Robotic lateral heller myotomy without fundoplication for achalasia
2020-07-30FaridGharagozlooNabihaAtituzzmanBasherAtiquzzman
Farid Gharagozloo, Nabiha Atituzzman, Basher Atiquzzman
Center for Advanced Thoracic Surgery, Global Robotics Institute, Advent Health Celebratio n University of Central Florida, Celebration, FL 34786, USA.
Abstract
Keywords: Achalasia, robotic, heller myotomy, laparoscopic, eckhardt score
INTRODUCTION
Achalasia is characterized by abnormal relaxation of the lower esophageal muscle and absence of progressive peristalsis in the body of the esophagus[1].In patients with achalasia, histopathologic studies of the lower esophagus have shown depletion of the ganglion cells and inflammation of the myenteric plexus[2-3].Since the function of the lower esophageal myenteric plexus cannot be restored, presently, the treatment of achalasia is palliative.The therapeutic options include medical therapy, botulinum toxin injections, pneumatic dilation, and distal esophageal myotomy by laparoscopy or endoscopy.
Although laparoscopic anterior esophageal myotomy with a Dor anterior fundoplication is the most commonly performed surgical myotomy procedure, several controversies persist, including the ideal operative approach, anteriorvs.lateral esophageal myotomy, the extent of esophageal myotomy, and the need for the addition of an antireflux procedure.
Elliset al.[4]reported that, after a lateral esophageal myotomy without an antireflux procedure performed through a left thoracotomy, there was 96% relief of dysphagia and 3.5% rate of post myotomy gastroesophageal reflux.An anterior myotomy is thought to divide the gastroesophageal valve at its midpoint, necessitating an antireflux procedure.However, by performing the myotomy laterally and preserving the antireflux barrier, a fundoplication may be unnecessary.On the other hand, a lateral myotomy by thoracoscopy has been associated with high rates of post-myotomy reflux[5].These results have been attributed to the shortcomings of conventional videoendoscopic visualization and instruments.By virtue of high definition magnified 3D visualization and precise instrument maneuverability in a small space, it has been reasoned that a surgical robot can enable the lateral myotomy procedure to be performed by laparoscopy.We studied our experience with robotic laparoscopic lateral Heller myotomy without an antireflux procedure for achalasia (RLHM).
METHODS
A retrospective review was conducted of the patients with achalasia who underwent RLHM.Diagnosis of achalasia was made by esophagogram, endoscopy, and manometry.Patients who had previously undergone a myotomy or had a hiatal hernia were excluded from this study.Patients who had undergone a previous myotomy underwent redo myotomy by left thoracotomy, and patients with a hiatal hernia underwent an anterior myotomy with repair of the hiatal hernia and Dor fundoplication.All patients completed a subjective dysphagia score questionnaire, received an Eckardt score, and underwent manometry and pH testing preoperatively.The dysphagia score, manometry, and pH testing were repeated at 6 months following the myotomy procedure.The validated dysphagia score instrument scores subjective severity and frequency of dysphagia on a scale from 0 to 5 with a total possible Score of 0-10 for each individual[6].The dysphagia score is presented as median and range.The Eckardt achalasia scoring instrument scores dysphagia, regurgitation, retrosternal pain, and weight loss from 0 to 3 with a total possible score of 0-12 for each individual[7].In addition, the Eckardt score was tabulated at 1 and 12 months after RLHM.The Eckhardt score is presented as mean ± SE.Failure of myotomy was defined as an Eckhardt score of ≥ 3.
The study was reviewed and determined to be exempt from institutional review board approval under 45 CFR 46.101 (b).
