Preparation of Fe3O4@PCDP nanocomposite and its application in Fenton-like system*
2020-09-17CHENFengxiaMAJiahai
CHEN Fengxia, MA Jiahai
(1 Sino-Danish College, University of Chinese Academy of Sciences, Beijing 100190, China;2 School of Chemical Sciences, University of Chinese Academy of Sciences, Beijing 100049, China)
Abstract In this study, a Fe3O4@PCDP nanocomposite is prepared as a highly efficient and eco-friendly heterogeneous Fenton-like catalyst that is used for the degradation of Rhodamine (RhB). The structure of the Fe3O4@PCDP nanocomposite is characterized by XRD, FTIR, and SEM, and the results demonstrate that it is synthesized successfully. The catalytic capacity of Fe3O4@PCDP is evaluated on the basis of various parameters, including the concentration of H2O2 and the pH value. The removal rate of RhB reaches 87.25 % 42 h after the reaction under the optimal condition. The structure of Fe3O4@PCDP does not change and the catalytic performance is stable after the third cycle.
Keywords Fe3O4@PCDP; catalyst; Fenton reaction
With the development of industry and economy, the environmental problems, such as haze, greenhouse effect, destruction of the ozone layer, land desertification, and water pollution, have seriously threatened the development of society and human health. Among them, the water pollution has become one of the main causes of human death and disease. Textile and dyeing industry is a traditional pillar industry and one of the oldest national industries in China. A large quantity of textiles is produced and sold around the world every day. However, the printing and dyeing wastewater is produced at the same time, and most is discarded without purification resulting in serious water pollution[1-4].
Fenton oxidation technology can be used to degrade dye pollutants on account of the mild reaction condition, fast reaction rate, and simple equipment. The basic mechanism is that Fe2+can be used as catalyst to decompose H2O2into hydroxyl radical (·OH), which degrades dye pollutants. The Fe3+generated in the reaction reduces to Fe2+by reacting with H2O2. So the iron cycle is built[5-6].
However, the traditional Fenton technology generates a large amount of hydroxide precipitation which leads to secondary pollution. It has been extensively demonstrated that Fe3O4can serve as heterogeneous Fenton-like catalyst due to the low toxicity, abundant resources, and low price. The possible basic mechanism is
The previous study showed that Fe3O4adsorbs H2O2on its surface to form ≡ FeIIIH2O2complex (Eq. (1)), then ≡ FeIIIH2O2converts into ≡ FeIIand ·HO2(Eq. (2)) and the generated ·HO2further reduces≡ FeIIIto ≡ FeII(Eq. (3)). All the obtained ≡ FeIIdecomposes H2O2into ·OH (Eq.(4)), which reacts with organic contaminants into carbon dioxide and water (Eq. (5)). However, Fe3O4nanoparticles tend to aggregate together in the water system due to strong anisotropic dipolar interaction, which reduces the dispersibility and catalytic activity of the nanoparticles. Therefore, Fe3O4nanoparticles are considered to be fixed on special substrates, such as graphene oxide, SiO2, molecular sieve, activated carbon, etc., to improve the catalytic performance.
Cyclodextrin crosslinked polymer is a kind of eco-friendly, biodegradable, and low cost mesoporous material, and the catalytic active sites can load in the mesostructure in a highly dispersed state to guarantee the catalytic performance[7]. In addition, cationic organic pollutants can be trapped by the inner cavity of β-CD to form complex, which allows the adjacent ·OH to degrade pollutant directly[8]. Hence, cyclodextrin polymer is considered as catalyst supports for Fe3O4, and the strong force between hydroxyl group and Fe3O4fixes the Fe3O4nanoparticles on cyclodextrin polymer to form Fe3O4@cyclodextrin polymer composite.
