Antiphospholipid syndrome and its role in pediatric cerebrovascular diseases:A literature review
2020-09-14BeataSareckaHujarIlonaKopyta
Beata Sarecka-Hujar,Ilona Kopyta
Beata Sarecka-Hujar, Department of Basic Biomedical Science,School of Pharmacy with the Division of Laboratory Medicine in Sosnowiec,Medical University of Silesia in Katowice,Sosnowiec 41200,Poland
Ilona Kopyta, Department of Paediatric Neurology,School of Medicine in Katowice,Medical University of Silesia in Katowice,Sosnowiec 41200,Poland
Abstract
Key words:Antiphospholipid syndrome;Antiphospholipid antibodies;Lupus anticoagulant;Anti-β2-glycoprotein-1;Children;Thrombosis
INTRODUCTION
Antiphospholipid syndrome(APS)(Hughes syndrome)is an autoimmune disease that is a very rare condition in children.It occurs in approximately 3% of patients before 15 years of age.APS can affect both arterial and venous circulation.It is characterized by recurrent fetal loss,and persistent circulating antiphospholipid antibodies(aPLs),i.e.,circulating lupus anticoagulant(LA),anticardiolipin antibodies(aCLs)and anti-β2-glycoprotein-1(anti-β2GP-1)[1,2].A diagnosis of APS can be either primary,if it occurs in the absence of any underlying disease[primary antiphospholipid syndrome(PAPS)],or secondary,when it occurs in association with another autoimmune disorder,most commonly systemic lupus erythematosus(SLE)or lupus-like disease.In a Canadian study,based on 149 pediatric patients with SLE,16% of children were LA positive[3].In Brazilian children with SLE,aPLs were present in 75.4% of patients,and the positivity for these antibodies fluctuated during the course of the disease[4].The authors diagnosed APS in 14% of the cases,on average three years after the diagnosis of SLE.In turn,in the study of Avcinet al[5],almost 50%of APS patients had underlying autoimmune disease.The specific form of APS is Sneddon's syndrome,defined as livedo reticularis and stroke.This syndrome is again a very rare disease,and the prevalence of aPLs in patients with Sneddon's syndrome is estimated to be up to 40%[6].
APS was found to be more frequent in female pediatric patients.Avcinet al[5]demonstrated a female:male ratio of 1.2:1,whereas in adults,this ratio was reported to be significantly higher,reaching 5:1[7].
Earlier studies showed an important role of APS in the pathogenesis of pediatric thromboembolism as well as ischemic stroke[8].However,because the scientific assessment of APS in children using available diagnostic and therapeutic methods was difficult due to the relatively low incidence of the disease as well as its heterogeneity,a special European registry was created in 2004(the Ped-APS Registry)[5].In the cohort of 121 pediatric patients with APS from 14 countries,Avcinet al[5]observed several thrombotic events,including deep vein thrombosis in 40% of patients,cerebral ischemic stroke in 26% of cases and cerebral sinus vein thrombosis(CSVT)in 7% of cases.
The aim of the present literature review was to discuss available literature data concerning antiphospholipid syndrome in children and its role in cerebrovascular diseases,including pediatric arterial ischemic stroke(AIS),migraine and cerebral venous thrombosis.Additionally,we analyzed the possible role of polymorphisms of genes encoding proteins involved in the prothrombotic state in the occurrence of cerebrovascular diseases in children.
METHODOLOGY
The search of the articles was performed until mid-December 2019 using the following databases:PubMed,Scopus,Embase and Google Scholar.The following combinations of keywords were used in the search process:“antiphospholipid syndrome” or“APS”,or“antiphospholipid antibodies” or“aPL” and“arterial ischemic stroke” or“ischemic stroke” or“stroke”,or“cerebrovascular disease” and“cerebral vein thrombosis” or“cerebral venous thrombosis(CVT)”,and“children” or“pediatric”.The pediatric populations were aged younger than 18 years.In the present literature review,the role of APS in reference to pediatric,perinatal and neonatal AIS as well as CVT was discussed.Finally,our literature review included those published results,which we believed were the most interesting or relevant.