Surgical technique
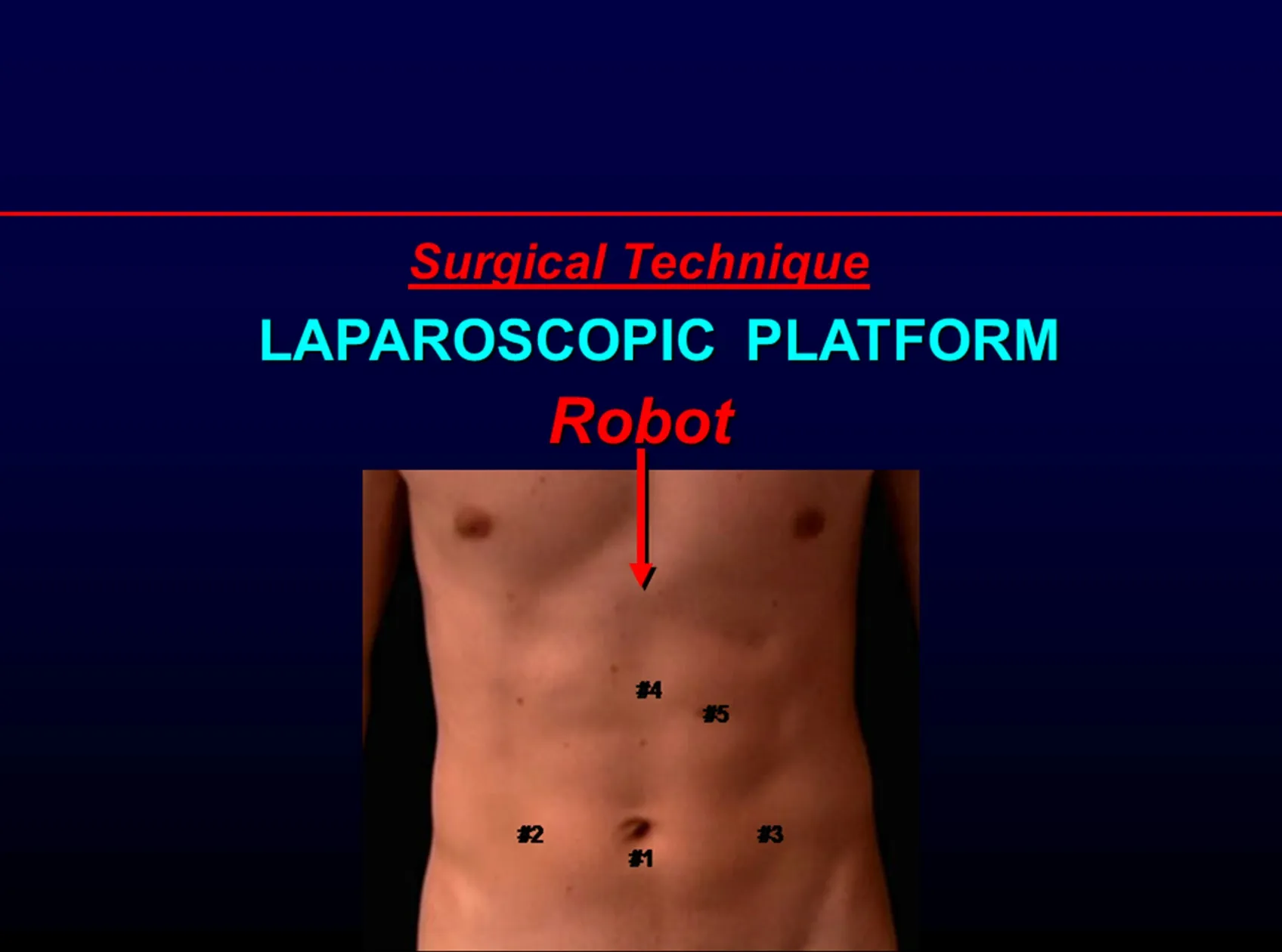
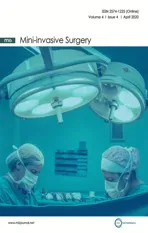
Figure 1.Positioning the robot and trocars for the robotic approach
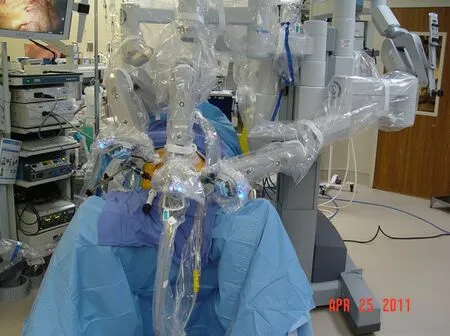
Figure 2.Positioning the robot for the robotic laparoscopic approach
The procedure is performed on a laparoscopic platform [Figure 1].Preoperative upper gastrointestinal endoscopy is performed and the gastroesophageal junction is examined by the retroflexed endoscope.Two Laparoscopic CO2insufflators are used.Port 1 (Camera Port) is placed inferior to the umbilicus.Pneumoperitoneum is created.The table is placed in a steep reverse Trendelenberg position.Port 2 is placed in the right paraumbilical region at the right mammary line.Port 3 is placed in the left paraumbilical region in the left mammary line.An Endo-Paddle paddle retractor (Medtronic, Norwalk, Conn.) is introduced through Port 2 and used to place upward traction on the left lobe of the liver.Port 4 is placed in the subcostal region halfway between the umbilicus and the xiphoid just to the left of the midline.This port is aligned with the right limb of the right crus of the diaphragm.Port 5 is placed in the subcostal region two finger-breaths to the left and caudad to Port 4.Port 5 is aligned with the left limb of the right crus of the diaphragm.
