Unlikely role of glycolytic enzyme α-enolase in cancer metastasis and its potential as a prognostic biomarker
2020-07-29LachIanSchofieIdLisaLinczKathrynSkelding
LachIan SchofieId, Lisa F. Lincz,3, Kathryn A. Skelding
1Faculty of Health and Medicine, Priority Research Centre for Cancer Research, Innovation and Translation, School of Biomedical Sciences and Pharmacy, University of Newcastle, Callaghan, New South Wales 2308, Australia.
2Hunter Cancer Research Alliance and Cancer Research Program, Hunter Medical Research Institute, New Lambton Heights,New South Wales 2305, Australia.
3Hunter Haematology Research Group, Calvary Mater Newcastle Hospital, Waratah, New South Wales 2298, Australia.
Abstract Reliance on glycolysis for energy production is considered a hallmark of cancer and the glycolytic enzyme α-enolase is overexpressed in a range of cancer types. However, recent studies have revealed that α-enolase is involved in a variety of unrelated physiological processes and can be found in multiple unexpected cellular locations. This review focuses on the unlikely role of α-enolase as an extracellular plasminogen-binding receptor localised to the plasma membrane. Conversion of plasminogen to plasmin on the surface of cancer cells enhances their ability to invade through stroma by activating collagenases and degrading fibrin as well as extracellular matrix proteins. Increased expression of α-enolase is associated with increased migration and invasion of cancer cells, and decreased metastasis-free survival in patients with several cancer types, including non-small cell lung,pancreatic, breast and colorectal cancers. Due to its overexpression in a range of cancer types and multi-functional roles in key areas of tumour metabolism and metastasis, α-enolase may be useful as a universal cancer prognostic biomarker or therapeutic target.
Keywords: Alpha-enolase, ENO1, metastasis, migration, invasion, proliferation, plasminogen-binding receptor
INTRODUCTION
All cancer cells have a high energy demand due to their increased rate of proliferation[1]. Increased glycolysis is considered a hallmark of cancer[2]and investigating glycolytic enzymes may yield new therapeutic approaches for cancer treatment. Enolases are key glycolytic enzymes[3], and increased expression of one isoform, α-enolase, has been identified in several cancer types[4-22]. This review provides an overview of the expression of α-enolase and key functions it controls in cancer cells, with a focus on the potential role of α-enolase as a cancer prognostic biomarker or therapeutic target.
ENOLASE IS A GLYCOLYTIC ENZYME THAT HAS THREE ISOFORMS
Enolases (EC 4.2.1.11) are metalloenzymes that catalyse the dehydration of 2-phospho-D-glycerate to phosphoenolpyruvate in the glycolysis pathway [Figure 1], and catalyse the hydration of phosphoenolpyruvate to 2-phopho-D-glycerate in the reverse anabolic pathway during gluconeogenesis[3].In mammals, the three genesENO1,ENO2,ENO3encode three isoforms, with expression being regulated in a tissue-specific manner. Alpha-enolase (ENO1) is ubiquitously expressed, whereas γ-enolase (ENO2)is primarily expressed in neurons and neuroendocrine tissues, and β-enolase (ENO3) in muscle tissues[23].Active enolase consists of a dimer in which two subunits face each other in an antiparallel formation[24],and requires two non-covalently bound magnesium ions as cofactors for enzyme activity[25].
ALPHA-ENOLASE IS A MULTI-FUNCTIONAL PROTEIN
Although many glycolytic enzymes are considered to be housekeeping proteins, α-enolase expression can vary dramatically depending on the stress, metabolic, or pathological state of the cell. A retrospective proteomic meta-analysis identified that α-enolase was the most differentially expressed protein in humans and rodents irrespective of tissue type and pathological condition[26]. Disrupted expression and/or activity of α-enolase has been reported in several pathologies with distinct aetiologies, including Alzheimer’s disease, systemic sclerosis, rheumatoid arthritis, bacterial infections and hepatic fibrosis[27-38].
