Mesenchymal stem cells and mesenchymal stem cell-derived extracellular vesicles: Potential roles in rheumatic diseases
2020-08-10JingHanYangFengXiaLiuJingHuaWangMinChengShuFengWangDongHuaXu
Jing-Han Yang,Feng-Xia Liu,Jing-Hua Wang, Min Cheng, Shu-Feng Wang,Dong-Hua Xu
Jing-Han Yang, Jing-Hua Wang, Dong-Hua Xu, Central Laboratory of the First Affiliated Hospital, Weifang Medical University, Weifang 261000, Shandong Province, China
Jing-Han Yang, Jing-Hua Wang, Dong-Hua Xu, Department of Rheumatology of the First Affiliated Hospital, Weifang Medical University, Weifang 261000, Shandong Province, China
Feng-Xia Liu, Department of Allergy, Weifang People’s Hospital, Weifang 261000, Shandong Province, China
Min Cheng, Department of Physiology, Weifang Medical University, Weifang 261000, Shandong Province, China
Shu-Feng Wang, Medical Experimental Training Center, Weifang Medical University, Weifang 261000, Shandong Province, China
Abstract
Key words: Mesenchymal stem cell; Extracellular vesicle; Autoimmunity; Inflammation; Rheumatoid arthritis; Osteoarthritis
INTRODUCTION
Rheumatic disease is a group of diseases with high morbidity over the world that can affect the musculoskeletal system, leading to arthritis, joint damage, and joint disability[1,2].Rheumatoid arthritis (RA) and osteoarthritis (OA) are the two most prevalent rheumatic diseases with arthritis worldwide[1,2].RA is a common systemic autoimmune disorder characterized by hyperplasia of the synovial membrane, infiltration of inflammatory cells, bone and cartilage progressive damage, and multiple organ involvement[3].Uncontrolled and progressive inflammation and joint damage make RA patients have irreversible joint deformity and decreased life quality.Although disease-modifying anti-rheumatic drugs and nonsteroidal anti-inflammatory drugs have been routinely applied in the clinic to prevent or delay the progression of the disease, the effective therapy to cure RA patients is still absent.Another prevalent joint condition in the elderly is OA, a disease marked by irreversible degeneration of multi-articular cartilage, changes of the underlying bone structure, synovitis, and osteophyte formation[4].Various pro-inflammatory mediators are deemed to participate in the pathogenesis of OA, such as matrix metalloproteinases (MMPs), tumor necrosis factor-α, and interleukin (IL)-6.The contribution of imbalance between anabolism and catabolism in the joint as well as the load of mechanical stress to OA has been shown in a previous study[5].Despite advances in the treatment of rheumatic diseases, their pathogenesis remains largely unknown.
The immune regulatory and regenerative effects of mesenchymal stem cells (MSCs) provide new insight into the treatment of rheumatic diseases.It has been demonstrated that MSCs can be used to treat RA and OA by regulating both innate and adaptive immune cells[6,7].MSCs can suppress the multiplication and development of T cells and B cells, induce more regulatory T cells (Tregs), promote the polarization of M2 macrophages, impair the function of dendritic cells (DCs), as well as decrease the maturation and cytotoxicity of natural killer (NK) cells[8](Figure 1).In addition, it has been demonstrated that the predominant mechanism by which MSCs exert their effects is producing a large variety of paracrine, rather than contact-dependent, mediators[9], although MSCs can work either directly or indirectly.These mediators include growth factors, cytokines, chemokines and so forth[10], among which extracellular vesicle (EV) is one of the most important kind which can mimic the MSCbased immunomodulatory and regenerative effects by delivering bioactive factors, such as proteins, nucleotides, lipids and so on.
EVs are nanoscale vesicles enwrapped by phospholipid bilayers and can be purified from various body fluids such as blood, urine, synovial fluid, and saliva[11].It has been demonstrated that EVs play an essential role in cell-to-cell communication owing to their ability to encapsulate and deliver a variety of bioactive molecules, including proteins, lipids, mRNAs, microRNAs (miRNAs), and long noncoding RNAs, from parent cells to recipient cells[12].The specific components of their contents vary with environmental conditions[13].Almost all types of cells can generate and release EVs into extracellular space, which retain almost similar properties to their parental cells[14,15].MSC-EVs in rheumatic diseases have drawn increasing attention in the last decade.
