Combinatorial treatment of curcumin or silibinin with doxorubicin sensitises high-risk neuroblastoma
2020-07-28PamaliFonsekaLahiruGangodaMohashinPathanDiGiannataleAngelaSureshMathivanan
Pamali Fonseka, Lahiru Gangoda, Mohashin Pathan, Di Giannatale Angela, Suresh Mathivanan
1Department of Biochemistry and Genetics, La Trobe Institute for Molecular Science, La Trobe University, Melbourne, Victoria 3086, Australia.
2Department of Hematology/Oncology, Bambino Gesù Children’s Hospital, IRCCS, Rome 00165, Italy.
#Authors contributed equally.
Abstract Aim: Neuroblastoma is a pediatric cancer of the sympathetic nervous system. Using various parameters including stage of the disease, amplification status of N-Myc, DNA index and histopathology, neuroblastoma can be stratified into low- and high-risk groups. Recent advances in treatment have significantly improved the survival rate of lowrisk neuroblastoma patients. However, the overall survival rate of high-risk neuroblastoma group, especially N-Myc amplified patients, is poor. Moreover, the survivors of both low- and high-risk neuroblastoma manifest adverse side effects to chemotherapy and thus their quality of life is impaired. Considering all these factors, there is an urgent need to develop therapeutic strategies with natural compounds to improve the survival rate and to reduce the side effects. In this study, we hypothesised that the mesenchymal nature of neuroblastoma cells is a reason, at least in part, for the aggressive and treatment resistant phenotype. Method: In order to validate our hypothesis, we used publicaly available RNA-Seq data, in vitro assays and xenograft mouse models.Results: Using a combinatorial treatment of mesenchymal-to-epithelial inducers (curcumin or silibinin) with doxorubicin significantly increased the cell death in a panel of neuroblastoma cells in vitro. Follow up analysis in vivo, confirmed the therapeutic benefit of utilising the combination of curcumin with doxorubicin. The combinatorial therapy significantly reduced the tumor burden and increased the survival of mice implanted with high-risk neuroblastoma cells. Conclusion: Taken together, this study shows the efficacy of using curcumin in combination with doxorubicin to improve the survival rate and has the potential to enhance the quality of life of neuroblastoma patients.
Keywords: Neuroblastoma, epithelial-to-mesenchymal transition, curcumin, silibinin, combinatorial therapy
INTRODUCTION
Neuroblastoma is the most common extracranial solid tumour that occurs in childhood[1]. It usually arises from the sympathetic nervous system and originates from the neuroepithelial cells of neural crest tissues[2]. The clinical behaviour of this complex disease is highly diverse, ranging from a benign tumour mass with no symptoms to a progressive and fatal disease with resistance to current treatments[2,3]. This aggressive cancer is the most common cancer diagnosed during the first two years of human life[4]. The most important prognostic factor in neuroblastoma is the stage of the disease while the age of the patients remains an independent prognostic factor. Interestingly, infants less than 12 months with stages beyond 1, have significantly better disease-free survival rates than older children who are diagnosed with the same stage[5-8]. The histopathologic features of tumours are also classified as favourable or unfavourable depending on the differentiation of neuroblasts and Schwannian stroma content[9]. In addition, the presence of amplified transcription factor N-Myc in neuroblastoma patients (about 20%) strongly correlates with poor prognosis and tumour dissemination. As amplification of N-Myc has a profound effect on clinical outcome in neuroblastoma patients, N-Myc copy number has been used as a biomarker[10-12]. The heterogeneity of neuroblastoma has resulted in decrease in the effectiveness of the therapeutic strategies over the past decades. However, tumours with favourable prognostic markers do not require as intense chemotherapy as the tumours with adverse factors. Hence, the Children’s Oncology Group has developed a risk group stratification system in order to categorise the treatment strategies[13]. This system is mainly based on the stage of the disease, amplification status of N-Myc, DNA index and histopathology.
