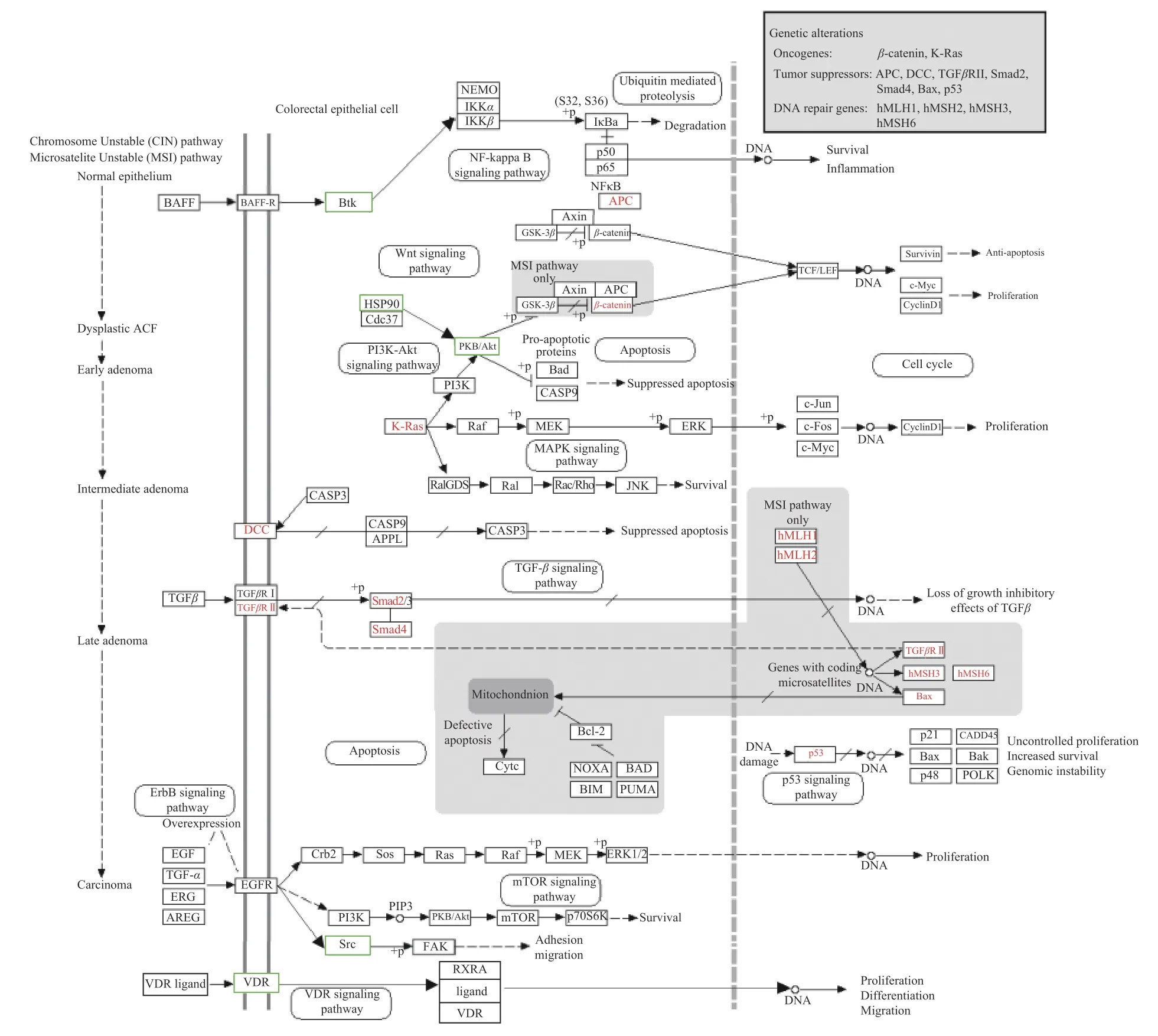
Figure4 Important targets of monotropein mapped to colorectal cancer related pathway
HSP90AA1, also known as heat shock protein HSP 90-alpha, is a molecular chaperone that promotes the maturation, structural maintenance, and proper regulation of specific target proteins involved in cell cycle control and signal transduction.HSP90AA1 is the target of investigational antitumor agents such as SNX-5422, tanespimycin and alvespimycin[17].These are inhibitors of HSP90AA1 and cause the degradation of important HSP90AA1 client proteins, such as human epidermal growth factor receptor 2, epidermal growth factor receptor, AKT, and extracellular signal-regulated kinase.These client proteins are also referred to as oncoproteins and are involved in cellular signalling pathways that drive cellular proliferation and counteract apoptosis.
VDR is also reported to be closely related to colorectal cancer.PALMER et al.[18]generated human SW480-ADH colon cancer cells that ectopically express mouse hemagglutinin-tagged Snai1 protein(SNAIL-HA).The overexpression of Snai1 in these cells results in lower VDR mRNA and protein expression and inhibits the induction of E-cadherin and VDR by 1,25(OH)2D3.A 1,25(OH)2D3analogue inhibits tumour growth in immunodeficient mice injected with mock cells, but not in those injected with SNAIL-HA cells.The authors concluded that the balance between VDR and SNAI1 expression is critical for E-cadherin expression, which influences cell fate during colon cancer progression.