Currently, as there is no cure for RA and OA, searching for novel and effective treatment to attenuate pain and stop further damage has become a goal of the treatment of rheumatic diseases.Existing studies have demonstrated the significant advantages and great potential of MSCs and their EVs in immunomodulation and tissue damage repair.Targeting MSCs and MSC-derived EVs may be a more promising treatment for rheumatic diseases.This review summarizes recent advances in the functional roles and mechanisms of MSCs and EVs generated from MSCs in rheumatic disease, with a special focus on their potential therapeutic effects, providing rationalities for further research of MSCs and MSC-derived EVs in this field.
MATERIALS AND METHODS
Search strategy
The keywords of “mesenchymal stem cell, extracellular vesicle, autoimmunity, inflammation, rheumatoid arthritis, osteoarthritis, and rheumatic disease” were used alone or in combination to retrieve articles related to “immunomodulation” and “tissue regeneration and repair” in PubMed.Papers published in English language from January 1999 to February 2020 and available in full text were under consideration.Preliminary screening of papers concerning analysis of “immunomodulatory function” or “regenerative function” by scrutinizing the titles and abstracts of the literature, excluded the papers not related to the subject of the article.Some other related studies were obtained by manually retrieving the reference lists of papers that meet the selection criteria, and these studies were screened to meet the final selection and exclusion criteria.
Study eligibility criteria
The selection criteria were: (1) The subjects of research cover MSCs or EVs from MSCs with regard to mechanisms of immune regulation or tissue regeneration and repair; (2) The literature deals with relevant research and clinical application of MSCs or EVs from MSCs in the treatment of RA, OA, or other related diseases; (3) Articles recently published or published in authoritative and professional journals in the same field; and (4) High-quality articles with reliable arguments.
The following search records were excluded: (1) The content of research is repetitive and obsolete; (2) Literature unrelated to the treatment of MSCs or MSC-derived EVs for RA or OA; (3) Full text not available or those published in non-English language; and (4) Review, meta-analysis, and protocols.
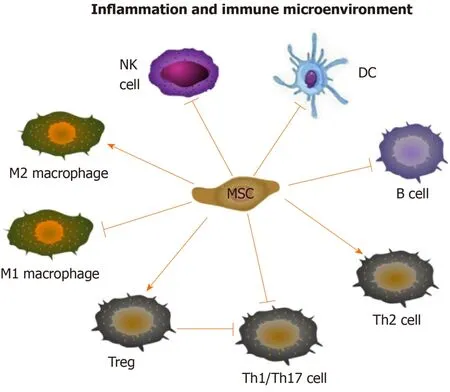
Figure 1 Immunomodulatory effects of mesenchymal stem cells.
Quality assessment
According to the inclusion criteria, two authors first scrutinized the titles and abstracts of the literature selected using the relevant keywords for preliminary screening to assess the effectiveness and applicability of the included literature.And to exclude articles that are inconsistent with the subjects of the study or that are repetitive, all authors read through the full text according to the exclusion criteria.Finally, 86 papers were selected for review and analysis.
Statistical analysis
This study is a systematic review of the literature, which did not involve any available statistical methods.
RESULTS
A total of 86 articles were included in the analysis after completing all the retrieval and review work (Figure 2).And a few articles were obtained by manually retrieving the reference lists of papers that comply with the selection criteria, and these studies were screened to meet the final selection and exclusion criteria.Figure 2 shows the process of literature retrieval.The great potential demonstrated in the literature of MSCs and MSC-derived EVs in modulating immune inflammation and promoting tissue regeneration supports their use in rheumatic disease.
At present, there is increasingly literature about the potential therapeutic value of MSCs in RA or OA.The application of MSCs in RA has primarily concentrated on their immunomodulation, and regenerative potential of MSCs has been intensively studied in experimental models of OA.A single intraperitoneal administration of MSCs could prevent further damage of articular bone and cartilage in a collageninduced arthritis (CIA) mouse model representing human rheumatoid arthritis, proving that the joint protective effect is caused by the immunomodulation mediated by MSCs[16].The beneficial effect of MSCs on RA is being gradually identified, from RA-like inflammatory models to refractory RA patients.Single intravenous injection of bone marrow-derived MSCs (BM-MSCs) to nine refractory RA patients without any other rheumatic diseases acquired a significant improvement of clinical symptoms[17].The exciting results of MSC regenerative potential have been obtained in preclinical models as well as in patients with OA or damage of joint surface.The suppression of synovial activation, ligament related enthesophyte formation, and cartilage damage can be observed after intra-articular infusion of adipose tissue-derived MSCs (ADSCs) to mouse models with OA[18].In addition, the similar effect of MSCs for cartilage regeneration also appeared in larger OA models such as the donkey and goat[19,20].