Even though the overall outcome of the neuroblastoma patients has improved recently, the survival rates of the children with high-risk neuroblastoma have not shown a substantive improvement[14,15]. Hence, there is a need for better therapeutic avenues to treat high-risk neuroblastoma[1,16,17]. Regardless of the efforts and current developments in treating high-risk patients, most of them relapse due to acquired drug resistance. Moreover, the survivors of high-risk neuroblastoma have manifested adverse effects to current therapeutics, which in turn has impaired their quality of life. Some of the uncovered effects from radiotherapy and surgery are damages to eyes, osteoporosis and various other musculoskeletal abnormalities[18,19]. Unfortunately, damages to renal tubes, chronic abnormalities in electrolytes, impaired sexual maturation, premature menopause and growth hormone deficiency can also occur due to long-term exposure to chemotherapy[20-25]. When considering all these factors, there is an urgent need of developing therapeutic strategies with natural compounds that increase the anti-cancer activity with lower side effects to enhance the quality of life of neuroblastoma patients.
Epithelial-to-mesenchymal transition (EMT) is a cellular process where epithelial cells lose their adhesion properties and turn mesenchymal. This highly plastic and dynamic shift towards the mesenchymal state is considered as EMT, wherein the expression of the adhesion proteins are downregulated so as to promote migration and invasion[26,27]. On the contrary, mesenchymal-epithelial transition (MET) is the reverse of EMT, wherein the expression of adhesion proteins are upregulated and the cells lose the migratory phenotype[28]. It is well established that EMT regulates cancer metastasis. Recent evidence suggests that EMT also regulates chemotherapeutic drug resistance in several cancer types. Human colorectal cancer (CRC) cell lines KM12L4 and HT29 displayed EMT because of oxaliplatin resistance by translocating β-Catenin to the nucleus[29]. Hepatocellular carcinoma cells resistant to 5-Fluorouracil (5-FU) also showed induction of EMT with the upregulation of Twist and the down regulation of E-Cadherin[30]. Similarly, activation of Snail in CRC cell lines led to increased motility and invasiveness with increased resistance to 5-FU[31]. Collectively, these data suggest that EMT plays a role in chemoresistance. Neuroblastoma cells, due to their origin, are more mesenchymal and it is highly likely that the inherent resistance to treatment could be partly attributed to EMT. It is unclear whether the utility of MET inducers along with standard chemotherapeutic drugs could increase the sensitivity of the neuroblastoma cells.
Curcumin and silibinin are natural components that have exhibited anti-cancer activities and can induce MET in many adult cancers with less or no toxicity[32,33]. These natural active ingredients have been implicated in suppressing various growth and pro-invasive signalling pathways as well as inducing cell death in a variety of cancer cells[34-36]. In non-N-Myc amplified SH-SY-5Y neuroblastoma cells, curcumin was shown to induce cell deathin vitro[37]. However,in vivostudies that highlight the therapeutic potential of curcumin and silibinin in treating high-risk neuroblastoma cells are currently lacking. In addition, the utility of these natural compounds in combinatorial therapy with doxorubicinin vivohas not been examined. In this study, we examined the combinatorial effect of curcumin or silibinin in sensitising neuroblastoma cells to the chemotherapeutic drug doxorubicin bothin vitroandin vivo.
METHODS
RNA-Seq analysis
The RNA-Seq data for NBL and CRC cell lines and tissues were downloaded from published literature[38]. The mRNA expression (log2RPKM values) of genes categorised as epithelial and mesenchymal were plotted using MATLAB. Genes (315) classified as epithelial and mesenchymal were downloaded from published literature[39]. Statistical analysis to calculate the significance was performed using the Pearson’s chi-square test.P-values less than 0.05 were considered statistically significant.
Cell culture
The neuroblastoma cell lines SK-N-BE2, SH-SY5Y and SK-N-AS were cultured in 150 cm2tissue culture flasks (BD FalconTM) in Dulbecco’s Modified Eagle Medium (DMEM) (GIBCO, Life Technologies) medium supplemented with 10% (v/v) fetal calf serum (GIBCO, Life Technologies) and 100 units/mL of penicillinstreptomycin (GIBCO, Life Technologies). IMR32 neuroblastoma cells were cultured in Minimum Essential Medium (GIBCO, Life Technologies) medium. The cells were incubated at 37 °C with 5% CO2. SK-N-BE2 (CRL-2271TM) and IMR32 (CCL-127TM) cells were purchased from ATCC®, while SH-SY5Y and SK-N-AS cells were kindly gifted by Dr Julie Atkin.