Figure 3.Laparoscopic view of the completed lateral esophageal myotomy prior to the re-approximation of the left limb of the esophageal crus
The surgical robot (Da Vinci Si, Intuitive Surgical, Sunnyvale, CA) is docked using the “side docking” technique [Figure 2].A 30-degree down-viewing robotic binocular camera is used, which is introduced through Port 1.The right robotic arm with a hook cautery instrument is introduced through Port 3.The left robotic arm with a Debakey grasper instrument is introduced through Port 2.The entire dissection uses electrocautery and meticulous hemostasis.An Endo-Paddle Retract Retractor (Covidian, Norwalk, Conn, USA) is introduced through Port 5 by the assistant and is used to provide appropriate counter traction and exposure at the esophagogastric junction.
The left limb of the esophageal crus is identified, and the muscle is divided perpendicular to the direction of the fibers for half the width of the crus.Care is taken not to enter the pleura, which resides just under the crus.The left limb is not transected completely.This allows for partial retraction of the muscle away from the lateral aspect of the gastroesophageal junction while at the same time facilitating repair of the left limb at the end of the procedure.The hook cautery is set at 30 cut/30 coagulation with blend setting.The stomach is retracted inferiorly, thereby straightening the gastroesophageal (GE) junction.Care is taken to stay on the left lateral aspect of the gastroesophageal valve.Theoretically, by preserving the gastroesophageal valve and the phreno-esophageal ligament, the antireflux mechanism is kept intact.The muscle of the esophagus is divided to the level of the mucosa.The hook cautery them completes the myotomy approximately 2 cm onto the cardia of the stomach.Myotomy is discontinued when the submucosal vascular plexus of the stomach wall is visualized [Figure 3].The myotomy is extended cephalad on the esophagus to the level of the pleura.The total length of the myotomy is approximately 6 cm.
At this point, an assistant who is positioned at the head of the patient advances the gastroscope past the GE junction into the stomach.The ease of movement of the gastroscope into the stomach and the lack of resistance further confirms the complete division of the esophageal muscles at the GE junction.Furthermore, the gastroscope is retroflexed to view the GE junction from a caudad to cephalad direction [Figure 4].Observation of the trans-illuminated mucosa of the proximal portion of the gastric cardia from the light of the robotic camera serves as the final confirmation for the completion of the esophageal myotomy.The retroflexed view further confirms that the myotomy is lateral to the gastroesophageal valve.Following the completion of the myotomy, the area is filled with saline and the gastroscope is used to insuラate air into the stomach and esophagus in order to rule out any mucosal perforation.
Following a satisfactory myotomy, the partially transected left limb of the esophageal crus is reapproximated with two O-Ethibond sutures (Ethicon, Inc.Sommerville, NJ) with 2-cm square absorbable pledgets cut from Vicryl mesh (Ethicon, Inc.Sommerville, NJ).
A video of the procedure can be accessed at https://www.youtube.com/watch?v=WUEuHSioodY&feature=youtu.be.