Figure 2 Flowchart for literature retrieval.
EVs isolated from MSCs, directly or loaded with therapeutics, such as specific miRNA[21], have also become the hotspot of recent research.Although MSC-derived EVs are not as commonly used in RA or OA as MSCs, it is clear that this cell-free therapy may become an alternative to MSC-based cell therapy.For example, MSCderived EVs (exosomes and microparticles) efficiently ameliorated the inflammatory symptoms of CIA modelsviaexerting an immunosuppressive effect on T-cell and Bcell[22].In another study, MSC-derived exosomes were applied to rat models with osteochondral defects by intra-articular administration[23].The results showed that the defects of rats in the experimental group recovered and finally proved the feasibility of MSCs in promoting cartilage repair.At present, research on MSC-derived EVs in RA and OA is far from enough, but a small part of the current research has aroused exciting interest.
DISCUSSION
MSCs and rheumatic diseases
MSCs are pluripotent progenitor cells that possess all the commonalities of stem cells, namely, self-renewal and multi-directional differentiation[24].Over the last decades, MSCs are well known not only for their regenerative activity but also for their strong immunosuppressive property.MSCs can differentiate into three cell lineages of mesodermal organin vitro, namely, osteoblasts, adipocytes, and chondrocytes[25].Two important prerequisites for the application of MSCs in experimental research and clinical application are as follows: MSCs can be easily amplifiedin vitro; they can be present in a plenty of tissues including bone marrow[26], adipose tissue[27], Wharton’s jelly[28], umbilical cord (UC-MSC)[29], umbilical cord blood[30,31], synovial membrane (SMSC)[32,33]and others, and among them bone marrow and adipose tissue are two commonly used tissues for therapeutic utilization[34].Combing the above factors, MSC becomes the preferred seed cell for tissue engineering study.
A growing body of evidence has demonstrated that progressive immune inflammation contributes significantly in rheumatic diseases pathogenesis[35,36].The chronic inflammation within the joint contributes to irreversible joint destruction.And the balance between joint destruction and tissue reconstruction as well as tissue repair determines the outcome of arthritis.It has been well known that MSCs can mediate a wide spectrum of immunoregulatory and tissue damage repairing activities, which support their use as a novel treatment option for rheumatologic disorders[37,38].During the last few years, accumulating studies have been carried out to confirm the therapeutic value of MSCs for different rheumatic diseases, such as RA[39], OA[40], systemic lupus erythematosus[41], and ankylosing spondylitis[38].Clarifying the mechanism of MSCs is crucial for identifying novel MSC-based strategies for these diseases.
Immunomodulatory effect of MSCs: MSCs have immunoregulatory bioactivity.The main immunological characteristics of MSCs are low immunogenicity and high immunosuppressive ability.The precise molecular mechanisms of the immunomodulation effects of MSCs have not been fully elucidated.However, currently available data have suggested that MSCs play an immunosuppressive role mainly through intercellular contact and the secretion of soluble factors encapsulated by EVs[42].Numerous MSC-EVs derived soluble factors can participate in the immunomodulatory process, such as nitric oxide (NO), prostaglandin E2 (PGE2), indoleamine 2-3-dioxygenase (IDO), IL-10, transforming growth factor (TGF)-β and so on[8](Figure 1).They may come directly from MSC, or be produced by the paracrine of immune cells, including T cells, B cells, DCs, and NK cells.Accumulating studies have disclosed that MSCs can regulate immune and inflammatory response, including inhibition of proliferation and differentiation of T helper (Th)1, Th17 cells and B cells, induction of activation of Tregs, suppression of maturation of DCs, promotion of the polarization of macrophages to M2, and inhibition of the functions of NK cells[8](Figure 1).