DIAGNOSTIC CRITERIA OF ANTIPHOSPHOLIPID SYNDROME
There are no pediatric-specific APS diagnostic criteria.The original APS criteria were formulated in 1998 in Sapporo during the Eight International Congress on Antiphospholipid Antibodies and then published in the subsequent year[9].For this reason,the criteria were called Sapporo criteria.In 2004,they were revised during the Eleventh International Congress on Antiphospholipid Antibodies in Sydney;updated criteria were published in 2006[1].Currently,APS may be diagnosed if at least one clinical criterion and one laboratory criterion based on the study by Miyakiset al[1]are met(Figure 1).
The most typical clinical presentation is vascular thrombosis in arterial or venous or small vessels;thrombosis must include one or more thrombi in the patient,which must be confirmed by the imaging results or by histopathological examination results.In the latter,no inflammatory features within the tested tissue or organ are crucial,as APS is defined as a noninflammatory state[1].
The other typical clinical presentation of APS is obstetric failure,defined as at least one unexplained death of a morphologically normal fetus at 10 wk or beyond of gestation,and the morphology of the fetus must be confirmed by clinical imaging,such as ultrasonography,and/or by direct examination.
Three or more consecutive spontaneous miscarriages before 10 wk of gestation,with the exclusion of any anatomical or hormonal maternal problems,and when both parents' chromosomal examination excluded potential genetic causes,is the other clinical criterion of APS.Then,three or more premature(before the 34thweek of gestation)deliveries of a normally developed fetus due to eclampsia or severe preeclampsia or recognized features of placental insufficiency is another criterion[1].Laboratory criteria focus on the presence of aPLs,i.e.,lupus anticoagulant,anticardiolipin antibodies or anti-β2 glycoprotein-1 antibodies[1].
ROLE OF ANTIPHOSPHOLIPID ANTIBODIES IN THROMBOTIC EVENTS
Antiphospholipid antibodies indirectly increase the generation of thrombin and induce changes on the surface of endothelial cells by introducing a hypercoagulable state[10].The highest risk for venous and arterial thrombosis is observed for patients with triple positivity,i.e.,LA positive,aCL positive,anti-β2GP-1 positive[11].
The exact mechanism of action of antiphospholipid antibodies leading to thrombosis as well as their direct predilection to neurons leading to neuropsychiatric symptoms without central nervous system thrombosis is not well understood and complex.In patients presenting neuropsychiatric manifestations of APS,white matter lesions on magnetic resonance were found[12].In animal models of experimental APS,histopathological signs of meningeal and perivascular inflammatory processes were seen;the number of animal models,especially human models,is small[12].The prothrombotic action of aPLs may disrupt the kinetics of pro- and anticoagulant reactions on the cell surface;then,the stimulation of cells may lead to changes in the secretion of various molecules[13].aPLs inhibit anticoagulant reactions interfering with the pathway of protein C,antithrombin and β2 GPI activity[13].The antibodies affect the blood cell surface,mostly in endothelial cells,monocytes and platelets[12].The autoantigen crucial for aPL binding on the surface of target cells is β2 glycoprotein-1[14].
On endothelial cells,aPLs bind domain I of β2-GP1 receptors,which are subsequently dimerized.Consequently,the dysfunction of endothelial cells through endothelial NO synthase inhibition is induced.An experimental study in mice demonstrated aPL-mediated endothelial NO synthase inhibition as the molecular basis of endothelial dysfunction,increased leukocyte-endothelial cell adhesion,and thrombus formation in APS[15].In turn,there is increased expression of the adhesion molecules endothelin-1 and tissue factor,eventually leading to thrombus formation[16].
When circulating aPLs bind the I domain of β2-GP1 receptors on platelets,increased production of thromboxane B2,the adhesion of platelets to collagen and platelet aggregation are observed.All of these mechanisms induce venous and/or arterial thrombosis.Earlier,it was suggested that some aPLs,i.e.,circulating aCLs and anti-β2GP-1 antibodies,may play a role in forming foam cells by mediating the internalization of oxidized low-density lipoprotein-β2-GP1 complexes into macrophages[17].Circulating aPLs are also involved in the activation of the classical complement system.Previous data demonstrated a role of uncontrolled complement activation in fetal death in mice with aPLs;mice deficient in complement C3 were found to be resistant to fetal injury induced by aPLs[18].A study by Shamonkiet al[19]analyzing tissue samples of placentas from 47 females with aPLs and 23 healthy control patients reported that complement activation and deposition were associated with placental injury in women with APS.Figure 2 presents the schematic role of aPLs in the pathogenesis of thrombosis.

