A Green Strategy to Synthesize Ag/Ag3PO4/Chitosan Composite PhotocataIysts and Their PhotocataIytic Degradation Performance under VisibIe Light Irradiation
2020-07-21PengChengWuHuiLiPengYuHanWuLeiLiXiaoRuiHaoBangHuaPengGuiHuaMengJianNingWuZhiYongLiu
Peng-Cheng Wu | Hui-Li Peng | Yu-Han Wu | Lei Li | Xiao-Rui Hao |Bang-Hua Peng | Gui-Hua Meng | Jian-Ning Wu | Zhi-Yong Liu
Abstract—In this work,the plasmonic Ag/Ag3PO4/chitosan (Ag/Ag3PO4/CS) composite photocatalyst was prepared by a low-temperature strategy.Environmentally friendly CS plays triple vital roles in this composite.First,it was devoted to in situ reducing metallic silver from silver ions of Ag3PO4.Also,as the carrier of Ag/Ag3PO4 nanoparticles,CS can effectively prevent aggregation.Furthermore,benefitting from the settlement of hydrophilic CS,the prepared composite could be easily separated and recovered from the solution system.X-ray diffraction (XRD),the scanning electron microscope,energy-dispersive X-ray spectroscopy (EDS),ultraviolet-visible (UV-vis) diffused reflectance spectroscopy,and X-ray photoelectron spectroscopy (XPS) were employed to characterize the properties of materials.The results of photo-decomposition testing showed that the Ag/Ag3PO4/CS composite possessed good activity for the decomposition of Rhodamine B (RhB) under visible light.
1.Introduction
Nowadays,the environmental pollution and energy crisis are the main challenges for the future of humanity.How to address these two issues efficiently and simply has become the focus of research.Photocatalysis,a “green” technology,has been considered to be one of the most promising and effective methods for environmental treatment and energy conversion,including decomposition of organic pollution,reduction of high toxicity heavy metal ion,water splitting,and carbon dioxide reduction[1],[2].Among various photocatalysts,owing to its low cost,good stability,high activity,and non-toxicity,inorganic TiO2is the most studied[3]-[5].However,it is worth noting that the photocatalytic activity of TiO2is extremely limited by its intrinsic property of the large bandgap (Eg=3.2 eV),indicating that it only responds to ultraviolet (UV) light (less than 5% of the solar spectrum).As is known,the visible light is about ten times abundant than UV light (~48%),hence the development of visible-light responsive photocatalysts arouses wide research interest in recent years[6].
In 2010,Ye’s group firstly explored and certificated the photooxidation property of silver phosphate(Eg=2.36 eV) under visible-light,after that this material has been widely studied in the field of oxidation of water and photodegradation of organic pollution[7].When the wavelength of irradiation light is longer than 420 nm,Ag3PO4has excellent quantum efficiency and photocatalytic activity[8].Additionally,there are many experimental results showing that the surface plasmon resonance (SPR) effect of Ag0could drastically improve the activity and stability of Ag3PO4
[9]-[11].However,nano-sized Ag/Ag3PO4is easy to aggregate into micrometer-sized particles in aqueous solutions,which would greatly decrease the photocatalytic activity.However,nano-photocatalysts are difficult to isolate and recover from the reaction medium in a simple way during the practical application that will lead to secondary contamination.To solve these issues,immobilizing nano-photocatalysts onto/into supporting materials is considered as one of the easiest and most effective ways.Until now,various kinds of materials have been developed as carriers,such as the inorganic clay[12]-[16],carbonaceous materials[17]-[21],metal oxide[22],[23],and organics[24]-[26].As mentioned above,the SPR effect could enhance the photocatalytic performance of Ag3PO4,but the progress of reducing Ag+to Ag0always meets some challenges.UV irradiation and high-temperature polyol reduction are the most general fabrication methods,but they are costly,tedious,and time-consumed.Therefore,the development of suitable carriers and facile reducing approaches is necessary and valuable.