In this work, β-cyclodextrin is crosslinked with tetraflorophenedionitrile to form polymer (PCDP) which serves as support material. Fe3O4nanoparticles are fixed on PCDP to prepare Fe3O4@PCDP composite catalyst. The composite is characterized by XRD, FTIR, and SEM. A common cationic dye Rhodamine B (RhB) is selected as targeted pollutant, and the result demonstrates that heterogeneous Fenton-like catalyst Fe3O4@PCDP is superior to Fe3O4while degrading RhB. The catalytic effect of Fe3O4@PCDP is evaluated on the basis of various parameters, including the concentration of H2O2and pH. The reusability of Fe3O4@PCDP is also investigated.
1 Experimental section
1.1 Materials
Ferric trichloride (99%) was from Tianjinfucheng. Iron(II) sulfate heptahydrate (99%). Hydrogen peroxide (27%) and potassium carbonate (99%) were from Alfa. Tetrafluoroterephthalonitrile (99%) and β-cyclodextrin (97%) were from Sigma. Dichloromethane (AR) and tetrahydrofuran (AR) were from Aladdin. Rhodamine B was from Amresco.
1.2 Synthesis of Fe3O4@PCDP
Fe3O4: 50 mg anhydrous ferric chloride and 76.8 mg iron (II) sulfate heptahydrate were added in 50 mL ultrapure water. Using mechanical agitation at 90 ℃ in nitrogen atmosphere and adding ammonia to adjust pH to 9.0, then heating 0.5 h. After cooling the system to room temperature and separating by magnet, the obtained product was washed by water and ethanol for 2 times and dried in the vacuum drying oven[9].
PCDP: 0.8 g β-cyclodextrin, 0.4 g tetrafluoroterephthalonitrile, and 1.2 g potassium carbonate were mixed in 30 mL tetrahydrofuran. The reaction occurred in N2atmosphere and was stirred for 48 h under 80 ℃. The obtained orange suspension was filtered and washed by 0.1 mol/L HCl to remove K2CO3. After washing by water, tetrahydrofuran, and dichloromethane, the obtained yellow solid was dried in the vacuum drying oven at 40 ℃ for 2 d[10].
Fe3O4@PCDP: 300 mg Fe3O4was dispersed in 25 mL PBS and 50 mg PCDP was dispersed in 200 mL water, and then PBS was added into the water. The mixed system was stirred for 3 h and centrifuged for 15 min (4 000 rpm), and the solid was dried in the vacuum drying oven at 37 ℃ for 24 h[11].
1.3 Characterization
The FTIR spectrum was measured by a Nicolet VERTEX 70 (Bruker Crop) spectrometer. KBr was used as diluent.
The nitrogen adsorption-desorption isotherms was carried out on a Quantachrome NOVA 2000e.
Power X-ray diffraction (XRD) data was measured by a Bruker D8-Advance. The scan range was form 5° to 80°.
The SEM image was performed by Hitachi S-4800 Field Emission Scanning Electron Microscope.
1.4 Fenton-like degradation experiments
25 mg catalyst Fe3O4@PCDP was added in 50 mL RhB solution (200 mmol/L); the pH of the system was adjusted to 3; and the concentration of H2O2in the system was 20 mmol/L. The reaction system was put on an oscillator to make the reactants mixed well. The concentration of RhB was measured at 3, 6, 18, 30, and 42 h by a TU-1900 UV-Visible spectrophotometer at 554 nm.
2 Results and discussion
2.1 Characterization of Fe3O4@PCDP
In FTIR spectra (Fig. 1(a)), the characteristic peaks appeared at 2 240, 1 265, and 1 485 cm-1are assigned to the vibrations of C≡N, C-F, and aromatic skeleton of tetrafluoroterephthalonitrile. 2 935, 3 418, and 1 027 cm-1are attributed to the stretching vibrations of C-H, O-H, and C-O of β-Cyclodextrin in polymer. This indicates that the polymer PCDP is synthesized successfully. The nitrogen adsorption-desorption isotherms (BET) is shown in Fig. 1(b). The BET surface of synthesized PCDP is 46 m2/g which is lower than the number in the literature. It is maybe because the different dried methods are used. The pore-size distribution of synthesized PCDP is in a range from 1.8 to 3.5 nm, which is similar to that in the literature. In conclusion, PCDP was prepared successfully[10].