Whole cell lysate preparation
Neuroblastoma cells were treated with 10 µM curcumin and 100 µM silibinin for 24 and 48 h prior to preparation of cell lysates. Cells were lysed as described previously[40]using 4 × sodium dodecyl sulfate (SDS) loading dye [2% (w/v) SDS, 125 mM Tris-HCl pH 7.4, 12.5% (v/v) glycerol and 0.02% (w/v) bromophenol blue]. Briefly, loading dye (1.5 mL) was added to culture dishes and evenly spread using the cell lifter (Fisher Biotec). The lysate was then collected in thick wall polyallomer tubes (Beckman Coulter) and centrifuged at 100,000gfor 1 h (TLA 100.2, Beckman). Supernatant was collected and stored at -80 °C for further analysis.
SDS-PAGE and Western blotting
Equal amount of protein samples was prepared in 4 × SDS loading buffer with 100 mM DTT (Astral). Samples were then denatured by heating at 95 °C for 2 min and were run on a NuPAGE® 4%-12% Bis-Tris precast gel (Life Technologies). The gels were run at 150 V for 1 h in NuPAGE® MES SDS Running Buffer (Life Technologies). Proteins were transferred on to nitrocellulose membrane using iBlot dry blotting system (InvitrogenTM) at 20 V for 7 min. The membrane was incubated with blocking solution containing 5% (w/v) skim milk in Tris-buffered saline with 0.05% Tween 20 (TTBS) [100 mM Tris-HCl pH 7.5, 150 mM NaCl and 0.05% (v/v) Tween 20] for 45 min. The membrane was washed three times with TTBS (10 min each) and probed with the relevant primary antibody overnight at 4 °C. The membrane was again washed with TTBS over 30 min. Subsequently, the blot was probed with appropriate IRDye (LI-COR®) or peroxidase (Sigma-Aldrich®) conjugated secondary antibody for 1 h at room temperature. The blot was then washed three times with TTBS over 30 min. For the visualisation of the protein bands probed with IRDye (LI-COR®), ODYSSEY CLx (LI-COR®) machine was used.
Cell death assay
Cells (5 × 103per well) were seeded in a 24-well plate in 500 µL DMEM medium and allowed to adhere for 48 h at 37 °C in the presence of 5% CO2. Cells were then treated with or without doxorubicin (1 µM) and incubated for 48 h. For combinational treatment studies, curcumin (10 µM) or silibinin (100 µM) treatment was performed 24 h before the addition of doxorubicin (pre-treatment) as well as on the same day of doxorubicin treatment (combinational). After 48 h of cancer therapeutic drug treatment, cells were scraped and resuspended. Supernatant (300 µL) was then transferred in to a 96-well plate and spun at 300gfor 5 min before discarding the supernatant. The remaining pellet was then resuspended with 200 µL of propidium iodide (PI) buffer [0.1% (w/v) TritonX 100 and 50 µg/mL Propidium iodide (Sigma Life Science®] and was incubated overnight at 4 °C. For this, 2 × 104cells were used in the analysis, Results from fluorescence activated cell sorting CANTO II (BD Biosciences) were then analysed using FlowJo (TreeStar).
Establishment of tumour xenografts
SK-N-BE2 cells (5 × 106) were subcutaneously injected to athymic Balb/c nude female mice (8 weeks old). The cells were suspended in Matrigel before the injections. After formation of tumours, the mice were injected intraperitoneally (i.p.) with dimethyl sulfoxide (DMSO) (control), curcumin (40 mg/kg), doxorubicin (5 mg/kg) and combinational treatment of doxorubicin and curcumin three times a week. Tumour size and the weight of the mice were measured daily. The tumour volume was calculated according to the formula ½(W2× L). According to the Australian code of practice for the care and use of animals for scientific purposes and La Trobe Ethics Committee guidelines (AEC 14-15), mice were sacrificed when the tumour size reached 1500 mm3.
Statistical analysis
Statistical significance of experiments was analysed by studentt-test andPvalues less than 0.05 were considered to be statistically significant. CI (Combination index) was calculated using Chou-Talalay method[41]. A violin plot was generated using MATLAB and thePvalue of the violin plot was also calculated using MATLAB[42].