Chitosan (CS),a hydrophilic and cationic polymer with lots of hydroxyl and amino groups,is the second most abundant natural biopolymer on the earth.CS is usually used as an adsorbent in environmental science.These functional groups could provide plentiful chelation sites for metal ions and organic contaminants[27],[28].In the domain of catalysis,CS has been selected as carriers to synthesize composite catalysts for the Suzuki coupling and catalase-like reaction[29],aerobic oxidation of alkyl arenes and alcohols[30],reduction of aromatic nitro compounds[31],advanced oxidation processes[32],and so on.Besides,previous work had demonstrated that CS could serve as an effective reduction agent and stabilizing agent to prepare noble metal nanoparticles.Qian and co-worker[33]employed CS as a mediator agent under the quiescent crystallization condition to synthesize Ag and Au nanoparticles.Weiet al.[34]took CS as a reductant and scaffold to prepare metal nanoparticles-CS bioconjugates.Taking advantage of these features of CS,our group synthesized three composite catalysts,Ag nanoparticles/Ndoped carbon[35],Ag/AgCl/N-doped carbon[36],Ag/AgCl/CS[37],and applied them for the degradation of organic pollutants.
In light of the problems of Ag/Ag3PO4nanoparticles and the characteristics of CS,in this work,we prepared an Ag/Ag3PO4/CS composite photocatalyst via a “green” strategy for photo-decomposition of organic dye.During the preparation process,Ag+is partially in situ reduced to Ag0by CS at low temperature; therefore,the reductant or UV light is no longer needed.As the carrier,CS could effectively prevent the agglomeration of Ag/Ag3PO4nanoparticles.The photodegradation experiments indicated that the Ag/Ag3PO4/CS composites possess the superior photocatalytic activity in RhB degradation under visible light.Also,benefitting from the settlement of CS,the Ag/Ag3PO4/CS composite photocatalysts could be easily separated from the medium solution and reused in the next experiment.
2.ExperimentaI
2.1.MateriaIs
CS powder was bought from Shanghai Lanji Technological Development Co.,Ltd.The degree of deacetylated is >90.0% and the viscosity is <100 cps.Silver nitrate and sodium chloride were obtained from Xi’an Chemical Reagent Factory.RhB was purchased from Tianjin Guangfu Fine Chemical Research Institute.Anhydrous ethanol came from Tianjin Fuyu Fine Chemical Reagent Company.All the reagents are analytical grade and without further purification for use.
2.2.Synthesis
The preparation process of Ag/Ag3PO4/CS is illustrated in Fig.1.All the samples were prepared by the green and facile water bath heating method.Briefly,0.5 g CS was dispersed in a certain concentration of the silver nitrate aqueous solution (40 mL) with continuously magnetic stirring for 2 h at room temperature.This progress is to reach the adsorption-chelation balance between CS and Ag+.Then,the 1.1 fold stoichiometric Na2HPO4aqueous solution was slowly impregnated.The prepared mixture was heated to 80 °C and kept 3 h.Finally,the production was collected and rinsed with the deionized water and anhydrous ethanol for several times,respectively.After drying in the vacuum overnight at 50 °C,the samples were obtained.They were designated as 40-Ag/Ag3PO4/CS,60-Ag/Ag3PO4/CS,and 80-Ag/Ag3PO4/CS,and the corresponding mass ratios of Ag3PO4to CS were 40%,60%,and 80%,respectively.80-Ag3PO4/CS was prepared in the same method and the difference is without heating.What needs special emphasis is that all the steps need to be covered with the tin foil to make sure Ag3PO4would not be reduced by natural light.
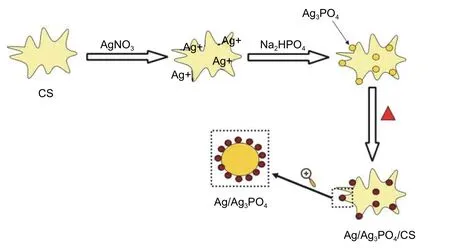
Fig.1.Schematic illustration for the synthesis of Ag/Ag3PO4/CS composites.