Apart from its role in the glycolytic pathway, recent studies have revealed that α-enolase is a multifunctional protein that controls a variety of cellular processes, including proliferation, survival, migration and invasion. Additionally, using an alternative transcription start codon, theENO1gene can produce a 37 kDa protein, c-myc promoter-binding protein (MBP-1). MBP-1 localises to the nucleus, where it acts as a transcription repressor by binding to the c-myc P2 promoter[39], helping regulate and maintain the function of the glycolysis pathway.
ALPHA-ENOLASE EXPRESSION IS ALTERED IN TUMOURS AND VARIES WITH CANCER TYPE
The overexpression of α-enolase is associated with tumour development via a process known as aerobic glycolysis or the Warburg effect. The Warburg effect has been hypothesised to be an adaptation mechanism in cancer cells to support the biosynthetic requirements of rapid proliferation. Alpha-enolase expression has been shown to be altered at the mRNA and/or protein level in a range of tumours [Table 1], and generally upregulated in most, including acute myeloid leukaemia (AML), glioma, melanoma, lymphoma,and colorectal, endometrial, gastric, head and neck, liver, ovarian and pancreatic cancer[4-22].
ALPHA-ENOLASE CAN SHUTTLE BETWEEN CELLULAR COMPARTMENTS
Alpha-enolase can be localised to the cytoplasm and plasma membrane, as well as secreted in exosomes,and its location varies with cancer type. For example, in pancreatic, breast and lung tumours, α-enolase is localised to the plasma membrane[22,44,45], whereas in melanoma, mesothelioma, non-small cell lung,colorectal and prostate cancer α-enolase is also secreted and found in exosomes[43,46-49]. Alpha-enolase can shuttle between compartments, performing different functions when in different subcellular locations,such as surface membrane plasminogen binding, controlling the overall metabolic state of the cell, stressrelated or acting as a heat-shock protein, RNA transport, mitochondrial membrane stability, and cell cycle control[50-55].

Figure 1. The glycolysis pathway. Enolases catalyse the dehydration of 2-phospho-D-glycerate (2-P-glycerate) to phosphoenolpyruvate(P-enolpyruvate) in the glycolysis pathway
INCREASED ALPHA-ENOLASE EXPRESSION ENHANCES CELL PROLIFERATION IN A VARIETY OF CANCERS
In most solid tumours, the Warburg effect causes an increase in total glycolysis under both hypoxic and normoxic conditions [Figure 2]. Enhanced cell proliferation leads to increased anabolic needs, and cancer cells remodel metabolic processes by diverting nutrients to anabolic pathways to satisfy increased cellular energy demands[56]. Therefore, the Warburg effect may provide cancer cells with an advantage when competing with non-cancerous tissues for nutrients. This suggests that increased α-enolase expression will contribute to enhanced proliferation commonly observed in cancer cells.
Indeed, upregulated α-enolase expression has been shown to regulate cell proliferation in various solid tumoursin vitro[11,13,57-61], and to increase tumour growth in a HCT116 colorectal cancer xenograft modelin vivo[11][Table 2]. Conversely, silencing of α-enolase in glioma, pancreatic, lung, endometrial,colorectal and breast cancer cells was found to induce cell cycle arrest and senescence, and also to reduce tumour volume in CFPAC-1 pancreatic, MDA-MB-231 breast and U-87MG glioma xenograft modelsin vivo[6,11,12,62,63]. Furthermore, α-enolase is also implicated in the control of apoptosis and sensitivity to chemotherapeutic agents, as silencing ofENO1in cancer cells induced apoptosis and increased sensitivity to cisplatin and 5-fluorouracilin vitro[13,62,64]. Unexpectedly, cells respond to α-enolase silencing by inducing catabolic adaptations that lead to restoration of pyruvate, acetyl-CoA bulk and oxidative phosphorylation,and exhibit an increased expression of proteins involved in both oxidative stress- and sirtuin-induced autophagy[62]. Taken together, these studies demonstrate that α-enolase is an important regulator of tumour cell metabolism, proliferation and survival, which by definition make it a perfect target for anticancer therapy.