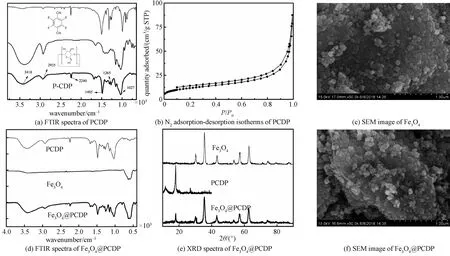
Fig.1 Characterization of PCDP, Fe3O4, and Fe3O4@PCDP
In Fig. 1(d), a strong characteristic peak appeared at 570 cm-1is attributed to the Fe-O stretching vibration. Besides, the synthetic Fe3O4has five XRD characteristic peaks, and they are (2 2 0), (3 1 1), (4 0 0), (5 1 1), and (4 4 0) (Fig. 1(e)), respectively. The diameter of the synthetic Fe3O4is about 20-50 nm (Fig. 1(c)), which means uniform Fe3O4nanoparticles were synthesized[9].
In Fig. 1(d), the characteristic peaks of Fe3O4and PCDP are both displayed in Fe3O4@PCDP and no new characteristic peak appears. In addition, the XRD spectrum of Fe3O4@PCDP also shows the characteristic peaks of Fe3O4and PCDP. There is no new substance produced (Fig. 1(e)). This is because the combination of Fe3O4and PCDP is attracted by the strong force between Fe and -OH. The SEM images show Fe3O4nanoparticles is dispersed on PCDP uniformly (Fig. 1(f)).
2.2 Catalytic properties of Fe3O4@PCDP
Comparative experiments were performed to access the performance of Fe3O4@PCDP in RhB degradation (Fig. 2(a)). The reaction was carried out at pH=3. The catalytic efficiency of Fe3O4@PCDP is higher than Fe3O4. About 87.25% RhB was degraded while Fe3O4only degraded less than 40% after 42 h. Remarkably, the RhB degradation curve of Fe3O4@PCDP/H2O2system is similar to the adsorption curve of Fe3O4@PCDP in the first 6 h, which indicates at the beginning of the reaction, most pollutant was adsorbed by Fe3O4@PCDP rather than degraded due to the excellent adsorption of PCDP. In addtion, it seems that the increase of the removal rate of Fe3O4@PCDP/H2O2system is only due to the improvement of catalyst adsorption capacity rather than catalytic efficiency. This can be better discussed later.
The effect of the H2O2concentration on the RhB degradation was investigated (Fig. 2(b)). When the concentration of H2O2rose from 5 mmol/L to 30 mmol/L, the degradation rate of the pollutants increased at first and then decreased. When H2O2concentration was 20 mmol/L, the removal rate of RhB was the best and reached 87.25%. At first, the concentrations of H2O2and the generated hydroxyl radicals increased. So the removal rate of RhB became higher. However, when the concentration of H2O2reached a certain extent, the excess H2O2might consume the generated hydroxyl radicals, which would reduce the degradation rate of RhB[12].
The effect of pH on Fenton reaction was also explored (Fig.2(c)). When pH value increased from 3 to 9, the degradation rate of RhB gradually decreased. The removal rate of pollutant could reach 87.25% after 42 h, while the initial pH of the reaction system was 3. Because the pH of the solution system can affect the Fenton reaction (Eq. (4)), the equation tends to occur at acdic condition and more hydroxyl radicals are generated. In addition, when pH is greater than 5, iron ions will precipitate in the form of hydroxide, which reduces the degradation rate of pollutants[13].