2.3.Characterizations
Crystal structures of the samples were identified by X-ray diffraction (XRD) with Cu Kα radiation in a scanning range of 10° to 80° on an X-ray diffractometer (Bruker D8 Advance,Germany).Surface morphologies of the samples were observed by scanning electron microscope (SEM,Hitachi,S4800).X-ray photoelectron spectroscopy (XPS) was measured by using an axis ultra spectrometer with monochromatized Al Kα X-ray as the excitation source (225 W).The UV-visible diffuse reflection spectra (UV-vis DRS) were used to study the optical property by a UV-vis spectrophotometer (UV-2 450,Corporation,Shimadzu).
2.4.PhotocataIytic Activity
The photocatalytic experiments were carried out by the degradation of RhB (C0=10 mg/L) using a 150 W Xe lamp with a cutoff filter to filter the UV light (λ<420 nm).Typically,50 mg as-prepared photocatalysts were dispersed in a quartz vessel with the RhB aqueous solution of 50 mL,and the suspensions were magnetically stirred in the dark for 60 min to establish an adsorption-desorption equilibrium.The distance between the center of the quartz vessel and the lamp kept about 20 cm.Then turned on the cooling water and the light,about 2.5 mL aliquots were pipetted from the mixture every 10 min.To remove the residual photocatalyst thoroughly,the suspension was immediately centrifuged at 3 000 rpm for 3 min.The RhB remnant concentration was recorded by a UV-vis spectrophotometer (TU-1901,Beijing) at its characteristic wavelength of 553 nm.
3.ResuIts and Discussion
3.1.PhotocataIyst Characterization
Figs.2 (a) to (d) show the SEM images of CS and different mass ratio composite photocatalysts.In comparison with the other three samples,CS has a rather smooth surface.After the reaction,many particles can be clearly found on the surface of CS,and as the ratio increased,the more particles were loaded on the CS.It is worth noting that the particles on the CS surface do not have extensive agglomeration,which is attributed to the abundant hydroxyl and amino groups of the CS anchor silver ion before it reacts with sodium hydrogen phosphate.These well-dispersed nanoparticles will make the photocatalytic effect more efficient.The energy-dispersive X-ray spectroscopy (EDS) confirms the elemental composition of the composite (Fig.2 (e)),including C,N,and O that come from CS,while Ag and P could be attributed to Ag/Ag3PO4.
The XRD measurement was performed to confirm the crystal phase of the prepared sample (Fig.2 (f)).The diffraction peaks at 21.17°,29.82°,33.49°,36.87°,42.66°,47.99°,52.92°,55.27°,57.57°,61.93°,70.18°,and 71.13° are well agreement with the crystal planes of (110),(200),(210),(211),(220),(310),(222),(320),(321),(400),(420),and (421) of the body-centered cubic phase Ag3PO4(Joint Committee on Powder Diffraction Standards (JCPDS),No.06-0505).However,there are not any obvious diffraction peaks corresponding to metallic silver,a kind of reasonable explanation is that the content of silver is low or the size of Ag particles is too small.The similar situation had also been reported in the previous work of Ag/AgX(X=Cl,Br,or I)[38],[39].
XPS was used to study the elemental composition and chemical status of Ag/Ag3PO4/CS,and prove the existence of metallic silver.Fig.3 (a) displays the fully scanned spectra of 80-Ag/Ag3PO4/CS from 0 to 1 200 eV,it is clearly seen that the sample mainly composed of C,N,O,Ag,and P elements,indicating that there are no other impurities in the sample.The detailed spectra of Ag are shown in Fig.3 (b).Two bands at 367.2 eV and 373.2 eV are clearly observed,ascribing to Ag 3d5/2and Ag 3d3/2binding energy,respectively.These two peaks can be further divided into two separate peaks.Based on the research result of Ferrariaet al.[40],the Ag+photoelectron chemical shift is slightly lower than metallic silver.The predominant cause of this peculiar shift is due to initial state factors of the ionic charge and lattice potential[40].Therefore,the peaks at 367.7 eV and 373.7 eV can be attributed to Ag0,indicating the existence of metallic silver in the prepared sample.And the peaks at 367.1 eV and 373.1 eV are assigned to Ag+of Ag3PO4.Fig.3 (c) shows a broaden peak in the range of 130 eV to 135 eV of the P 2p spectrum that is corresponding to the phosphorous of Ag3PO4.