Currently, many antitumor agents commonly used in clinical practice are single-target drugs,which can cause side effects and drug resistance.For example, cetuximab and panitumumab, whose target is EGFR, may cause different degrees of skin toxic reaction; fluorouracil and capecitabine, whose target is thymidylate synthase, can cause myelosuppression and digestive tract toxicity; and irinotecan,whose target is topoisomerase I, may cause delayed diarrhoea and neutropenia.In the process of cancer treatment, the side effects of drugs are usually unbearable for patients.Furthermore, the efficacy of these drugs is not satisfactory enough for their high sensitivity to patient variability.However, multi-target drugs can affect multiple pathways at the same time and sometimes produce synergistic effects among the multiple pathways.Thus, multi-target drugs produce fewer side effects and are less sensitive to patient variability[19].Multi-target drugs possess advantages over single-target drugs in clinical practice[6].
According to the results of our study, monotropein has the potentiality to be a multi-target drug for the treatment of colorectal cancer.Indirect interaction targets and direct targets together constitute the target network of monotropein and this contributes to the therapeutic effect of monotropein on colorectal cancer in a multi-dimensional manner.Although the indirect interaction targets are involved in pathways closely related to colorectal cancer, they are not known therapeutic targets.For example, the important potential targets of monotropein, AKT1,SRC and VDR, are indirect targets.Currently, there is no available single-target drug for these targets.This may be because these targets have a wide range of functions and single-target drug that aims at them will probably cause a variety of side effects.Additionally, the regulation of these targets alone may not be enough to produce therapeutic effects.
As for one of the challenges faced by network pharmacology, target prediction, there are some ready-made tools available online, which can be divided into four categories according to their principles.These are target prediction tools based on reported literature, molecular docking, molecular similarity, and pharmacophore modelling.These methods have their advantages and disadvantages.Several target databases are based on reported literature.The targets in these databases have been verified by experiments.Unfortunately, the known information is limited.Compounds may have unreported targets and there is no information about new compounds.
Molecular docking-based target fishing is a broadly used structure-based method based on protein-ligand interactions.There are three types of docking approaches, rigid docking, semi-flexible docking, and flexible docking.The rigid docking approach does not take into account the possible conformational changes of small molecules during docking to their putative targets, thus, the calculation cost and accuracy are low.However, a flexible docking approach consumes a lot of time because of the large amount of calculation required; thus, it is only suitable for accurate intermolecular recognition.Semiflexible docking offers a compromise between the two.Molecular similarity-based target prediction is a ligand-based method that can be divided into two types: two-dimensional and three-dimensional similarities.This method relies on the prior knowledge of biologically active ligands and cannot find new targets.Furthermore, similar parts of similar molecules may not be related to their activities, which may lead to misleading results.In this study, the potential targets of monotropein were identified by PharmMapper using a pharmacophore mapping approach.Pharmacophore modelling has the advantages of both structure-based and ligand-based methods and not only considers complementary pairing principles of 3D chemical structures but also avoids the influences caused by the differences in conformations of binding sites residues.According to a paper published by the PharmMapper team[20], the accuracy of PharmMapper for target prediction can be expressed by receiver operating characteristic enrichment(ROCE).ROCE is defined as the ratio of true positive rate (TPR)to false-positive rate (FPR)at a given screening stage at which a percentage of the rankings has been returned.The values of ROCE increase as the score ranking decreases, which indicates that the ratio of TPR and FPR is much higher when the ranking is near the top.The higher the accuracy of the target prediction, the more valuable the results of the PPI network analysis.In this part of the study, the top 50 predicted drug targets were selected to increase the number of true positive results as much as possible and avoid excessive false-positive results.
Another challenge faced by network pharmacology is information interpretation and this study attempted to use a preliminary strategy to analyse the data.First, a network of drug targets and disease-related targets was constructed.Then, the important nodes in the network were screened using a network topology parameter calculation.Signal pathways involved in drug targets related to colorectal cancer were identified through pathway enrichment analysis.According to the importance of candidate drug targets in the network and the matching degree of drug binding, the targets were scored and sorted.The scoring system takes advantage of the concept of vectors.Finally, the possible targets of monotropein for the treatment of colorectal cancer were predicted according to the results of pathway enrichment analysis and comprehensive scoring.Although the purpose of this part of the study was to identify and establish a suitable analysis method theoretically, the predicted results still need to be further verified in cell and animal models.
5 Conclusions
The aim of this study was to discover a method to solve two of the problems faced by network pharmacology: target prediction and information interpretation.A computational strategy was used to identify important candidate targets of monotropein that may act on colorectal cancer.This study offered clues for the mechanism of the bioactivities of monotropein against colorectal cancer by network analysis.Monotropein has the potential to be a multitarget drug against colorectal cancer, which lays the foundation for its clinical applications and further study.
Acknowledgements
We thank for the funding support from the Joint Research Fund for Overseas Chinese, Hong Kong and Macao Young Scholars of National Science Foundation of China (No.81929003), the Science and Technology Development Fund, Macau SAR(No.0027/2017/AMJ)and the National Key Research and Development Program of China (No.2017YFE0119900).
Competing Interests
The authors declare no conflict of interest.
杂志排行
Digital Chinese Medicine的其它文章
- Instructions for Authors
- Discussion on Etiology and Pathogenesis of Corona Virus Disease 2019 from “Cold-dampness and Insidious Dryness”
- Clinical Intelligent Diagnosis Path Based on the Chief Complaint
- Effect of Electroacupuncture on Platelet-derived Growth Factor and the Ultrastructure of Mitochondria in Rats with Diabetic Gastroparesis
- Regularity of Wind-dispelling Medication Prescribed by LI Dong-Yuan: A Data Mining Technology-based Study
- Research on the Correlation Between Physical Examination Indexes and TCM Constitutions Using the RBF Neural Network