RESULTS
Neuroblastoma cells exhibit mesenchymal signature
Prior to commencement of the experiments, publicly available RNA-Seq data for neuroblastoma tissues and cells were queried for the expression of EMT genes to examine the hypothesis of whether the neuroblastoma cells are mesenchymal. To validate the expression of epithelial and mesenchymal genes, publicly available RNA-Seq data for neuroblastoma cell lines and tissues were examined. In total, 315 genes implicated in EMT were retrieved from the public literature[43]and the expression profile of these genes were plotted in a panel of neuroblastoma cell lines and tissues [Figure 1]. Interestingly, agreeing with our hypothesis, neuroblastoma cell lines (n= 8) and tissues (n= 157) exhibited a high mesenchymal and low epithelial gene expression. These results suggest that neuroblastoma cells are mesenchymal and hence could contribute to the aggressive phenotype.

Figure 1. Neuroblastoma cells and tissues have a high mesenchymal gene signature. Violin plot representation of epithelial and mesenchymal gene expression in neuroblastoma cell lines (n = 8) and tissues (n = 157) is depicted. The red line represents the median. P value was determined by Mann-Whitney test
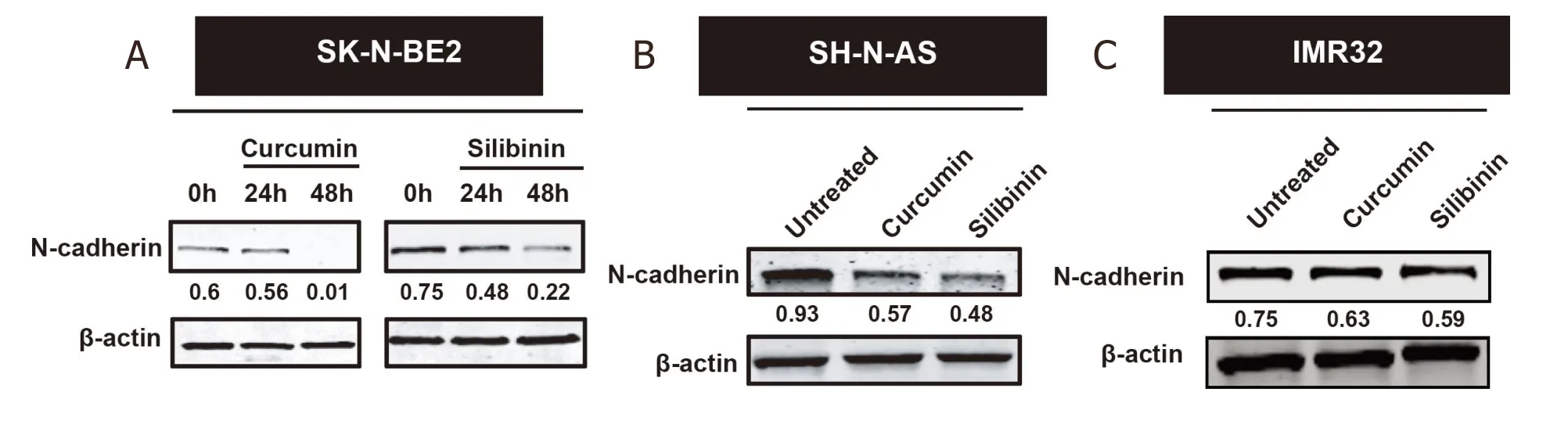
Figure 2. Curcumin and silibinin reduced the expression of the mesenchymal marker N-Cadherin. A: Western blot analysis for N-Cadherin in SK-N-BE2 cells; B: Western blot analysis for N-Cadherin in SH-N-AS cells; C: Western blot analysis for N-Cadherin in IMR32 cells. Drug concentration: 10 μM curcumin or 100 μM silibinin. β-actin was used the loading control. The N-cadherin band intensities are normalised to respective band intensities of β-actin
Curcumin and silibinin reduce the expression of mesenchymal marker N-Cadherin
If the mesenchymal nature of neuroblastoma cells account for the treatment resistance, induction of an epithelial phenotype by MET inducers could sensitise the neuroblastoma cells to doxorubicin. To test this hypothesis, MET inducers such as curcumin and silibinin were utilised in this study. It has been previously established that curcumin and silibinin can reverse EMT in certain cancer types[44,45]. Consistent with the literature, treatment of the cancer cells with curcumin and silibinin reduced the expression of mesenchymal marker N-Cadherin [Figure 2A-C][46,47]. N-cadherin is a well-established mesenchymal marker that promotes cell motility and migration[48]. The reduction of N-Cadherin levels was only observed at 10 µM for curcumin and 100 µM for silibinin. Epithelial markers E-Cadherin and Keratin 18 could not be detected in the neuroblastoma cells with or without the MET inducers. Similarly, the expression of another mesenchymal marker, Vimentin, did not change in the neuroblastoma cells treated with or without curcumin or silibinin (data not shown).