The photo-absorption properties of 80-Ag/Ag3PO4/CS,80-Ag3PO4/CS,and CS were measured by UV-vis DRS(Fig.4).As a blank,the carrier CS has a very weak absorption.Compared with 80-Ag3PO4/CS,80-Ag/Ag3PO4/CS exhibits stronger absorbance in the whole region,especially,in the visible light region (at the range of 400 nm to 500 nm),which may arise from SPR of Ag nanoparticles.This is beneficial for making full use of solar light.In addition,the photographs of the sample before and after reaction would provide more intuitive evidence (insert images in Fig.4) to verify the formation of metallic silver.The sample without water-bathing heating shows the typical golden color of Ag3PO4,while the sample after reaction exhibits brownish yellow,indicating the production of metallic silver species during the reaction.Combining the optical properties with the XPS results,it could be confirmed that the existence of metallic silver in the composite[41],[42].
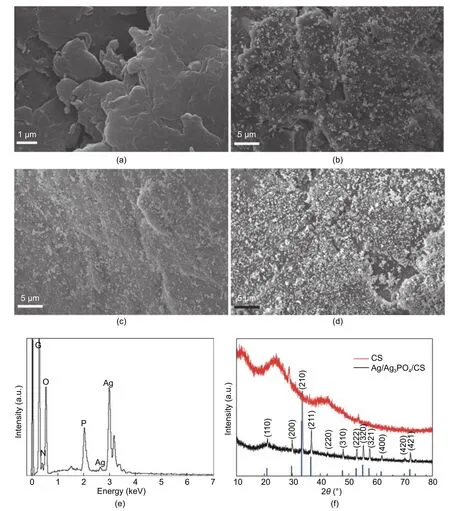
Fig.2.SEM images of (a) CS,(b) 40-Ag/Ag3PO4/CS,(c) 60-Ag/Ag3PO4/CS,(d) 80-Ag/Ag3PO4/CS,(e) EDS spectrum of 80-Ag/Ag3PO4/CS,and (f) XRD patterns of natural CS and Ag/Ag3PO4/CS.