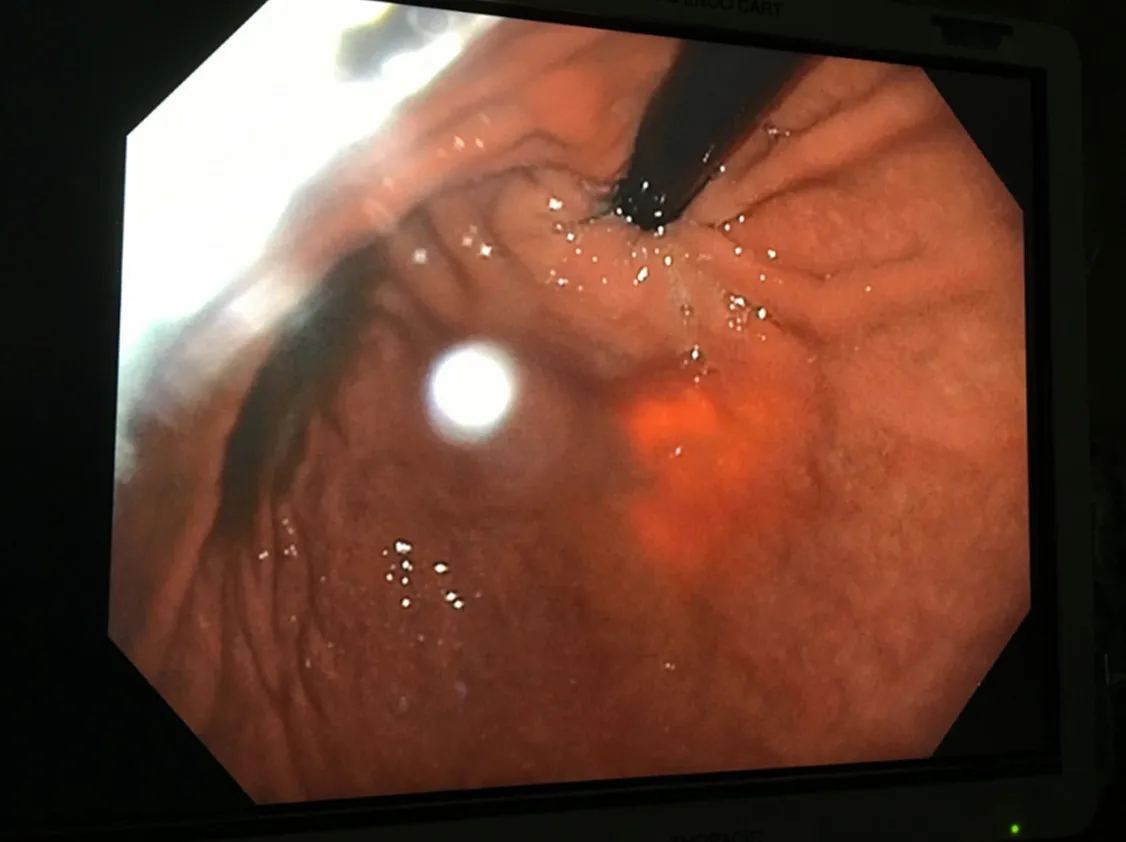
Figure 4.Retroflexed endoscopic view of the intact gastroesophageal valve and trans-illuminated lateral esophageal myotomy
RESULTS
Forty-eight patients underwent RLHM.There were 25 men and 23 women with a mean age of 48 ± 21 years.Median OR time was 85 min (range 60-132 min).There was no conversion to a laparotomy.
Median hospitalization was 2 days (range 2-3 days).There were no mucosal perforations, complications, or deaths.Manometry data are shown in Table 1.
Following RLHM, the Lower esophageal (LES) Pressure decreased from 35 mmHg (range 18-120 mmHg) to 13.2 mmHg (range 9.8-16.6 mmHg) (P< 0.0001).The length of the LES high-pressure zone decreased from 5.5 cm (range 4-9 cm) to 2.2 cm (range 1.5-2.8 cm) (P< 0.0001) [Table 2].
Following RLHM, based on the DeMeester score, two patients (4.2%) had pathologic gastroesophageal reflux.Median acid exposure in all patients was 0.4% (range 0%-17.8%), and the median Demeester score was 7.5 (range 2-125).
Following RLHM, the dysphagia score decreased from 9 (range 8-10) to 1 (range 0-1) (P= 0.01) [Table 3].Eckardt scores are shown in Table 4.Following RLHM, the Eckardt score decreased from 6.3 ± 1.8 to 0.8 ± 1.8 (P< 0.0001) at 1 month and 0.8 ± 1.1 at 12 months (P< 0.0001).Postoperatively, all patients had an Eckhardt score of less than 3.