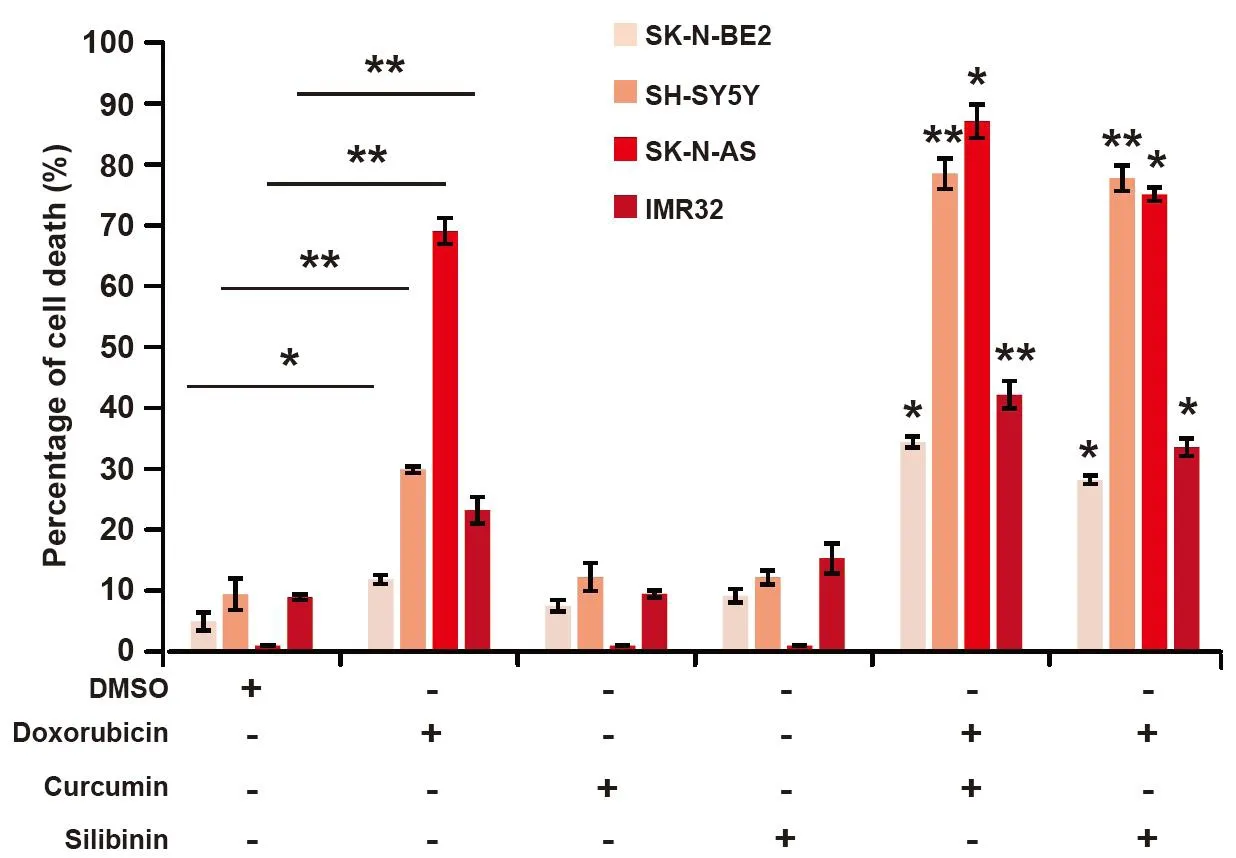
Figure 3. Curcumin and silibinin increases the sensitivity of neuroblastoma cells to doxorubicin. FACS cell death assay on a panel of neuroblastoma cells treated with 10 μM curcumin or 100 μM silibinin in the presence of 1 μM doxorubicin (n = 3). Data are presented as mean ± SEM, *P < 0.05; **P < 0.01 as determined by Student’s t-test. Significance of the percentage of cell death in combinational treatments was calculated in comparison to respective doxorubicin only treatments. DMSO: dimethyl sulfoxide; FACS: fluorescence activated cell sorting
Combinatorial treatment of curcumin or silibinin with doxorubicin sensitises neuroblastoma cells
Curcumin and silibinin are natural compounds that have many anti-cancer properties including induction of MET[34-36]. As neuroblastoma cells, especially high-risk ones, are aggressive and resistant to treatment, the combinatorial effect of curcumin or silibinin with doxorubicin was examined to understand their therapeutic potential. A panel of four neuroblastoma cells, namely SH-SY-5Y, SK-N-AS, SK-N-BE2 and IMR32, was selected for thein vitroanalysis. Among these neuroblastoma cells, SH-Y-5Y and SK-NAS cells are low-risk and do not contain N-Myc amplification[49]. On the contrary, SK-N-BE2 and IMR32 cells are high-risk neuroblastoma cell models with N-Myc amplification (> 0 copies)[49]. Consistent with the literature, treatment of the low-risk neuroblastoma cells (SH-SY-5Y and SK-N-AS) with the chemotherapeutic agent doxorubicin (1 µM) induced significant cell death [Figure 3]. Whilst the basal cell death for SK-N-AS cells was about 1%, incubation with doxorubicin induced nearly 70% cell death, an increase by 70-fold. Similarly, SH-SY-5Y cells exhibited more than three-fold cell death (30%) upon doxorubicin treatment. However, the