3.2.PhotocataIytic Activity and Mechanism

Fig.3.XPS spectra of 80-Ag/Ag3PO4/CS:(a) XPS fully scanned spectrum of 80-Ag/Ag3PO4/CS,and high-resolution XPS spectra of (b) Ag 3d and (c) P 2p.
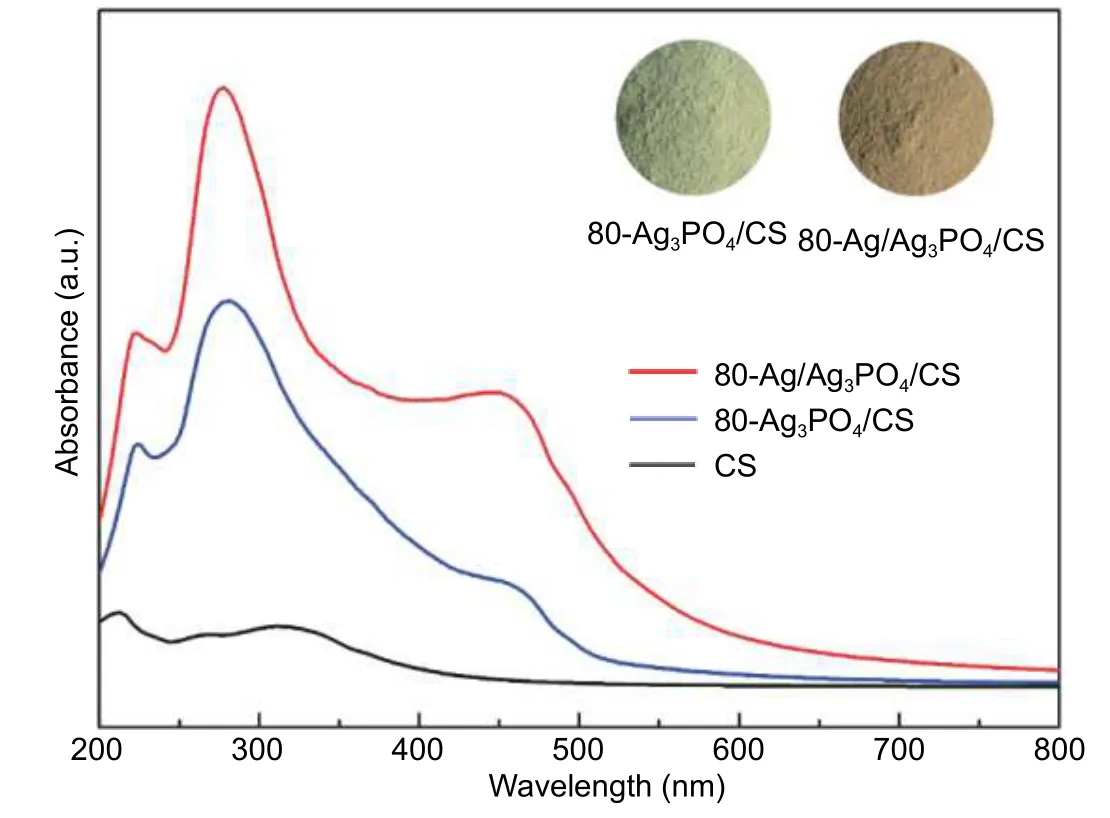
Fig.4.UV-vis DRS of 80-Ag/Ag3PO4/CS,80-Ag3PO4/CS,and CS.
The photocatalytic activity of the CS,80-Ag3PO4/CS,and 80-Ag/Ag3PO4/CS composites was firstly evaluated in terms of the degradation of RhB that is a kind of chemically stable and difficult degraded organic dye,under simulated visible light (λ>420 nm).As the blank test shown (Fig.5 (a)),the self-degradation of RhB is negligible.CS possesses the absorption capacity of RhB to a certain extent,but lack of photodegradation activity.The 80-Ag/Ag3PO4/CS exhibited much higher photocatalytic efficiency than that of 80-Ag3PO4/CS during the same light irradiation time,which could be attributed to the SPR effect of nano-sized metallic Ag.Therefore,the better photocatalytic activity of 80-Ag/Ag3PO4/CS further confirms that the formation of Ag nanoparticles in the production.Fig.5 (b) shows the changes of the RhB aqueous solution in the absorption spectra at different times in the presence of 80-Ag/Ag3PO4/CS.At