Figure 1 Classification criteria for antiphospholipid syndrome.APS:Antiphospholipid syndrome;ELISA:Enzyme linked immunosorbent assay.
EPIDEMIOLOGICAL DATA ON ANTIPHOSPHOLIPID SYNDROME IN ADULTS AND CHILDREN
Antiphospholipid syndrome occurs in 1%-5% of the general population and increases the annual risk of thrombosis to 2%-5%[20-22].The annual incidence of APS in the population aged ≥ 18 years is approximately 2 new cases per a population of 100000,resulting in a prevalence of approximately 50 APS patients among 100000 individuals in the general population[23].The most typical clinical presentation was venous thrombosis,and in nearly 20% of APS patients,the criteria to diagnose SLE were met.The incidence rate of APS was similar in both sexes;mortality did not differ between the group of patients with APS and the general population[23].
The most affected population is people between the 15thand 50thyears of life;most researchers underline the prevalence of APS in men[20].In primary syndrome,the male:female ratio is 1:3.5,and in secondary APS,it is 1:7[24].After the exclusion of obstetric failure and SLE,the male:female ratio was 1.0[20].
Pediatric manifestation of APS is rare;for this reason,from a formal point of view,it is important to define it properly.For most researchers,the term "pediatric APS"applies if the diagnostic criteria of APS are met in patients below the age of 18 years.However,in some papers,cutoffs such as 16 years or 21 years are taken into consideration[25,26].
As there are no APS criteria specific for children,the problem seems to be underdiagnosed in this population.The mean age of the first manifestation of APS in childhood is between 9 and 14 years[25,27-32].
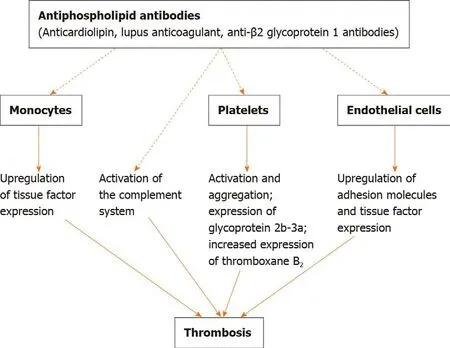
Figure 2 Role of antiphospholipid antibodies in the pathogenesis of thrombosis.
The APS feature specific for its pediatric occurrence has a high tendency to progress into connective tissue disease;within the next 6 years,up to 21% of children with PAPS progress to secondary APS[33,34].This is why the proper classification of pediatric APS,primary versus secondary,in individual children should be delayed.
CLINICAL PRESENTATION,OTHER THAN CEREBROVASCULAR,OF ANTIPHOSPHOLIPID SYNDROME IN THE PEDIATRIC POPULATION
One of the most dramatic and common first APS manifestations in children is catastrophic antiphospholipid syndrome(CAPS);in children,nearly 90% of APS cases at onset are CAPS,whereas in adults,the value is approximately 50%[35].CAPS is defined as a life-threatening clinical situation of the microvascular,and occlusions spread to crucial organs such as the heart,lungs and kidneys.In pediatric CAPS,the most important triggering factor is infection,which is specific for APS patients at pediatric age and is in opposition to CAPS features in adults[35].The prognosis of CAPS in children is poor,as approximately one-fourth of CAPS patients present fatal outcomes;most affected children are females with primary APS.
Other than CAPS and cerebrovascular thromboses in pediatric APS,deep venous thrombosis is most common in the lower limbs(approximately one-third of children),followed by much rarer manifestations of venous thrombosis,such as upperextremity thromboses and thromboses within abdominal organs,but thromboses mostly occur in the kidneys,digits or heart.Thromboses localized in arterial vessels other than the brain are of renal,myocardial and splenic onset[5].
Neuropsychiatric APS manifestation in children diagnosed with APS is most common in secondary syndrome in the course of SLE;however,the number of papers in this field is very numerous,and the size of the described groups is scant.Extrapyramidal disturbances such as athetosis and/or chorea,seizures and epilepsy,transverse myelitis,increased intracranial pressure without central nervous system pathology on neuroimaging(pseudotumor cerebri)and cognitive impairment are most commonly found in up-to-date literature.There is also an association between anti-β2GP-1 and neuropsychiatric symptoms in a cohort of SLE children diagnosed and treated at the Hospital for Sick Children in Toronto,Canada[5].