DISCUSSION
The surgical therapy of achalasia has evolved with a better understanding of the disease process, the anatomy of the GE junction, and the nature of the “antireflux barrier”, as well as advances in technology.
Over the years, surgical therapy for achalasia has been controversial.The controversy has centered on the ideal operative approach, the extent of esophageal myotomy, and the need for the addition of an antireflux procedure.With minor changes, presently, the same controversies continue.
A better understanding of the antireflux barrier has been crucial in understanding the reasons for the controversies.The antireflux barrier, which corresponds to the high-pressure zone on esophageal manometry, seems to be the result of the following:
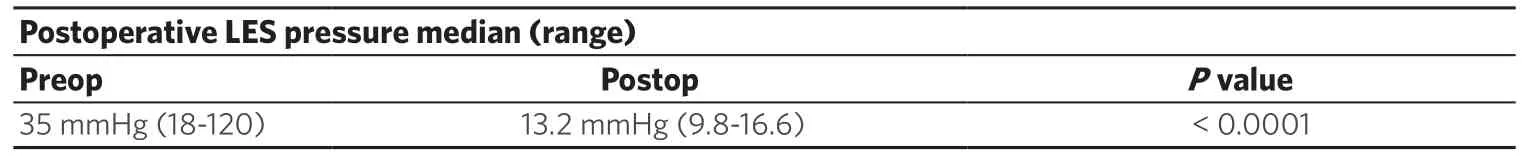
Table 1.Comparison of preoperative and postoperative LES high-pressure zone pressure (6 months)

Table 2.Comparison of preoperative and postoperative LES high-pressure zone length (6 months)

Table 3.Comparison of preoperative and postoperative dysphagia score (6 months)
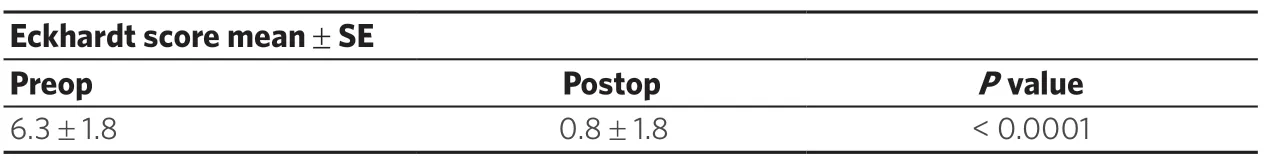
Table 4.Comparison of preoperative and postoperative (12 months) Eckhardt score
(1) The anterior and lateral intussusception of the esophagus into the stomach, extending 270 degrees from the right limb of the right crus to the left limb of the right crus of the diaphragm.
(2) The crural sling exerts pressure in an anterior to posterior direction onto the GE junction and creates a slight angulation.This angulation at the GE junction serves to hold the intussuscepted esophagus in place and provides a slight resistance to reflux at the GE junction.
(3) The entire “antireflux” mechanism is held in place by the phreno-esophageal ligament and the tissues at the esophageal hiatus.
(4) Disruption of the esophageal hiatus, either with a hiatal hernia or at the time of surgical dissection, leads to the straightening of the GE junction, reduction of the anterior esophageal intussusception, and the creation of gastroesophageal reflux.
Prior to the advent of the laparoscopic approach to achalasia, the most commonly performed procedure for this disease was the transthoracic modified Heller myotomy with or without an antireflux procedure.The transthoracic approach was preferred to the transabdominal approach due to the technical difficulties of exposing the gastroesophageal junction and the distal esophagus by an open abdominal procedure.Elliset al.[4]advocated transthoracic esophageal myotomy without an antireflux procedure with very low rates of postoperative reflux[8,9].The advent of laparoscopy obviated the morbidity of a thoracotomy, and laparoscopic Heller myotomy with an anterior Dor fundoplication became one of the more frequently adopted surgical techniques for treating esophageal achalasia[10-18].