T cells: T cells are of critical importance in adaptive immunity, whose dysregulation contributes to the pathogenesis of rheumatic diseases.MSCs can prevent pathogenic T cell expansion and induce Tregs activation.Inhibiting the proliferation of Th1, Th17, and granulocyte-macrophage colony-stimulating factor-expressing CD4+T cells is the most significant effect of MSCs on T cells.Apart from depressing Th1/Th17 subtypes, MSCs also induce Th2, an anti-inflammatory subtype[43].Human adipose tissuederived MSCs have been shown to decrease the level of granulocyte-macrophage colony-stimulating factor-expressing CD4+T cells in peripheral blood and the spleen while increase the level of Tregs in CIA mice model[44], which suggests the immunosuppressive role of MSCs in suppressing CD4+T cells in RA pathogenesis[45].The study by Maet al[46]has demonstrated that human umbilical cord MSCs can reduce Th17 cell percentageviadownregulating RORγt, and upregulate Foxp3 to augment Treg percentage in the spleen in RA[46].Rashediet al[47]have reported that MSCs can either increase the level of Treg cells by directly interacting with Tregs through the Notch signaling pathway or indirectly induce CD4+lymphocytes to differentiate into Treg cells[47].In addition, bone marrow-derived MSCs can also inhibit the production of inflammatory cytokines by T cells in RA[48].The significant anti-inflammatory role of MSCs on T cells is mainly dependent on hindering the nuclear factor-κB signaling pathway[49], which has been well recognized as the pivotal downstream signaling pathway involved in rheumatic disease pathogenesis[50].Accordingly, the interaction between MSCs and T cells is involved in RA pathogenesis, providing novel strategies for the immunological treatment of rheumatic diseases.
B cells: MSCs can inhibit the multiplication and differentiation of B lymphocytes, even the production of immunoglobulins[51].Cheet al[52]has found that the suppressive effect of MSCs on B cell multiplication and differentiation is attributed to the downregulation of Blimp-1 and upregulation of PAX-5 in B cells[52].Besides, it has also been well documented that MSCs exert effects on B cells by regulating interactions between programmed death 1 (PD-1) and its ligands PD-L1 and PD-L2[53].MSCs can indirectly inhibit the effect of B cells through T cells[54].Follicular helper T cells are also involved in the immunosuppressive process of MSCs by delivering proliferative signals to B cells in the secondary lymphoid tissues[55], which strongly supports that the suppressive activities of MSCs on B-cell also depend on the interaction between MSCs and T cells.Taken together, MSCs are involved in autoimmune disorders by influencing B cell proliferation, differentiation, and function.
Macrophages, DCs, and NK cells
MSCs play a role as immune suppressive cells in rheumatic diseases.MSCs can reprogram the functions of the macrophage by inducing the switch of activated macrophage from pro-inflammatory phenotype (M1) to an anti-inflammatory phenotype (M2)[4,56]viainhibiting nuclear factor-κb/p65 and activating signal transducer and activator of transcription 3 signaling pathways[57].DCs, the main antigen presenting cells that initiate T cell immune response, have been widely recognized in regulating inflammation and autoimmunity.It has been confirmed that the inhibitory impacts of MSCs on lipopolysaccharide-elicited DC activation and maturation can be mediated by PD-L1 as well as NO, PGE2, and adenosine in canine immune-mediated disease models[58].The blocking effect of MSCs on DC differentiation and maturation ultimately leads to inhibition of the T cell response[59].Mediators of IDO and PGE2 generated by MSCs can restrain the extension and cytotoxicity of NK cells[60,61].Nevertheless, little is known about the immunomodulatory effect of MSCs in rheumatic disease mediated by intercellular communications with macrophages, DCs, and NK cells.Elucidating this issue is essential for identifying the immunological targets for the diagnosis and treatment of rheumatoid disease.
Soluble cytokines
MSCs exert immunomodulatory function not only relying on cell-cell contact, but by means of producing multi soluble factors such as NO, PGE2, IDO, TGF-β1, tumor necrosis factor-inducible gene-6, and human leukocyte antigen-G5[62-64].NO and PGE2 are essential for the suppression of T-cell expansion[65,66].Additionally, MSCs derived soluble factors PGE2 and TGF-β1 also participate in inducing CD4+CD25+Foxp3+Tregs, which are also involved in inducing the transition of M1 macrophages to an antiinflammatory M2 phenotype[43].The immunoregulatory effect of MSCs can be enhanced upon exposure to interferon-γ under the inflammatory microenvironment[67-69].Pro-inflammatory or anti-inflammatory mediators in the microenvironment can affect the function of MSCs[70].Pretreatment of ADSCs with pro-inflammatory RASF enhances their ability to trigger Tregs and inhibit activated macrophages[70].Some pro-inflammatory factors can sometimes interfere with the immunosuppressive effect of MSCs.The immunomodulatory property of MSCs is highly plastic in inflammatory microenvironment[71], in which the inflammatory cytokines act as a crucial switch, such as iNOS[72,73].MSCs possess a pro-inflammatory phenotype and elicit inflammatory response through activation of TLR4 following exposure to inflammatory cytokines[73-75].As a result, MSCs act as a double-edged sword in regulating immune responses.Given such plasticity in the immunomodulatory effects of MSCs, in-depth research is needed to determine the application of MSCs in treating rheumatic disease.