high-risk neuroblastoma cells (SK-N-BE2 and IMR32) showed lesser percentage of cell death (11.8% and 23%, respectively) when incubated with the chemotherapeutic agent doxorubicin (1 µM). This relates to a 2.3- and 2.6-fold increase in cell death in SK-N-BE2 and IMR32 cells, respectively.
Next, the combinatorial effect of curcumin or silibinin with doxorubicin was evaluated in the panel of neuroblastoma cells. As shown in Figure 3, neither curcumin (10 µM) nor silibinin (100 µM) induced significant cell death in any of the neuroblastoma cells. The concentration for curcumin and silibinin was chosen as they reduced the expression of N-Cadherin in neuroblastoma cells. When performing the combinational treatments, the cells were treated with curcumin and silibinin 24 h prior to doxorubicin treatment to allow the induction of MET. Nevertheless, combination of curcumin with doxorubicin induced significant cell death in both the low- and high-risk neuroblastoma cells. Compared to the doxorubicin alone treatment, a 2.9-, 2.6-, 1.3- and 1.8-fold increase in cell death was observed in SK-N-BE2, SH-SY-5Y, SK-N-AS and IMR32 cells, respectively. Moreover, the Combination index (CI) values obtained for the treatments were < 0.1, indicating a synergism. The CI values were 0.56 and 0.5 for SK-N-BE2 and SH-SY-5Y cells, respectively. Consistent with these results, combinatorial treatment of silibinin and doxorubicin also induced significant cell death, compared to doxorubicin alone, in the panel of neuroblastoma cells. Taken together, these data suggest that curcumin and silibinin can increase cell death induced by doxorubicin in both low- and high-risk neuroblastoma cells. The data also suggested that curcumin along with doxorubicin was more effective in inducing cell death in the neuroblastoma cells.