There are few very interesting data on the association of specific antibody presence and specific neuropsychiatric manifestations in children with APS.Anti-β2GP-1 was found in 26% of pediatric SLE patients presenting neuropsychiatric symptoms,whereas LA was more common in children with SLE at diagnosis and persisted over the course of disease in SLE children presenting chorea[5].This observation did not concern the association of aCLs,LA or anti-β2GP-1 and conditions other than chorea at neuropsychiatric presentation[5].
Recent data showed other manifestations of APS in adults,including the very rare Budd-Chiari syndrome,which is a disorder of blood outflow from the liver caused by thrombophlebitis.The role of aPLs in the pathogenesis of Budd-Chiari syndrome is controversial,and the syndrome is fatal[36].
CEREBROVASCULAR DISORDERS IN CHILDREN WITH ANTIPHOSPHOLIPID SYNDROME
APS may affect any organ of the body and exhibit a broad spectrum of manifestations.APS was suggested to play a significant role in the pathogenesis of cerebrovascular disorders both in children and adults.However,such data are not common,and most often,they are presented in case studies or result from studies performed in a low number of patients.
Arterial ischemic stroke
In pediatric patients,risk factors for ischemic stroke are still not well known and understood.It was,however,demonstrated that AIS or transient ischemic attack(TIA)is the initial presentation of APS both in pediatric patients and in adults[5,37].Since ischemic stroke usually arises from cerebral thrombosis,many mechanisms responsible for the occurrence of thrombosis can be considered risk factors,including increased levels of aPLs,homocysteine,fibrinogen,protein C,protein S,antithrombin III,or lipoprotein(a).
A study performed in a group of children from Israel reported a relation between aPLs and AIS[8].In nine children out of 65 children with stroke,aPLs were present and were demonstrated to significantly increase the risk of stroke(OR=6.08).Similarly,the data of a study by de Veberet al[38]indicated an 8-fold increase in the prevalence of aCLs in children with stroke compared with the control group.In this study,33% of analyzed children with stroke had aCLs,and LA was present in 8% of patients.
On the other hand,in a study from the United Kingdom,elevated aCL IgG at low titer was demonstrated in only one case out of 37(2.7%)[39].Ischemic stroke was found to be the initial manifestation of APS in 26% of pediatric patients[5].In a study by Pilarskaet al[40]performed in a group of children with AIS from northern Poland,significantly higher values of aCLs as well as anti-β2GP-1 in cases compared to controls were found.In Croatian children with AIS and TIA,aCLs were observed in the TIA group(1.3%)and in the control group(7.1%),whereas no pediatric patients with AIS had aCLs[41].On the other hand,the authors did not observe LA in any analyzed group of children.Earlier data demonstrated aPLs in two children in the acute phase of ischemic events and in six cases during follow-up[42].In this study,acute hemiplegia in one patient was linked to aPLs.The authors proposed the detection of aPLs in all children suffering from stroke or TIA[42].
In a case report study by Roldan-Molinaet al[43],aggressive initial manifestation of APS secondary to systemic lupus erythematosus was demonstrated in two 13-yearold girls.The first girl presented with bilateral amaurosis and ischemic cerebral lesions,whereas the second presented with cerebral venous sinus thrombosis and membranous glomerulonephritis.Both girls improved after treatment with anticoagulants and immunosuppressive drugs[43].