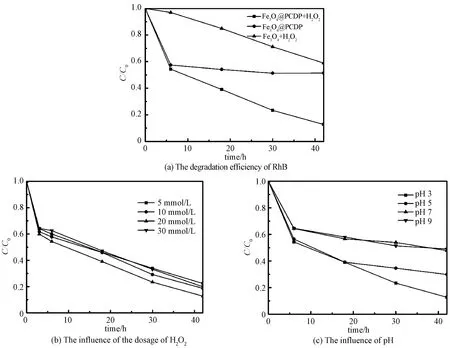
Fig.2 Catalytic performances of Fe3O4@PCDP under different conditions
2.3 Stability of Fe3O4@PCDP
In order to demonstrate the circulation ability of Fe3O4@PCDP, cyclic degradation experiments were studied(See Fig.3). After the third cycle, the removal rate can still reach 70%, which is still higher than Fe3O4, while the catalyst has reached adsorption saturation after the first cycle. It is evidenced that the increase of the removal rate is not only due to the improvement of catalyst adsorption capacity but also catalytic efficiency.

Fig.3 Recycling of Fe3O4@PCDP after the degradation of RhB
Wang et al.[8]reported that the pollutant was trapped in the β-CD cavity and form complex compound. So the generated hydroxy radical in proximity to β-CD can attack pollutant directly, which decreases the waste of hydroxy radical during delivery. That is also why the acceleration effect of Fe3O4@PCDP does not behave in the first cycle, and the hydroxyl radical tends to attack the RhB in the cavity (Scheme 1)[8]. Interestingly, compared with the second degradation curve, the third cycle removes more pollutant. Maybe because the leached iron ions were not released into the solution, the formed complex compound with the hydroxyl groups on PCDP participated in the subsequent cycle degradation, which improves the utilization rate of iron ions.
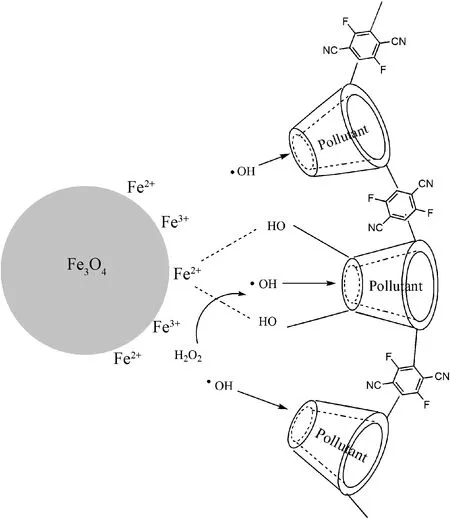
Scheme 1 Possible schematic diagram of the pollutant degradation in the Fe3O4@PCDP/H2O2 system
In Fig. 4 it is shown that the FTIR spectrum of the used Fe3O4@PCDP does not change after the catalytic reaction, which indicates that the structure of the catalyst is maintained. It is proved that the catalyst has good catalytic stability[14].
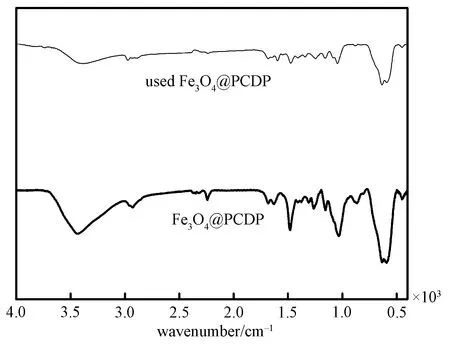
Fig.4 FTIR spectra of Fe3O4@PCDP and the used Fe3O4@PCDP
3 Conclusion
In this work, Fe3O4was fixed on the polymer PCDP to prepare a green and cheap heterogeneous Fenton catalyst Fe3O4@PCDP nanocomposite. The catalyst has a strong catalytic degradation effect on RhB due to the high dispersity and formation of complex between pollutant and β-CD,which decreases the waste of hydroxyl radical during delivery. By studying the influences of the H2O2concentration and pH on the catalytic efficiency, the optimal reaction conditions were obtained and are shown as follows. The concentration of H2O2was 20 mmol/L; the initial pH of the reaction system was 3; the amount of catalyst was 0.5 g/L; and the initial concentration of pollutant RhB was 200 umol/L. Under these conditions, RhB removal rate reached 87.25% 42 h after reaction. After three times of degradation, the catalyst Fe3O4@PCDP showed stable performance and still had good catalytic activity.