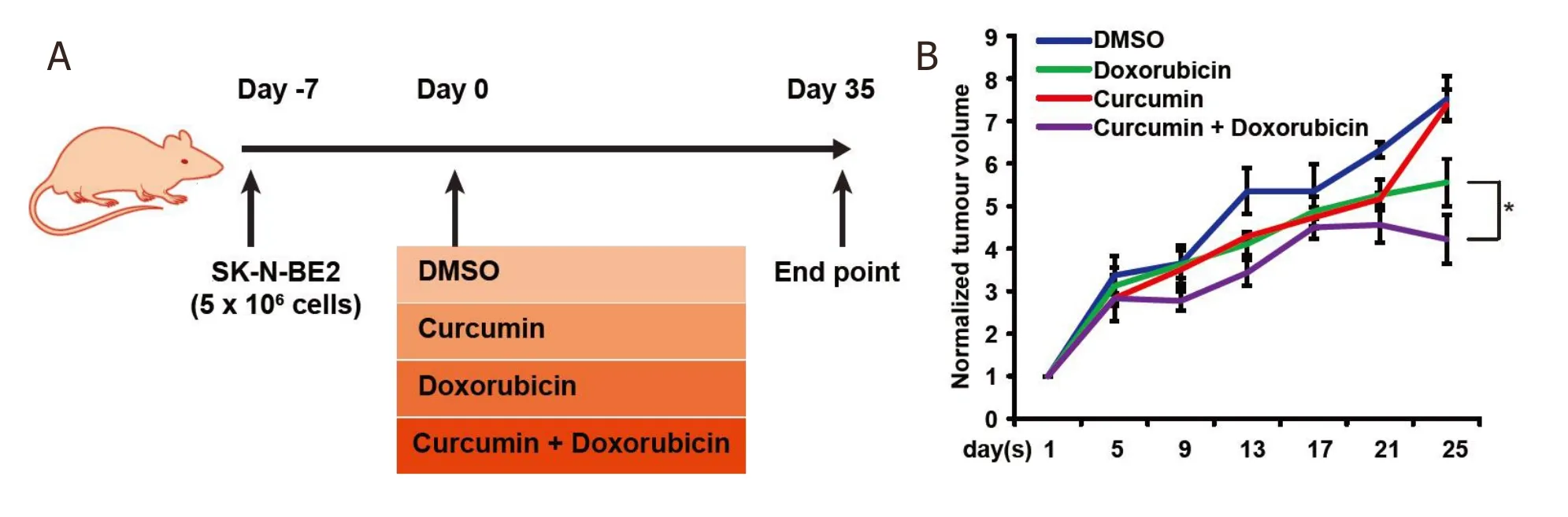
Figure 4. Combinatorial therapy reduces the tumour burden. A: schematic representation of in vivo work to examine the efficacy of combinatorial therapy on neuroblastoma tumour burden. B: relative tumour volume across a period in the presence of curcumin and/or doxorubicin (n = 3). Data are presented as mean ± SEM, *P < 0.05 as determined by Student’s t-test. DMSO: dimethyl sulfoxide
Combinational treatment reduced the tumour burden and increased the survival of mice implanted with neuroblastoma
Several studies have examined the effect of curcumin or silibinin on inducing cell death in neuroblastoma cells[37]. However, the therapeutic potential has not been examinedin vivo. Furthermore, it is unclear whether a combinatorial treatment that increases cell death in neuroblastoma cells also worksin vivo. Hence, to validate the effect of combinatorial treatmentin vivo, nude mice were injected with the highrisk SK-N-BE2 neuroblastoma cells (5 × 106) subcutaneously. N-Myc amplified SK-N-BE2 cells are more proliferative and resistant to doxorubicin treatment [Figure 3] than the other neuroblastoma cells used in this study, hence was chosen as a model cell line. After formation of tumours, the mice were administered (i.p.) with DMSO (control), doxorubicin, curcumin or a combination of doxorubicin and curcumin twice per week [Figure 4A]. Here, curcumin was chosen as the curcumin treatment had more effect on N-cadherin expression level [Figure 2A] and cell death [Figure 3] than silibinin. The tumour volume was monitored daily in the control and treatment groups. Tumour volume was the highest in mice that received DMSO or curcumin alone [Figure 4B]. Consistent with thein vitrodata and literature, mice that were treated with doxorubicin alone had a significant decrease in the tumour volume. Encouragingly, the tumour volume in mice treated with both doxorubicin and curcumin was significantly smaller compared to those receiving only doxorubicin. There were no visible side effects from any of the treatments and no change in body weight of the mice was observed [Figure 5A]. Importantly, mice that received combinational treatment exhibited a higher survival rate [Figure 5B]. Taken together, these results suggest that treatment of curcumin in combination with a chemotherapeutic drug may be a viable strategy to treat neuroblastoma patients, most importantly high-risk neuroblastoma patients with N-Myc amplification.