Regenerative property of MSCs in rheumatic diseases: In recent years, the value of MSCs in the application of regenerative medicine has been deeply studied (for review, see[76]).In arthritis, the balance between joint destruction and repair determines the outcome of arthritis.Failure to tissue repair leads to joint damage and disability.Currently, MSCs provide a new prospect for the healing of arthritis in RA and OA.The mechanisms supporting the application of MSCs in promoting joint repair may be as follows: First, MSCs secret a large number of trophic factors to promote angiogenesis, anti-fibrosis, anti-apoptosis and so on; Second, MSCs differentiate into chondrocytes or osteoblasts directly.In short, the differentiation potential and paracrine effect of MSCs make them suitable for the repair of joint defects[77,78].MSCs can differentiate into osteocytes and osteoblasts in osteoblast regulating medium containing inflammatory stimulants[79].MSCs are capable of inhibiting osteoclast formation by enhancing the expression of osteoprotegerin[80], suggesting the critical role of MSCs in tissue regeneration.
Available data have revealed that intra-articular administration of MSCs can control synovial inflammation, reduce osteophyte formation, inhibit cartilage degeneration, and stimulate chondrocyte proliferation[18,81].The important role of MSCs in OA cartilage regeneration has been well established in cartilage cellsin vitro[82-84].Besides, the regenerative potency of MSCs has been intensively studied in experimental models of OA and RA.Murphyet al[20]have first found that the administration of BM-MSCs exerts a regenerative effect in a caprine model with complete medial meniscus resection and anterior cruciate ligament resection[20].The articular cartilage defect can be ameliorated by intra-articular infusion of MSC hyaluronic acid suspension in miniature pigs with condylar cartilage damage[85].Similar use of MSCs has been investigated in other animal models of OA[19,86].A clinical trial has recently reported that intra-articular administration of autologous ADSCs into the OA knee can improve the functional status, relieve pain, and reduce cartilage defects without side effects[87].A two-year follow-up study conducted by Joet al[88]has also demonstrated the safety and efficacy of intra-articular infusion of autologous ADSCs into the OA knee[88].Accordingly, all these findings strongly support the regenerative efficacy of MSCs for promoting cartilage regeneration and protecting cartilage from degradation to impede the progression of arthritis.MSCs can be identified as a novel therapeutic strategy for those rheumatic disease patients particularly with arthritis and bone damages.
Several factors affecting the therapeutic effect of MSCs: The profound value of MSCs has aroused increasingly interests in immunomodulation and regenerative medicine, let alone in rheumatic disease.However, enormous challenges yet remain ahead of clinical application of MSC-based cell therapy due to their vulnerability.The action of MSCs, for instance, will differ according to MSC tissue origin, administration route, and others.
One of the most important reasons that MSCs can be extensively studied and applied is that MSCs can be purified from various tissues.But the most suitable cell source with the best therapeutic effect is still under study, due to the significant variation of MSCs from different sources in many aspects, including differentiation potential, immunomodulatory ability and so forth.BM-MSCs demonstrate a superior osteogenic and chondrogenic capacity, compared with ADSCs[89].Another study reported that SMSCs exhibit a greater capacity for chondrogenesisin vitroover other four kinds of MSCs, in which BM-MSCs, ADSCs, and periosteal MSCs were included[90].However,in vivo, the capacity in osteogenesis of SMSCs is inferior to that of periosteum-derived MSCs[91].Furthermore, the influence of MSC tissue origin on immunomodulatory ability was demonstrated by Meliefet al[92]- better immunosuppressive effect of ADSCs on T cells and monocytes than BM-MSCs was discovered in their study.No matter the variability between MSCs from different tissue sources, the similar immunosuppressive or beneficial effect to arthritis have been described[93-95].
The injection route of MSCs varies according to the pathological characteristics of different diseases.Generally speaking, diseases such as RA, which tend to involve multiple joints and are characterized by progressive inflammation caused by immune dysfunction, can be administered systematically (Figure 3).While diseases with limited lesions, such as OA, tend to be given locally.In contrast, part of research failed to demonstrate the improvement of arthritis by MSC-based treatmentviasystemic route[96,97].