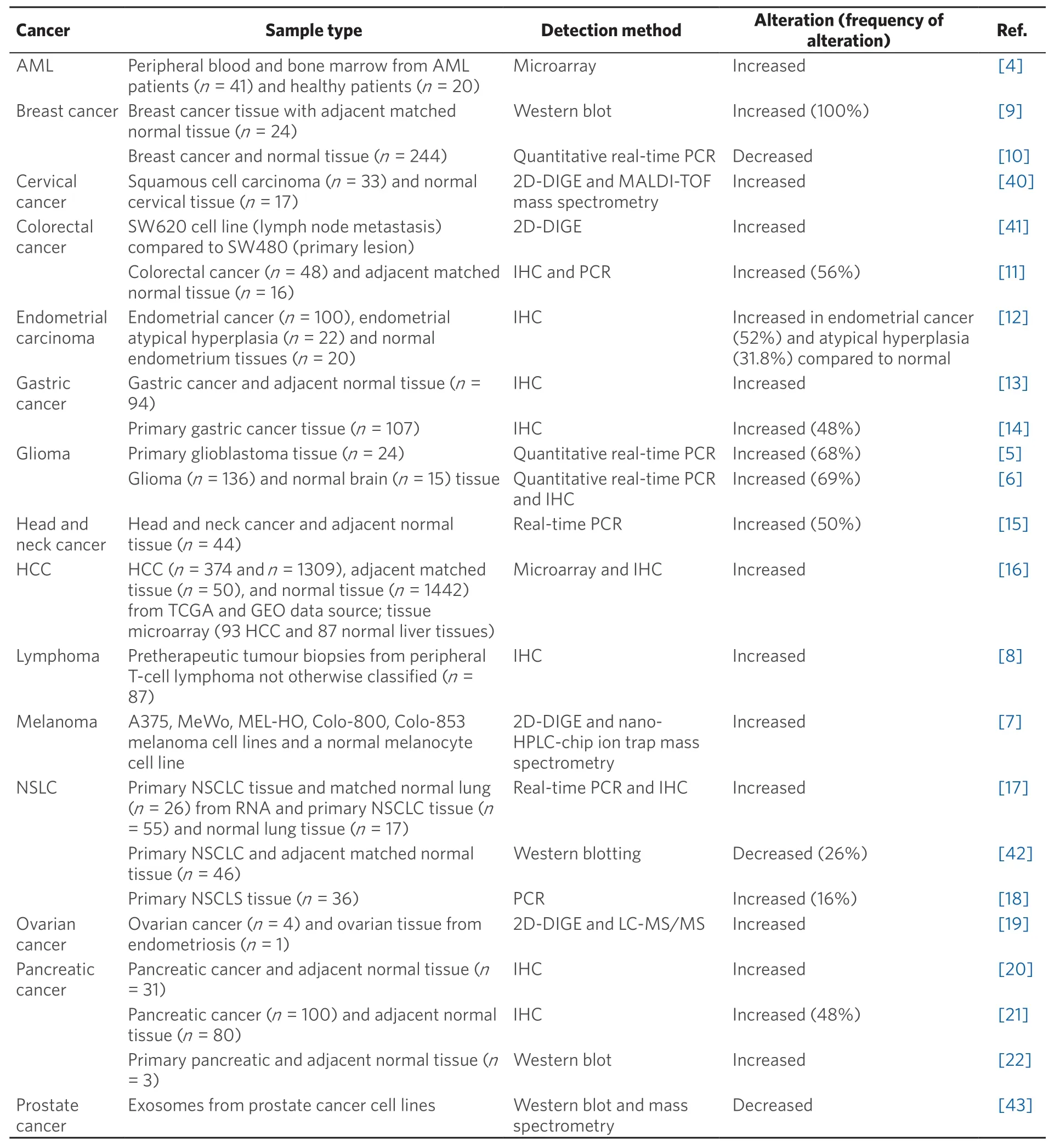
Table 1. The expression of α-enolase is altered in cancer
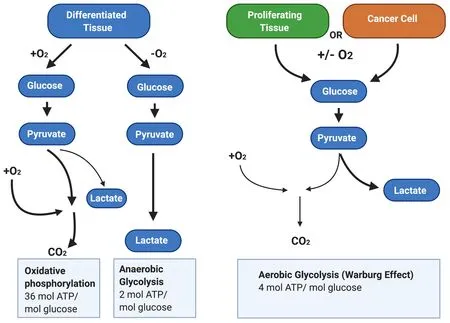
Figure 2. The Warburg Effect in cancer cells. In the presence of oxygen, differentiated tissues first metabolise glucose to pyruvate via glycolysis and then oxidise the majority of the pyruvate to carbon dioxide via oxidative phosphorylation. In situations where oxygen is low, cells redirect pyruvate generated by glycolysis away from oxidative phosphorylation by generating lactate via anaerobic glycolysis.By contrast, cancer cells convert most glucose to pyruvate regardless of whether oxygen is present. This allows cancer cells to meet the increased cellular energy demands
ALPHA-ENOLASE IS A SURFACE PLASMINOGEN-BINDING RECEPTOR
In addition to a role in cell proliferation and survival, α-enolase located on the plasma membrane acts as a plasminogen-binding receptor[55][Figure 3]. Plasminogen is a zymogen, which is converted to plasmin in the presence of the activators tissue plasminogen activator or urokinase-type