DISCUSSION
Neuroblastoma is the most common extracranial solid tumour in children under the age of five. Depending on risk factors, neuroblastoma can be divided in to low- and high-risk[50]. Even though the survival rates of low-risk neuroblastoma have improved significantly, the survival rates of high-risk neuroblastoma have remained poor[51]. Among the risk factors, amplification of the oncogene N-Myc is detected in about 20% of neuroblastoma patients and considered high-risk[52]. Hence, therapeutic strategies to manage high-risk N-Myc amplified neuroblastoma cells is needed.
Curcumin and silibinin have shown anticancer properties by modulating several signalling pathways[34,36,53-55]. More importantly, it is documented that curcumin and silibinin have the ability to repress proteins that are involved in EMT and metastasis[45,47,56]. We sought to elucidate the role of curcumin or silibinin in combination with doxorubicin.
First, the results from publicly available RNA-Seq analysis suggest that the mesenchymal-like phenotype exhibited by neuroblastoma cells could be one of the potential reasons for the aggressiveness. In the current study, we targeted the utility of curcumin or silibinin in sensitising neuroblastoma cells to doxorubicin. Combinatorial therapy of curcumin and doxorubicin sensitised the highly aggressive neuroblastoma cells bothin vitroandin vivo. Here, we utilised four neuroblastoma cell lines, among which SK-N-BE2 and IMR32 possess N-Myc amplification and hence are categorised as high-risk aggressive neuroblastoma. N-Myc is not amplified in SH-SY-5Y and SK-N-AS cells, which were used to identify the usage of combinational therapy in a wider range of neuroblastoma cells.
Agreeing with the previous literature, curcumin and silibinin reduced the expression of the mesenchymal marker N-Cadherin and induced MET[37]. However, the combinatorial effect with doxorubicin can also be attributed to other anti-cancer activities of these MET inducers, such as inhibition of p53, pAKt and STAT3 signalling pathway[34,53,57]. Moreover, recent findings also suggest curcumin and silibinin as agents that can inhibit the cancer stem cells growth[58,59]. Nevertheless, based on these results, curcumin and silibinin are interesting candidates for combination with standard chemotherapeutic drugs including doxorubicin for neuroblastoma treatment. As mentioned above, despite aggressive therapy, the overall survival for high-risk neuroblastoma patients is < 50% at five years[60]. In fact, the survival rate of relapsed stage 4 neuroblastoma patients as per the International Neuroblastoma Risk Group database between 1990 and 2002 is 8%[61]. Alarmingly, the survival rate drops below 4% for N-Myc amplified neuroblastoma patients.

Figure 5. Combinatorial therapy increases the survival of mice implanted with neuroblastoma cells. A: graphical representation of body weight of mice over the period in the presence or absence of curcumin and doxorubicin (n = 3). Treatment with curcumin and doxorubicin does not impart visible side effects in mice. B: Kaplan-Meier analysis of the survival rate of mice bearing neuroblastoma tumour undergoing treatment. Combinatorial treatment significantly enhanced the survival of mice (*P < 0.05). DMSO: dimethyl sulfoxide
Hence, the findings from this study have clear potential therapeutic benefits to increase the survival of the high-risk neuroblastoma patients. Importantly, as curcumin and silibinin have been shown to be safe and well-tolerated in randomised clinical trials[62-67], they can be readily utilised for neuroblastoma therapy.
DECLARATIONS
Author contributions
Conceived and directed the entire project: Mathivanan S
Performed the experiments: Fonseka P, Angela DG, Gangoda L
Performed bioinformatics analysis: Pathan M
Prepared the figures, drafted and finalized the manuscript with inputs from other authors: Fonseka P, Mathivanan S
Read and approved the manuscript: Fonseka P, Gangoda L, Pathan M, Angela DG, Mathivanan S
Availability of data and materials
Not applicable.
Financial support and sponsorship
Suresh Mathivanan is supported by ANZ Trustees and Ramaciotti Establishment Grant. Suresh Mathivanan is supported by Australian Research Council Future Fellowship (FT180100333) but the project was not funded by the basic science fellowship. Angela Di Giannatale is supported by a Grant from the Italian Ministry of Health (GR-2016-02364088). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Animal study was conducted according to Australian code of practice for the care and use of animals for scientific purposes and La Trobe Ethics Committee guidelines (AEC 14-15).
Consent for publication
Not applicable.
Copyright
© The Author(s) 2020.