The contradictory results show that the therapeutic potential of MSCs is disturbed by many factors other than tissue origin and administration route, which reveals the great challenge of current research, and more efforts are needed before MSCs can be put into clinical practice.
MSC-derived EVs in rheumatic diseases
EVs are well known for their great potential as a carrier for bioactive substances or biomarkers of diseases.According to their size and mode of biogenesis, EVs can be divided into three main categories: Exosomes, microparticles, and apoptotic bodies[98](Table 1).Exosomes (30-120 nm in diameter) originate from intraluminal vesicles inside of multivesicular bodies, which fuse with the plasmolemma and release exosomesviaexocytosis[99,100].They are packed with tetraspanins (CD9, CD63, and CD81) and heat-shock proteins such as Hsp60, Hsp70, and Hsp90[10,101,102].They also frequently express clathrin, alix, and tumor susceptibility gene 101[10,101,102].The size of microparticles, known as microvesicles, ranges between 100 and 1000 nm.They are producedviabudding directly from the plasma membrane of parent cells, which then are shed from the cell surface[10].There are no specific surface molecular markers for microparticles, but they express the surface markers of parent cells like exosomes[103].Apoptotic bodies (1000-5000 nm in diameter) are released by fragmenting apoptotic cells[104].The well-established methods for isolating and purifying EVs include precipitation, differential ultracentrifugation, density gradient ultracentrifugation, ultrafiltration, size exclusion chromatography, and immunoaffinity[105].EVs can encapsulate and deliver a variety of bioactive molecules, including proteins, lipids, and noncoding RNAs (ncRNAs)[12]from parent cells to recipient cells and participate in intercellular communications.Notably, miRNAs encapsulated by exosomes are a class of 20-22 nt small ncRNAs[106], which regulate targeted mRNAs at the posttranscriptional levelviabinding to 3’-untranslated region of the genes[106].Previously, our team has demonstrated the specific miRNA expression profile in RA patients and shown that exosomal miR-6089 regulates inflammatory reaction in RA by targeting TLR4[107], which suggests the potential role of exosomal miRNAs as diagnostic biomarkers and treatment targets for RA.Nonetheless, the role of ncRNAs in MSCderived EVs is unclear yet.
Recently, it has been supported that the effects of MSCs mediated by paracrine mechanisms are partly achieved through secretion of numerous EVs[108], although MSCs can also act directly (Figure 4).Growing evidence has revealed that EVs derived from MSCs also have immunomodulatory effects, and capacity of regeneration and repair of damaged tissues[80,109].Vonket al[109]have reported that EVs from BM-MSC can inhibit inflammation, promote regeneration, and repair cartilage damageviadecreasing COX-2 and other pro-inflammatory factors when co-cultured with OA chondrocytes[109].It has been recognized that EVs have the characteristics of selective assembly, targeted delivery, and stable preservation[110].MSC-derived EVs have a significant effect in mediating immunomodulation and tissue repair.MSC-EVs not only recapitulate the therapeutic functions of MSCs[111], but also have many advantages that MSCs do not have.EVs are more stable in nature and stronger in transmission ability, compared with MSCs[112].

Table 1 Classification and characteristics of extracellular vesicles

Figure 3 Administration routes of mesenchymal stem cells in rheumatoid arthritis and osteoarthritis.

Figure 4 Schematic representation of whole action mechanisms of mesenchymal stem cells.
MSC-EVs encapsulate diverse lipids, proteins, miRNAs, and mRNAs that originate from MSCs and are secreted into the extracellular microenvironment.Accumulating studies have implicated that MSC-derived EVs exert effectsviatransporting molecules with biological activity[113].Those EVs can interact with the recipient cells in a variety of ways, including fusing with the plasmolemma of recipient cells, interacting with target cell surface receptors, and internalizing by endocytosis, and subsequently deliver their contents to receptor cells, therefore modifying inflammatory and immune responses[114,115].
Immunoregulatory property of MSC-EVs: Recently, the role of MSCs in immune regulation has been demonstrated by mounting studies[116,117], however, the application of MSCs in the clinic remains limited due to their instability.During the past few years, EVs derived from MSCs have attracted increasing attention.Accumulating studies have implicated that MSC-EVs also possess similar immunomodulatory property as MSCs[118,119].MSC-EVs can also exert immunosuppressive effects on T cells[118], B cells[120], macrophages[121], DCs[122], and NK cells[123].