In 1991, Shimiet al.[19]reported the first laparoscopic experience for Heller myotomy.In one series of 133 patients who had undergone laparoscopic myotomy with a partial fundoplication, Pattiet al.[20]reported 11% persistent dysphagia, 17% new gastroesophageal reflux, and 5% mucosal perforations that were amenable to laparoscopic closure.Invariably, all series reporting the laparoscopic approach to Heller myotomy have shown excellent relief of dysphagia.The majority of difficulties with the laparoscopic approach have been related to reflux and the technical aspects of the fundoplication.In a series of 69 patients undergoing laparoscopic myotomy and fundoplication for achalasia, Finleyet al.[18]reported a median operative time of 1.9 h, one mucosal perforation that was amenable to laparoscopic repair, 96% patient satisfaction for relief of dysphagia, and a 9% rate of new postoperative gastroesophageal reflux.
A generous anterior myotomy including onto the gastric cardia has been advocated to prevent incomplete myotomy presenting as residual achalasia.To prevent postoperative reflux, a fundoplication should be performed as well.The fundoplication has also been demonstrated to prevent the formation of a mucosal diverticulum following myotomy, a condition which may have added to the problem of chronic dysphagia in these patients with compromised esophageal dysmotility[18].
On the other hand, the surgeons who have advocated myotomy without an antireflux procedure, most notably Elliset al.[4], have emphasized that, in their experience, fundoplication recreates the resistance to esophageal emptying and that, depending on the degree of resistance, fundoplication can lead to progressive esophageal dilation and ultimately the same sequalae as with untreated achalasia.Furthermore, based on performing a lateral esophageal myotomy, these authors have asserted that, in their experience, if the esophageal myotomy is carried onto the cardia by up to 2 cm, an antireflux procedure is not required.
The present understanding of the gastroesophageal antireflux barrier has served to explain the different observations and the discrepancy in the experience of the proponents versus the opponents of an added antireflux procedure to the modified Heller myotomy.Based on this understanding, by nature of not disrupting the three-dimensional relationship at the esophageal hiatus and performing a very careful and limited myotomy, the surgeons who did not add an antireflux procedure were able to preserve the antireflux barrier and accomplish the goal of the myotomy without the need for an antireflux procedure.On the other hand, surgeons who opened the esophageal hiatus and performed an extensive dissection of the gastroesophageal junction, thus disrupting the normal antireflux barrier, needed to add an antireflux procedure to the myotomy in order to prevent postoperative reflux.It is important to note that, to visualize an adequate length of esophagus, a transabdominal approach invariably needs to disrupt the anatomy at the gastroesophageal junction and the antireflux barrier.Consequently, all transabdominal approaches to esophageal myotomy have required the addition of an antireflux procedure.
This is a retrospective review of patients who underwent a robotic laparoscopic esophageal myotomy without fundoplication.RLHM was performed without complications or mortality.There was significant decrease in the pressure and length of the lower esophageal high-pressure zone on manometry.The manometry data correlated with the significant decrease in the subjective dysphagia score.In addition, the objective Eckhardt scores decreased significantly and remained unchanged at 12 months following RLHM, signifying the long-term efficacy of the procedure.The rate of pathologic reflux following RLHM was very low.This finding is further evidence that RLHM preserves the gastroesophageal valve and does not require a fundoplication.
Long-term results of the laparoscopic anterior esophageal myotomy with an antireflux are excellent.Theoretically, by virtue of three-dimensional high definition magnification, and precise instrument maneuverability, the robotic laparoscopic approach may be associated with better outcomes for a procedure that requires exceptional surgical precision and visualization.In addition, the use of the surgical robot in performing a lateral esophageal myotomy may obviate the need for a fundoplication.
Given the excellent relief of dysphagia, and very low incidence of post myotomy gastroesophageal reflux, RLHM should be considered in patients with achalasia.
Study limitations
The following limitations of this study should be considered before drawing definitive conclusions.The study was limited to a small number of patients.In addition, the study was retrospective and represented a highly selected group of patients.
Undoubtedly, the use of robotic technology adds greater cost.If the results of this study are validated by a randomized prospective study, this shortcoming may be offset by the greater accuracy of dissection, the high rates of relief of dysphagia, and the low incidence of pathologic reflux associated with robotic lateral Heller myotomy without fundoplication for achalasia.
DECLARATIONS
Authors’ contributions
Collection of data, planning and preparation of manuscript: Gharagozloo F, Atituzzman N, Atiquzzman B
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2020.