plasminogen activator(uPA)[65]. This cell surface interaction concentrates protease activity in the tissue surrounding the cell,protecting plasmin from inactivation by circulating α2-antiplasmin[66]. Plasmin activates collagenases and degrades fibrin and other matrix proteins, resulting in cell migration and invasion into tissue, ultimately underpinning cancer metastasis and relapse.
Role in invasion and migration
Overexpression of α-enolase has been shown to increase the migration and invasion of hepatocellular carcinoma, colorectal and gastric cancer cellsin vitro[11,58,60,61,67]and to enhance colorectal cancer metastasisin vivo[11], demonstrating that it is an important driver of metastasis in multiple cancer types [Table 3].Conversely, knockdown or pharmacological inhibition of α-enolase decreased the migration and invasion of glioma, colorectal, pancreatic and endometrial carcinomain vitro[6,12,63,68,69], and decreased tumourigenesis and metastasis of endometrial carcinomain vivo[12]. Furthermore, binding of recombinant α-enolase to the surface of prostate cancer cells was shown to promote cell migration via its plasminogen receptor activity[70]. By contrast, anti-α-enolase monoclonal antibodies inhibited plasminogen-dependent invasion of human pancreatic cancer cellsin vitroand metastasis formationin vivo[71]and also lung cancer cell invasionin vitroand growthin vivo[72][Table 3].
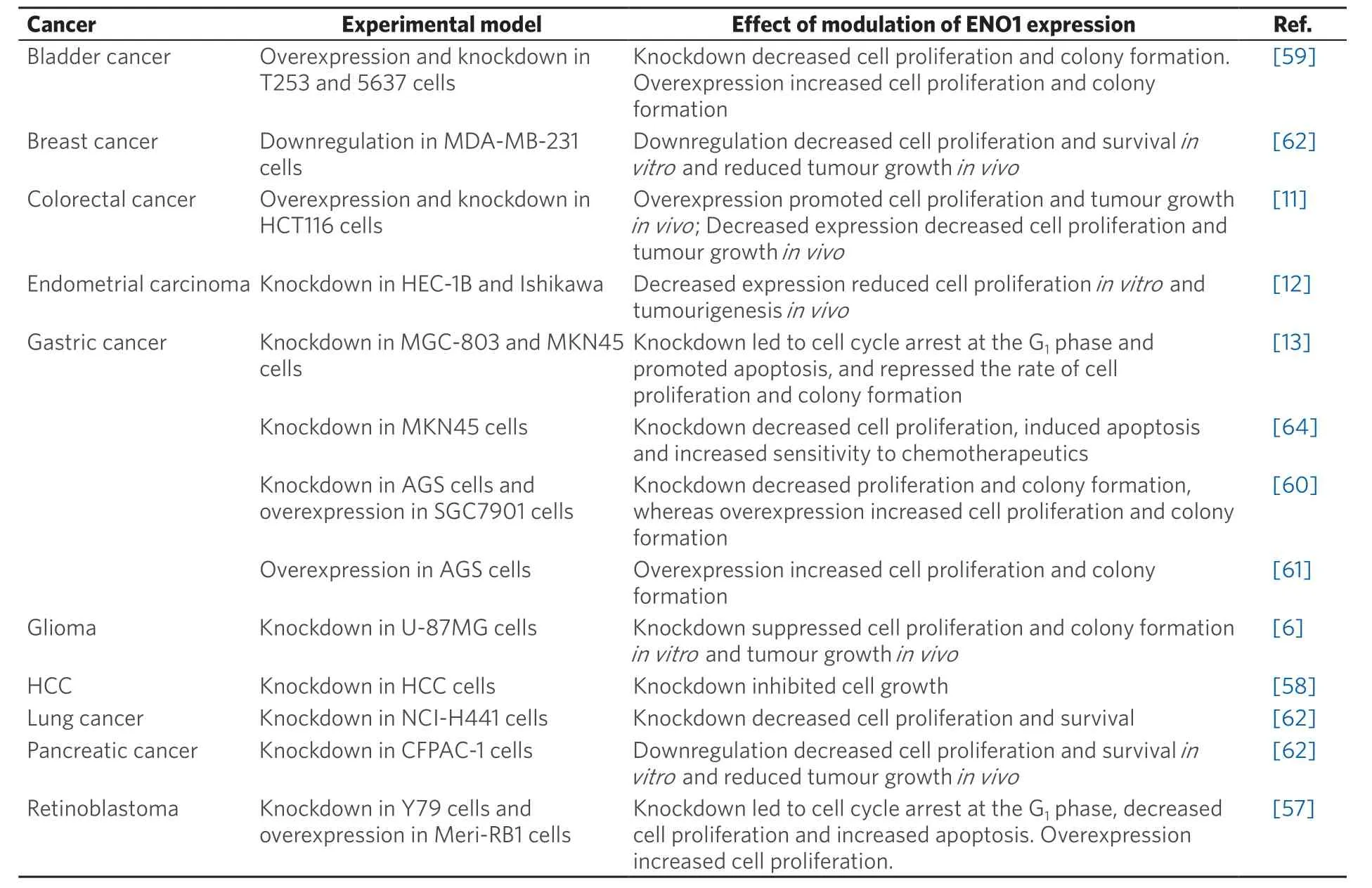
Table 2. Alpha-enolase controls cancer cell proliferation and survival
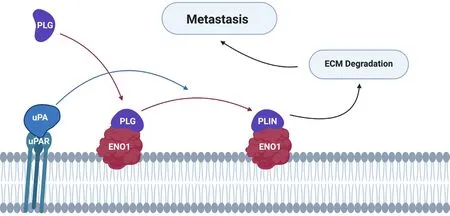
Figure 3. Alpha-enolase acts as a surface plasminogen-binding receptor to mediate cancer cell invasion and metastasis formation. PLG binds to its receptors and is subsequently converted to PLIN by plasminogen activators (e.g., uPA). Cell surface-associated plasmin facilitates degradation of the ECM, allowing tumour cells to invade and metastasise into other tissues. PLG: plasminogen; PLIN: plasmin;ECM: extracellular matrix; uPA: urokinase-type plasminogen activator
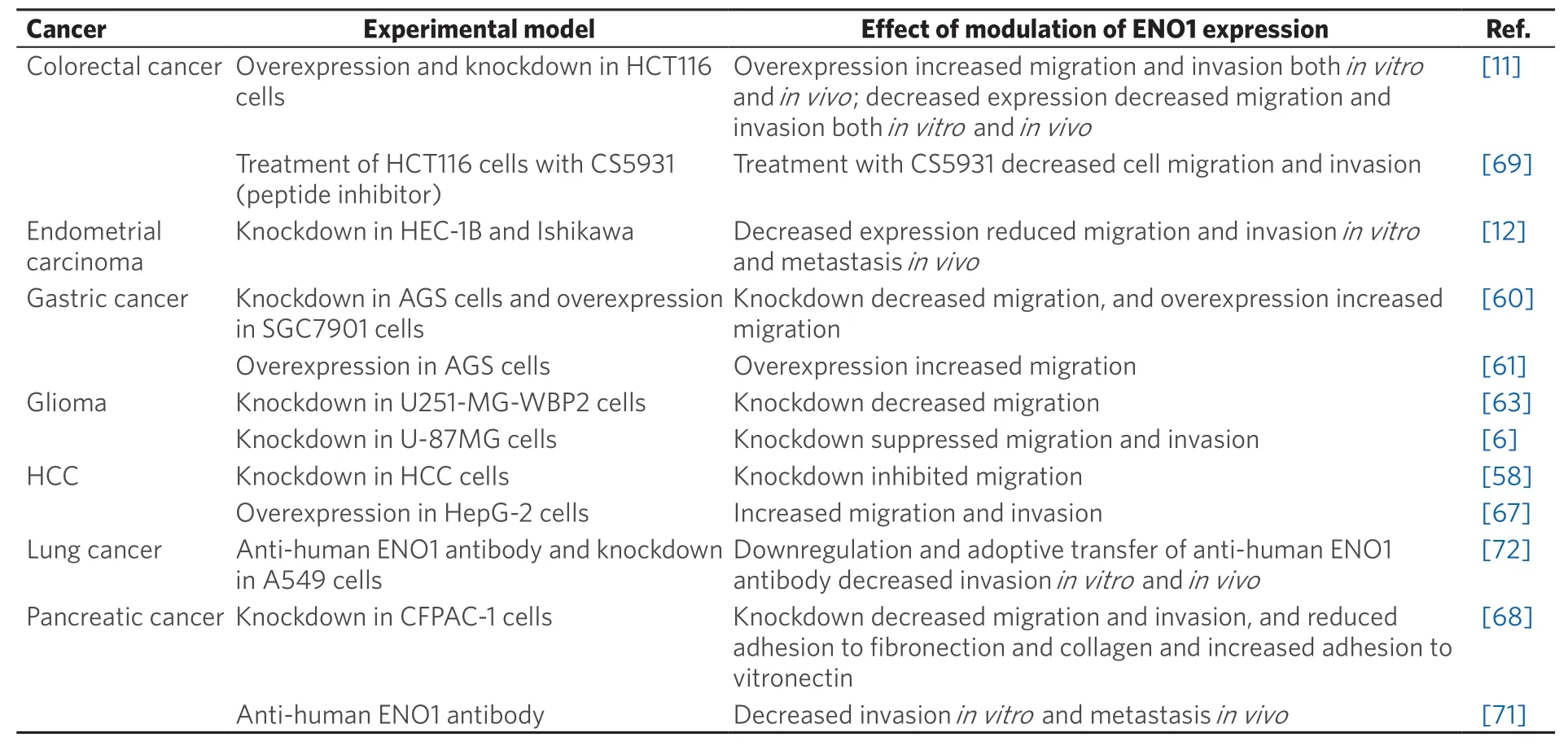
Table 3. Alpha-enolase is a potential regulator of metastasis
ALPHA-ENOLASE IS A TUMOUR-ASSOCIATED ANTIGEN
Externalisation of α-enolase by cancer cells exposes it to the immune system as a tumour-associated antigen that has been found to induce autoantibody production in cancer patients, including those with acute and chronic leukaemias, melanoma, and lung, breast, gastric and pancreatic cancers[22,45,73-79]. In pancreatic cancer, T cells activated by α-enolase-pulsed dendritic cells lysed pancreatic cancer cells, but not normal human keratinocytesin vitroand inhibited CF-PAC-1 tumour growthin vivo[22]. In oral squamous cell carcinoma, an HLA-DR8-restricted human α-enolase peptide was recognised by CD4+T cells and produced a cytotoxic response against OSC-20 cells[80]. Additionally, vaccination withENO1in KrasG12D/Cre and KrasG12D/Trp53R172Hmice prior to development of pancreatic carcinoma delayed tumour growth and increased survival[81]. Taken together, these studies suggest that immune responses directed against α-enolase may be immunostimulatory and ultimately beneficial to patients.