MSC-derived EVs are documented to restrain the multiplication of activated T cells and promote the production of tolerant Tregs[124].Similarly, MSC-EVs can inhibit the activation and development of T cells by decreasing interferon-γ generated by CD4+T cells[125,126].Exosomes from MSCs can also boost the production of CD4+CD25+Foxp3+Tregs[124].Besides, MSC-derived exosomes have been found to inhibit inflammation by promoting the levels of anti-inflammatory cytokines IL-10 and TGF-β1 in PBMCs and inducing the activation of Tregs[127].Reduced production of immunoglobulin and inhibited B cell proliferation and differentiation can be induced by MSC-EVs in B cells[128].The immunosuppressive role of MSC-EVs in macrophages is also well established.EVs derived from MSCs can be effectively internalized by macrophages, which also suppress the pro-inflammatory phenotype (M1) macrophage activation but promote the anti-inflammatory phenotype (M2) macrophage activation[129].However, the study by Monguio-Tortajadaet al[130]has reported that EVs released by UC-MSCs do not affect the polarization of mononuclear macrophages[130].The immunomodulatory effect of MSCs on peripheral blood leukocytes is significant[131,132], whereas no significant influence was observed in those leukocytes co-cultured with MSCderived exosomes[133].The difference in immunomodulatory mechanism between EVs and the receipt cells may be related to their tissue origins[118].In summary, these findings provide the evidence for the immunoregulatory effect of MSC-EVs.Nevertheless, more studies are warranted for a clear understanding of the roles and mechanisms of MSC-EVs in immune regulations.
Regenerative effect of MSC-EVs: The regenerative action of MSCs-EVs has been well documented in a previous published study[111].The critical role of MSC-EVs in tissue repairing is demonstrated in a femur fracture model of CD9-/-mice[134].The study by Zhanget al[23]has first demonstrated that exosomes from human embryonic MSC confer a protecting effect on cartilage repair[23].Exosomes released by both SMSCs (SMSC-Exos) and induced multipotent stem cell-derived MSCs (iMSC-Exos) can attenuate OA score in a mouse OA model, but a greater therapeutic effect of iMSCExos on OA than SMSC-Exos has also been demonstrated[126].Similar to the effects of MSCs on inflammatory arthritis, MSC-EVs also can help to relieve the pain and joint damage in OA and RAviathe protection against cartilage degradation.
Some well-established miRNAs delivered by exosomes have also been demonstrated in rheumatic diseases.MiR-320c-overexpressing hBMSC-Exos can induce cartilage regeneration in OA by promoting the proliferation of chondrocytes and decreasing MMP13 expression[135].A similar effect of miR-140-5p-overexpressing SMSC-Exos has also been documented, which protect against OA[136].Accordingly, MSC exosome-encapsulated miRNAs play a protective role in OA, which implicates a potential therapy for OA by targeting miRNAs delivered by MSC exosomes.However, more investigations are needed to clarify the underlying mechanisms of EVs in tissue damage, repair, and regeneration.
Although both MSCs and MSC-EVs have immunomodulatory and regenerative functions, the safety and efficiency of MSC-based cellular therapy should be seriously considered.Currently available data have demonstrated that the therapeutic activity of MSC-EVs may be superior to that of MSCs in terms of safety and versatility[115,137,138].MSC-EVs offer a promising cell-free restorative approach for regenerative medicine and immunomodulation, which may be a better option for patients with OA and RA, and even other rheumatic diseases.Additionally, EVs can act as drug carriers by encapsulating and delivering small molecules and particular nucleic acids to targeted cells to acquire the desired therapeutic effect in rheumatic diseases.Chen and colleagues have elucidated that miRNA-150-5p delivered by MSC-exosomes plays a therapeutic role in RA through modulating MMP14 and VEGF[21].Taken together[139-141], MSC-EVs-based therapeutic approach is promising for the treatment of rheumatic diseases because they offer the possibility to develop cell-free therapy.
At present, MSCs play important immunosuppressive and tissue regenerating roles through immune regulation, secretion of trophic factors, and multi-directional differentiation, which has attracted much attention in the field of rheumatism.At the same time, as a product of MSCs, EVs have a similar function to MSCs, and may have more advantages than MSCs in biomanufacturing, storing, and other aspects, which makes it get more and more attention.MSC-EVs may represent a more promising therapeutic strategy in immune regulation and tissue repair and regeneration.In summary, MSCs and MSCs derived EVs can be novel therapeutic strategies in rheumatic diseases.