the beginning (0 min),the characteristic absorption peak of the conjugated chromophore structure at 554 nm is very obvious and sharp.After the light on,this peak decreases progressively and accompanied by a blue shift,indicating RhB was degraded to a series of N-demethylation intermediates.
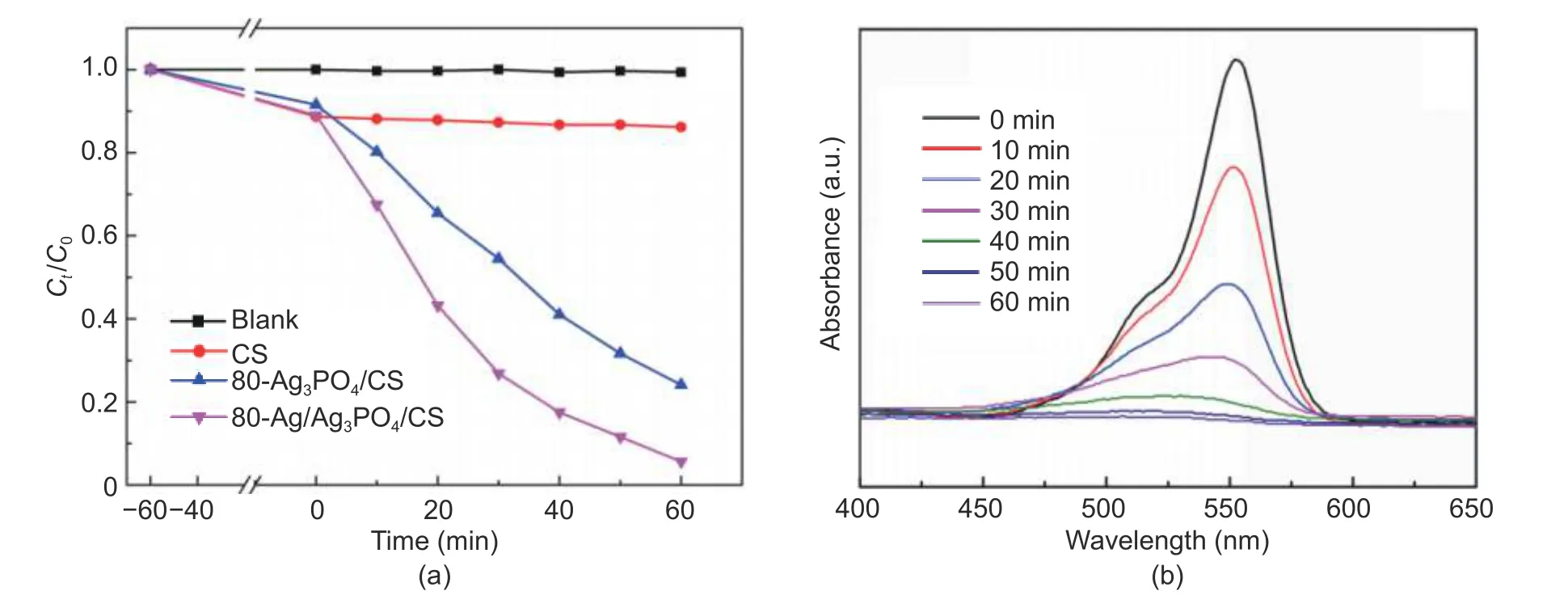
Fig.5.Contrast and blank experiments:(a) photocatalytic efficiency for the degradation of RhB and (b) UV-vis spectra changes of RhB during the photocataltic degradation by 80-Ag/Ag3PO4/CS.
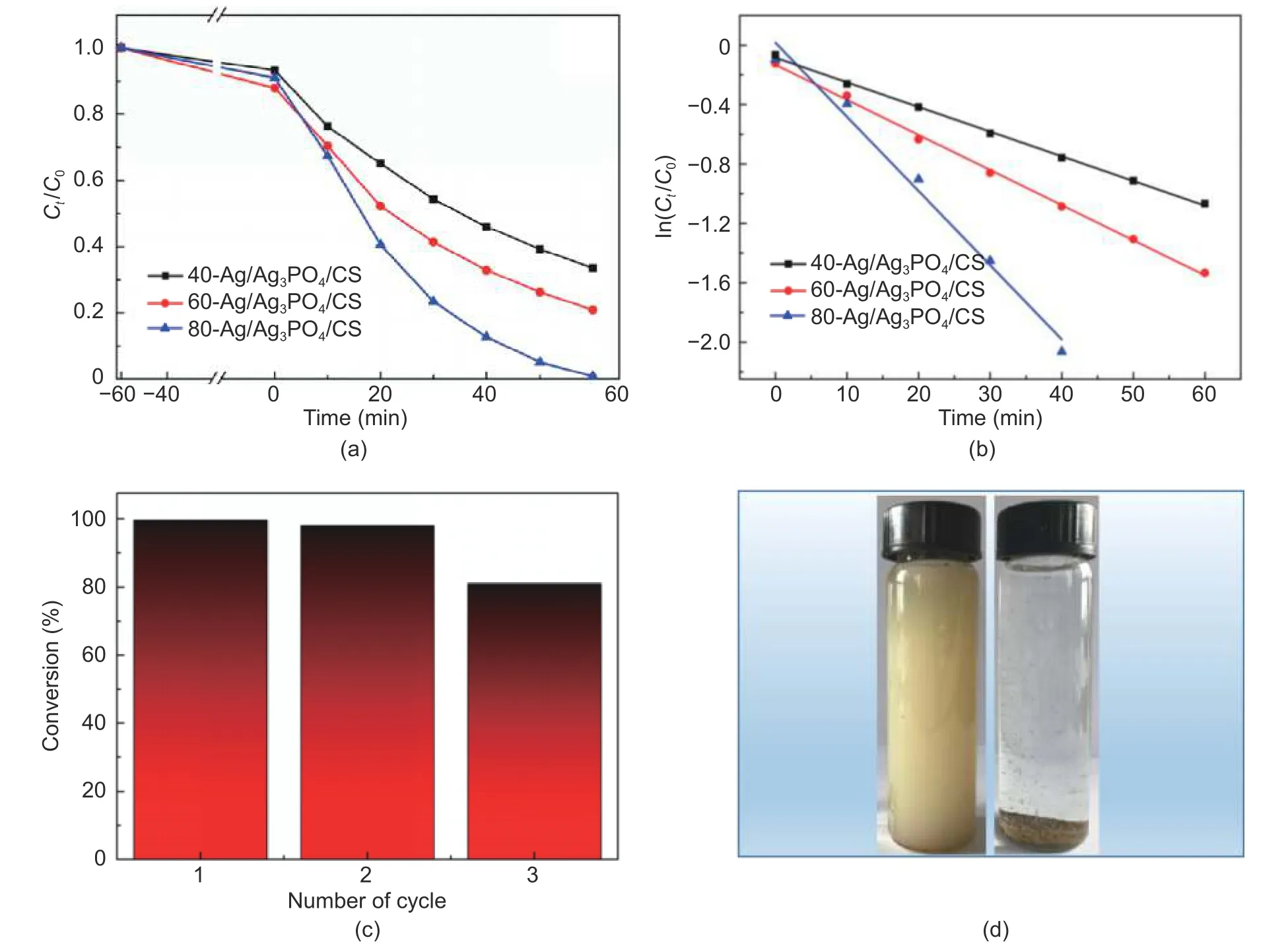
Fig.6.Photocatalytic activity for the degradation of RhB:(a) photocatalytic efficiency curves of as-prepared samples with different mass ratios,(b) pseudo-first-order kinetics curves of different samples under visible light irradiation,(c) repeated photodegradation of RhB by 80-Ag/Ag3PO4/CS,and (d) comparison photographs of pure Ag3PO4 (left) and 80-Ag/Ag3PO4/CS (right) dispersed in water after standing for 1 min.
Subsequently,the loading of the composite photocatalyst was investigated.The photodegradation curves of RhB as a function of time are displayed in Fig.6 (a).During the dark absorption progress,the prepared samples absorbed 5% to 10% RhB.All the samples had excellent photodegradation activity under visible light.When the reaction time reached 60 min,the degradation rates reach 66.48%,79.17%,and 99.19% for 40-Ag/Ag3PO4/CS,60-Ag/Ag3PO4/CS,and 80-Ag/Ag3PO4/CS,respectively.Fig.6 (b) is the degradation kinetic constants of RhB over different photocatalysts; they were calculated based on a pseudo-first-order kinetic model as shown in (1):

whereCtandC0are the time-dependent and initial concentration of RhB,andkis the rate constant.Thekvalues of 40-Ag/Ag3PO4/CS,60-Ag/Ag3PO4/CS,and 80-Ag/Ag3PO4/CS are 0.017 min-1,0.020 min-1,and 0.049 min-1,respectively.Table 1 summarizes the reported works on Ag3PO4-based photocatalysts for RhB degradation.The prepared-sample of this work has relatively good activity,although it is not the best.It must be emphasized that there are many factors could influence the result of the photocatalytic experiment,such as ambient temperature and light intensity.In addition,secondary pollution is one of the challenges in the field of photocatalyses; therefore,the separability of the nanoscale photocatalyst should be considered,it will be shown later.