ALPHA-ENOLASE IS A PROGNOSTIC FACTOR FOR MULTIPLE CANCER TYPES
In addition to being overexpressed in many cancers, α-enolase has been identified as a putative prognostic biomarker in a range of tumour types [Table 4]. WhilstENO1expression was not associated with tumour stage in colorectal cancers, it was significantly correlated with tumour size and presence of distant metastases[11]. Alpha-enolase expression was positively correlated with lymph node status in endometrial and gastric cancer patients[12,13,60], and increased α-enolase expression in endometrial, gastric,lung, lymphoma and hepatocellular cancer patients was associated with worse overall survival[8,12,13,16,73,82].Furthermore, increased α-enolase expression was correlated with worse distant metastasis-free survival in breast cancer patients[9], worse disease-free survival in hepatocellular carcinoma and chordoma[16,83]and worse progression-free survival in lung cancer patients[73]. By contrast, downregulation of α-enolase is a predictor of poor prognosis in clear cell renal cell carcinoma (ccRCC)[84], demonstrating that α-enolase may control different cellular functions in ccRCC when compared to other cancers.
Autoantibodies generated against α-enolase in its capacity as a tumour-associated antigen represent an additional type of prognostic biomarker that may be assayed in serum. The presence of autoantibodies against α-enolase correlated with longer disease-free survival and overall survival in pancreatic and lung cancer patients[45,85-87][Table 4]. Furthermore, compared with healthy individuals, α-enolase antibodiesare decreased in stage IV lung and breast cancers[88], and are lower in stage III/IV than in stage I/II lung cancer patients[89]. By contrast, the presence of anti-α-enolase antibodies in sera from chronic lymphocytic leukaemia (CLL) patients is predictive of a shorter time to first treatment[90], indicating that the presence of α-enolase antibodies are indicative of a disrupted immune system in CLL. Taken together, these studies suggest that autoantibodies against α-enolase are a good prognostic factor in pancreatic, lung and breast cancers, and provide further evidence that targeting α-enolase may be beneficial in solid tumours.

Table 4. α-Enolase is a prognostic biomarker for a range of cancer types
ENOLASE INHIBITORS ARE POTENTIAL ANTICANCER AGENTS
Due to its important cancer-related roles, enolase is one of several glycolytic enzymes being examined as a potential anticancer therapeutic target. Polyamine sulphonamide analogues have proven particularly effective at inhibiting α-enolase activity. Two such compounds have been further developed and shown to be cytotoxic to KG-1 (AML) cells and to the AML leukaemic stem cell fraction, with minimal effects on normal healthy stem cells[92]. This report highlights that α-enolase is an actionable therapeutic target that may be useful in the treatment of cancer, particularly AML.
CONCLUSION
Alpha-enolase plays a supportive role in cancer progression and has been implicated in three of the hallmarks of cancer: cellular energetics and metabolism; cell proliferation; and invasion and migration.In cancer cells, α-enolase is overexpressed and localised on the surface, where it acts as a key promotor of metastasis, driving invasion through plasminogen activation and extracellular matrix degradation. In several cancer types, patients develop an immune response against α-enolase, and anti-α-enolase antibodies can be detected in their sera. Increased expression of α-enolase mRNA, proteins or autoantibodies are associated with decreased metastasis-free survival in several cancer types, including non-small cell lung,pancreatic, breast and colorectal cancers. Future examination of the expression and function of α-enolase in cancers may ultimately result in α-enolase becoming a therapeutic target and prognostic biomarker for a range of cancer types.
DECLARATIONS
Authors’ contributions
Contributed to the drafting and editing of this manuscript: Schofield L, Lincz LF, Skelding KA
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was funded by grants from the Calvary Mater Newcastle and Hunter Medical Research Institute.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2020.