However, the current research is only the tip of the iceberg, from the point of view of clearly understanding the complete mechanisms of MSCs and EVs.At present, there are still many uncertainties in the precise roles and mechanisms of MSC-derived EVs in rheumatic diseases.Some current studies have shown that whether MSCs and EVs can play a full role in the treatment of diseases is affected by many factors.Obviously, in order to better understand their mechanisms of action, a large number ofin vivoandin vitrostudies need to be carried out in terms of tissue source, administration route, window of injection, injection dose and so on.Before application of MSCs and MSCderived EVs into the treatment of rheumatic diseases, a large number of preclinical studies and clinical studies are required to thoroughly assess their safety and efficiency.
ARTICLE HIGHLIGHTS
Research background
Mesenchymal stem cells (MSCs) have been widely investigated in rheumatic disease due to their immunomodulatory and regenerative properties.Recently, mounting studies have implicated the therapeutic potency of MSCs mostly due to the bioactive factors they produce.Extracellular vesicles (EVs) derived from MSCs have been identified as a prospective cell-free therapy due to low immunogenicity.Rheumatic disease, primarily including rheumatoid arthritis (RA) and osteoarthritis (OA), is a group of diseases in which immune dysregulation and chronic progressive inflammation lead to irreversible joint damage.Targeting MSCs and MSC-derived EVs may be a more effective and promisin g therapeutic strategy for rheumatic diseases.
Research motivation
MSCs and MSC-derived EVs have attracted increasing attention in rheumatic diseases due to their great potency in immunosuppression and tissue repair.Currently, it is of great significance to evaluate the therapeutic value by searching and summarizing the relevant literature.
Research objectives
To evaluate the potential therapeutic effectiveness of MSCs and EVs generated from MSCs in rheumatic diseases.
Research methods
One electronic database (PubMed) was searched for the relative literature using the corresponding search terms alone or in combination.Papers published in English language from January 1999 to February 2020 were in consideration.Preliminary screening of papers concerning analysis of "immunomodulatory function" or"regenerative function" by scrutinizing the titles and abstracts of the literature,excluded the papers not related to the subject of the article.Some other related studies were obtained by manually retrieving the reference lists of papers that comply with the selection criteria, and these studies were screened to meet the final selection and exclusion criteria.
Research results
Eighty-six papers were ultimately selected for analysis.After analysis of the literature,it was proved that both MSCs and EVs generated from MSCs exert great potential in multiple rheumatic diseases, such as RA and OA, in repair and regeneration of tissues,inhibition of inflammatory response, and regulation of body immunity via promoting chondrogenesis, modulating innate and adaptive immune cells, and regulating the secretion of inflammatory factors.But EVs from MSCs exhibit much more advantages over MSCs, which may represent another promising cell-free restorative strategy.Targeting MSCs and MSC-derived EVs may be a more efficient treatment for patients with rheumatic diseases.
Research conclusions
MSCs and MSC-derived EVs have demonstrated powerful regenerative potency, as well as their regulatory function for the innate and adaptive immune system.This study offers new ideas and possibilities for MSCs and EVs from MSCs to rheumatism treatment due to their enormous potential described above.However, more in-depth exploration is needed before their clinical application.
Research perspectives
The great potency of MSCs and MSC-derived EVs has been demonstrated, and they can be developed as a more effective and promising therapeutic strategy for rheumatic diseases.Before application of MSCs and MSC-derived EVs into the treatment of rheumatic diseases, a large number of preclinical studies and clinical studies are required.
ACKNOWLEDGEMENTS
We gratefully thank Dr.Feng-Xia Liu for insightful discussions over this work.
杂志排行
World Journal of Stem Cells的其它文章
- Application and prospect of adipose stem cell transplantation in treating lymphedema
- Role of stem cell therapies in treating chronic wounds: A systematic review
- Bone marrow mesenchymal stem cells induce M2 microglia polarization through PDGF-AA/MANF signaling
- Involvement of glycated albumin in adipose-derived-stem cell-mediated interleukin 17 secreting T helper cell activation
- Adipose-derived stem cell therapy shows promising results for secondary lymphedema
- Vitamin D and calcium signaling in epidermal stem cells and their regeneration