Genetic polymorphisms and APS in pediatric ischemic stroke
Polymorphisms within the genes encoding proteins involved in the regulation of the immune system and in the prothrombotic state are considered relevant factors that could predispose children and adults to clinical manifestations[44].The presence of aPLs and prothrombotic gene mutations was previously reported as a“two-stroke”model trigger in the pathogenesis of AIS[5,31].Gene candidates include the methylenetetrahydrofolate reductase(MTHFR)gene,the prothrombin(FII)gene and factor V Leiden.MTHFR is an enzyme involved in the remethylation of homocysteine(HCys)to methionine.Homocysteine exhibits toxicity towards the vascular endothelium,resulting in strong oxidative stress,which stimulates the production of proinflammatory cytokines and can also be the cause of chronic inflammation.In the N-homocysteinylation process,which occurs at high HCys concentrations,various proteins in the blood may be modified,including albumin,fibrinogen and hemoglobin as well as endothelial cell proteins,which then become susceptible to free oxygen radicals.The common 677C>T and 1298A>C polymorphisms in theMTHFRgene are important factors affecting the HCys level.The 20210G>A polymorphism of theFIIgene,in the noncoding 3' region,which most likely plays a role in the regulation of gene expression,is thought to potentially predispose children and adults to acute cerebral ischemia.The polymorphic variant 20210A of theFIIgene increases the prothrombin level,which,in turn,can lead to a prothrombotic state[45].The 1691G>A polymorphism in thefactor Vgene leads to the Arg506Gln substitution,which results in resistance to protein C and hence a prothrombotic state.Heterozygotes have an increased risk of venous thrombosis and a prothrombotic state,whereas in homozygotes,the risk is several-fold higher.
Rego Sousaet al[46]presented a girl with neonatal thrombotic stroke associated withde novosynthesis of antiphospholipid antibodies,homozygous for 1298CC in theMTHFRgene and double-homozygous in the plasminogen activator inhibitor 1 gene(PAI-1844A/A and 675 4G/4G polymorphisms).Mildly elevated aCL IgG and elevated anti-β2GP-1 IgG levels were detected in that girl,who was LA positive.Surprisingly,our previous meta-analysis demonstrated that the 1298A>C polymorphism within theMTHFRgene is not a risk factor for AIS in children,in contrast to theMTHFR677C>T polymorphism[47,48].Another case study described a 7-month-old boy withde novosynthesis of antiphospholipid antibodies and a TT homozygous state within theMTHFRgene as presumed prothrombotic risk factors[49].The authors also found that both a twin sister of a boy and his mother were positive for aPLs.On the other hand,a neonate girl from Italy developed AIS in the left middle cerebral artery and showed IgG anticardiolipin antibodies with a heterozygous genotype in theMTHFR677C>T polymorphism and prothrombin 20210G>A gene mutations[50].Simultaneously,the girl was 1691G>A factor V Leiden mutation negative.In the adult population,the abovementioned polymorphisms showed no relation between APS and cerebrovascular disease[51].In 44 patients with primary APS and cerebrovascular disease,mostly women,heterozygous mutations within factor V Leiden were found in 11% of patients and heterozygous prothrombin mutations were found in 9%,whereas carriers of the T allele(CT and TT genotypes)inMTHFRmutations were found in 59% of cases[51].However,the authors observed no relations between the analyzed mutations and the severity of cerebrovascular disease or the frequency of clinical manifestations related to non-cerebral arterial and venous thrombosis.It was found that in patients who were heterozygotes for factor V Leiden mutations,heterozygotes for prothrombin mutations or homozygotes for MTHFR polymorphisms,recurrent ischemic stroke occurred less frequently than in patients without these mutations(8%vs44%,respectively)[51].Similarly,in 75 patients with PAPS and 83 patients with SLE and aPLs with or without thrombosis followed at 2 university hospitals in Spain,factor V Leiden mutations were not significantly associated with vein thrombosis in patients with aPLs[52].In turn,theMTHFR677C>T polymorphism was found to be associated with the risk of recurrent thrombosis in patients with PAPS,secondary APS and SLE.Three or more episodes of thrombosis were registered in 17 of 40 patients with the MTHFR polymorphism and in 9 of 44 patients without the mutation(P=0.04)[53].
Polymorphisms,1691G>A in factor V Leiden as well asFII20210G>A,were also analyzed by Chopraet al[54]in 157 adult patients,of whom 94% had aCLs and 45% had LA.The Leiden mutation was present in 15% of patients with aCLs and arterial thrombosis,whereas it was present in 3.5% of patients with aCLs but without thrombosis(OR=4.9).In turn,the FII polymorphism was observed in 5 patients,none of whom had thrombosis[54].The authors performed an analysis that indicated that LA is one of the strongest risk factors for thrombosis,and polymorphisms withinFVorFIIgenes did not significantly increase this risk[54].
Interesting data by Aĭsinaet al[55]performed on adults with APS(78 patients,43 with PAPS and 35 with APS secondary to SLE)indicated that both arterial and,even more,venous thromboses were associated with the 4G/5G polymorphism of thePAI-1gene and high plasma levels of the inhibitor.The authors suggested that measurements of the levels of plasminogen and PAI-1 as well as the 4G/5G polymorphism of PAI-1 in APS patients could be practical indicators of patients at higher risk of thrombosis[55].