Table 1:Comparative representation photocatalytic performance of Ag3PO4-based catalysts reported in other similar works
We chose 80-Ag/Ag3PO4/CS as an assessment object to test the cycling stability.As shown in Fig.6 (c),after three times of successive cyclic experiments,the degradation rate was obviously decreased and still remained about 81.1%.There are three reasons may explain this phenomenon:1) owing to the problem of photo-corrosion of Ag3PO4,a part of Ag+was reduced to Ag0during the photodegradation; 2) small part photocatalysts were lost during the cycling experiments; 3) the absorption ability of the residual hydroxyl and amino group in CS decreased[46],[47].Apart from the stability of the photocatalyst,the property of separation and recovery from the reaction medium is an important parameter that must be considered.As shown in Fig.6,80-Ag/Ag3PO4/CS (right in Fig.6 (d) is very easy to separate from water,the majority of samples sink to the bottom of the bottle about 1 min,while the bottle with pure Ag3PO4(left in Fig.6 (d)) is still turbid.In the practical application of water purification,this property will drastically reduce the cost and avoid secondary pollution.
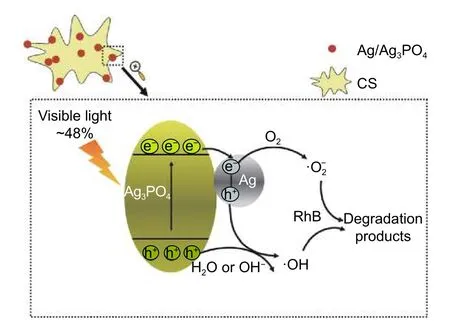
Fig.7.Proposed photodegradation mechanism of RhB on Ag/Ag3PO4/CS.
The photocatalytic mechanism of Ag/Ag3PO4/CS may be similar to previous related works (Fig.7)[45],[48].Both Ag3PO4and Ag nanoparticles can efficiently absorb visible light.Therefore,an amount of electronhole pairs could be generated in Ag3PO4.The electrons in the valence band will jump up into the conduction band (CB),and then these electrons will immediately transfer to Ag nanoparticles,while the holes will stay in the valence band of Ag3PO4.After light absorption in the Ag/Ag3PO4/CS nanostructures and SPR excitation,plasmons can decay,transferring the accumulated energy to electrons in CB of the Ag3PO4.The hot electrons produced by Ag nanoparticles and the photogenerated electrons would be trapped by O2on the surface of photocatalysts to createThese reactive oxygen species could effectively oxidize the dye molecules into intermediates,CO2and H2O.The photogenerated holes would react with the surrounding H2O or OH-,and create reactive intermediate species (·OH).There are two advantages of the existence of Ag nanoparticles.On the one hand,the excellent conductivity of silver nanoparticles enhances the interfacial charge transfer and stops the recombination of electron-hole pairs effectively.On the other hand,the hot electrons would be generated because of the SPR effect of Ag nanoparticles,which increases the performance of the composite catalyst.
4.ConcIusions
In this work,we synthesized a plasmonic Ag/Ag3PO4/CS composite photocatalyst by a simple and green strategy.Ag+was in situ reduced to Ag0by renewable natural CS.In this case,additional reduction conditions were not needed.As the carrier,CS effectively prevented the agglomeration of Ag/Ag3PO4nanoparticles.The results of photocatalytic experiments indicated that the Ag/Ag3PO4/CS photocatalysts have satisfying activity for organic contaminant degradation under visible light,but the stability needs further improvement.Therefore,the further work will focus on this issue.Fortunately,with the feature of easy separation,this kind of composite photocatalysts has a wide application prospect in water purification.
杂志排行
Journal of Electronic Science and Technology的其它文章
- RationaI Design of CoIIoidaI Core/SheII Quantum Dots for OptoeIectronic AppIications
- Recent Advances of Bismuth OxychIoride PhotocataIytic MateriaI:Property,Preparation,and Performance Enhancement
- Synthesis of Octapod Cu-Au BimetaIIic NanocrystaI with Concave Structure through GaIvanic RepIacement Reaction
- MoIecuIar Dynamics AnaIysis of the Effect of Laser and Defects on the Micro-Structure of Fused SiIica
- Random Group Recommendation ModeI Based on Fuzzy CIustering
- Robust Video Watermarking Using a Hybrid DCT-DWT Approach