Migraine
Previously,it was reported that aPLs may play a role in migraine in childhood,although the results are contradictory.Some studies demonstrated a significant relation,while others did not[40,56].Since the mechanisms underlying the association between migraine and ischemic stroke are not understood,APS was suggested to be the link between both disorders[57,58].APS introduces a hypercoagulable state,and persistent hypercoagulability may explain the tendency to thromboembolic cerebrovascular events,especially when other risk factors are present[59].A key role in determining migraine is decreased microvascular cerebral flow.In a study by Cavestroet al[60],adult migraineurs had a significantly higher prevalence of antiphospholipid antibodies.The authors demonstrated that 12% of adult patients with migraine had at least one aPL(LA,aCL IgG or anti-β2GP-1 IgG)detected compared with 3% of controls(OR=4.08).
In children with migraine from Naples,Italy,the mean values of aPLs in classes IgA,IgM,and IgG were not significantly different from controls[61].Simultaneously,LA was absent in all patients.In contrast,a study by Pilarskaet al[40]showed higher levels of aCLs and anti-β2GP-1 in children with migraine than in the control group.However,in this study,similar to the results obtained by Ferraraet al[61],LA was not detected.
Genetic polymorphisms and APS in pediatric migraine
Although in the study by Ferraraet al[61]no abnormal aPLs were found,35 children with headache were also tested for genetic polymorphisms within prothrombotic genes.Factor V Leiden andMTHFR677C>T polymorphisms were significantly more prevalent in migraine patients than in controls.However,polymorphisms in the factor II gene did not differ between the subgroups.
Cerebral venous thrombosis
The incidence of CVT in children is rare with multifactorial origin.In adults,risk factors for CVT include pregnancy and puerperium,oral contraceptives,and deficiencies in protein C,protein S and antithrombin III.Previously,CVT in association with the presence of APS was observed in non-SLE patients[62].
In a retrospective study by Carhuapomaet al[63],15 patients with CVT were analyzed,of whom 8 were aCL positive and 7 were negative.No patients had LA.Four patients with aCLs developed new onset or a worsening of preexisting migraine,2 developed recurrent peripheral venous thrombosis,and 1 went on to have intracranial hypertension.The authors suggested that aCLs may be an important factor contributing to the development of CVT even in the presence of other potential etiologies or risk factors[63].Unfortunately,the study analyzed patients with a wide age range,from children of 3 wk to adults over 50 years old.In addition,the study was retrospective,and the analyzed sample was very small.
In a study by Sébireet al[64],42 children with CSVT(median age 5.75 years,64%boys)from five European pediatric neurology stroke registries were studied.In this group,high aCL IgG(> 12 IU/dL)was detected in 3 children,while LA was detected in one child.In turn,a study performed on 23 Turkish children with CSVT(mean age 59.2 mo;56% of girls)revealed LA in one child out of two tested[65].On the other hand,a negative finding for aCLs and LA was seen in an 11-year-old boy diagnosed with CSVT after presenting with a long history of continuous headache[66].
In young adult patients with CSVT from Lebanon(mean age 22.9 years),positive aPLs were observed in 3 cases[67].Additionally,it was demonstrated that over 30% of patients carried the 1691G>A mutation of factor V,and the 677C>T polymorphism within theMTHFRgene was present in 50% of patients(2 of them were homozygous).Twenty-five percent of cases had both factor V Leiden andMTHFRmutations[67].
RISK OF THE RECURRENCE OF THROMBOTIC EVENTS IN CHILDREN WITH ANTIPHOSPHOLIPID ANTIBODIES
Studies on recurrent thrombotic events,including AIS in children with APS,are scarce.This is due to the rarity of the cooccurrence of these disorders in pediatric patients.In turn,in a situation where recurrences of thrombotic events are even rarer,pediatric APS with subsequent AIS is extremely rare.Moreover,an overwhelming quantity of pediatric AIS occurs in children at infancy and preschool age,while APS occurs most often in teenagers.Despite this,several data demonstrating quite a wide range of the frequency of subsequent thrombotic events in children with APS are available[68-71].However,it should be noted that these frequencies are calculated on the basis of the low number of examined groups in most studies.The study by Nageswara Raoet al[68]reported that the recurrence of thrombotic events occurred in almost 59% of children with AIS(out of 17 patients),with a median time for recurrence of 1.4 years.Five of the six patients suffering from previous arterial events showed recurrence[68].Similarly,in the study by Elwoodet al[69],recurrent thrombotic events occurred in 60% of pediatric patients with APS(12 out of 20 cases),in whom the first episode of thrombosis was diagnosed at a median age of 16.16 years.In the follow-up,20% of these patients suffered from recurrent AIS[69].Although APS was found to increase the risk of occurrence of the first thrombotic event,e.g.,AIS,it is not obvious whether APS may be considered a risk factor for recurrent stroke in children.The research by Lanthieret al[70]analyzed the role of aCL immunoglobulin G titers in the recurrence of thrombotic events in a significantly larger group of children surviving their first AIS/TIA.The authors demonstrated that AIS/TIA recurred in 26% of children with positive aCLs(out of 34 patients)and in 38% of children negative for aCLs(out of 151 patients),and none of the children developed sinovenous thrombosis or extracerebral thromboembolism[70].In a group of 301 German children with AIS,Sträteret al[71]observed no correlation between antiphospholipid antibodies and second stroke.Thus,antiphospholipid antibodies or APS do not seem be risk factors for the recurrence of thrombotic events in children.
Because of the lack of information on recurrent thrombotic pediatric events in APS,we decided to widen the discussion by analyzing adults.In adult patients with ischemic stroke from South Korea,neither an association between increased subsequent thrombotic events and aPL status nor between decreased time to thrombotic events and aPL status was observed[72].Similarly,according to Amoryet al[73],persistent aPLs as well as newer aPLs in ischemic stroke patients could not predict an increased rate of recurrent thrombo-occlusive events.In a group of 128 young patients(aged 18-45 years)with a recent TIA or ischemic stroke from the Netherlands,the relative risk for recurrent ischemic stroke or TIA was 0.7 in patients with aPLs[74].On the other hand,in the case of a young man(31 years old)presenting recurrent cerebrovascular incidents and during a recent one,the presence of LA,aCLs,and anti-β2GP-1 were within normal limits[75].
TREATMENT OF ANTIPHOSPHOLIPID SYNDROME IN THE PEDIATRIC POPULATION
For primary prevention in children with aPLs but without thrombosis,mostly patients diagnosed with lupus,a low dose of aspirin is advocated but not supported by evidence-based research data[76-78].
For secondary prevention in children with venous thrombosis,low-molecularweight heparin rather than unfractionated heparin is recommended for anticoagulation[76].Initial treatment with heparin is usually followed by oral anticoagulation with warfarin,a vitamin K antagonist;the target laboratory value for anticoagulation is an international normalized ratio value of 2-3[76].For secondary prevention after arterial thrombosis,the addition of antiplatelet therapy has been proposed[76].
CONCLUSION
Antiphospholipid syndrome in pediatric patients remains a complex problem in terms of its diagnosis,as no pediatric-specific diagnostic criteria exist.This is why in children with clinical features of APS,general criteria of the syndrome are used,and therapeutic procedures are followed by adult patient standards.Considering the age of pediatric APS onset,from the neonatal period until adulthood,the spectrum of clinical presentation is very wide,making diagnosis challenging.
Conflicting data regarding the relations between aPLs and cerebrovascular diseases in children may result from different study methodologies.However,the problematic issue in APS diagnosis is the number of diagnostic tests used to assess the level of aPLs.When using one laboratory test,the diagnosis of aPLs could be underestimated some patients.In contrast,when using three tests,the sensitivity of the detection of the antibodies is increased.
杂志排行
World Journal of Clinical Cases的其它文章
- French Spine Surgery Society guidelines for management of spinal surgeries during COVID-19 pandemic
- Prophylactic and therapeutic roles of oleanolic acid and its derivatives in several diseases
- Macrophage regulation of graft-vs-host disease
- Remotely monitored telerehabilitation for cardiac patients:A review of the current situation
- Keystone design perforator island flap in facial defect reconstruction
- Cross electro-nape-acupuncture ameliorates cerebral hemorrhageinduced brain damage by inhibiting necroptosis
