Exosomes as a promising diagnostic tool in head and neck squamous cell carcinoma?
2020-07-20KristinaPastorLauraBeneckeLaurentMuller
Kristina Pastor, Laura Benecke, Laurent Muller
Department of Biomedicine, University of Basel and Department of Otorhinolaryngology, Head and Neck Surgery, University Hospital of Basel, Basel4031, Switzerland.
Abstract Head and neck squamous cell carcinoma (HNSCC) is the 6th most frequently diagnosed malignancy and accounts for about 5% of all malignancies worldwide.There is a lack of biomarkers to monitor the status and progress of the disease.Therefore, it is of great importance to develop non-invasive diagnostic tools such as exosomes that monitor tumor changes and provide molecular information about the malignancy to identify the metastatic disease earlier and allow better therapeutic management.Thus, we aimed to review whether tumor-derived exosomes can predict disease progression in HNSCC and if and especially how they can be used as a diagnostic tool.
Keywords: Exosomes, head and neck squamous cell carcinoma, tumor-derived exosomes, liquid biopsy
CONSIDERATIONS ABOUT EXOSOMES AS BIOMARKERS IN HNSCC
Exosomes are virus-sized extracellular vesicles ranging from 30 to 150 nm in diameter that originate from the endosomal compartment of most eukaryotic cells[1-4].By fusion of cytosolic multivesicular bodies with the cell surface, they are released into the extracellular environment.Detectable in biological fluids such as serum/plasma, urine, saliva, cerebrospinal fluid, and cell culture medium, exosomes accumulate in the tumor microenvironment since tumor cells are highly active in producing exosomes[5].HNSCC patients with early or advanced disease severity show a significantly higher exosome number in their plasma in comparison to healthy patients[6,7].We can compare exosomes to hemerodromes in ancient Greece[8].
Pheidippides, one of the first marathon runners in 490 BC, is said to have run from Marathon to Athens acting as a messenger to provide news of the victory of the battle of Marathon.He ran approximately 240 km in two days from Athens to Sparta.Similar to the hemerodromes, exosomes serve as an intercellular communication system between different cells[9].Pheidippides died after completing his mission.Most probably, the exosomes are depleted as well once the message has been sent[8].They are produced by all cell types, both healthy and malignant cells[9].They contain a variety of functional proteins and genetic cargo such as DNA, mRNAs, miRNAs, and long non-coding RNAs.
Tumor-derived exosomes (TEX) share the characteristics of their parental cell but are known to enrich certain factors.It is thought that there is a new chapter in biology behind this: exosomes seem to be part of a messaging system and can deliver specific signals to distant cells[10].Because of these features, exosomes are ideal candidates as non-invasive biomarkers, also called “liquid biopsy”[10].It is essential to mention that exosome levels rise during pathological changes and decrease during convalescence[11], and therefore,their protein content is highly informative[12].Thus far, therapy monitoring tools lag in oncology.As such,PET/CT-scan is said to be one of the most sensitive imaging modalities in clinical routine: cancer detection is only visible on the scan above 4 mm, and it cannot be performed monthly.Besides, other current diagnostic tools such as endoscopy are invasive and may put the patient at risk of potential complications.These facts point towards the use of exosomes as a diagnostic tool as non-invasive biomarkers to set the patient at minimal risk[11].
TEX are highly enriched in HNSCC patients’ plasma.They carry several immunosuppressive proteins and molecules, facilitating the epithelial-to-mesenchymal transition (EMT).Recent analysis alludes that cancer therapies can affect the morphology and behaviour of tumor cells, somewhat encouraging rather than inhibiting EMT.Moreover, TEX participate in the regulation of EMT, as shown by Franzenet al.[13],Minet al.[14]and Theodorakiet al.[15].Most of the experiments on showing exosome involvement in EMT have been conducted with cell lines or in mouse models.Besides, it has been shown that TEX isolated from HNSCC patients’ plasma are highly immunosuppressive.TEX provoke immune cell dysfunction by acting on immune cells through several mechanisms: they hinder the functions needed for antitumor responses,programmed cell death of activated T effector cells, proliferation of suppressive activity in regulatory T and B cells, manipulation of cellular differentiation and transfer of cells to the tumor[16].They bear immune checkpoint proteins such as PD-1, PD-L1, and CTLA-4.PD-L1 exosomes produced by either normal or tumor cells can alter immune responses and therefore affect disease activity[17].Since PD-1 and especially PD-L1 inform about disease activity, it proves that exosomes can function as disease biomarkers.
TEX portray the communication network of the tumor to trigger autocrine, juxtacrine, and paracrine signaling between several cells[12].They convey autocrine signals that lead to tumor progression.Furthermore, they induce the production of cytokines, chemokines, growth factors and tumor necrosis factor alpha (TNF-α).These factors represent juxtacrine and paracrine signaling which is the communication with immune cells infiltrating tumors and stimulate the production of soluble factors enhancing tumor growth[12].
There is an immune response modulation from exosomes released by cancer cells.These exosomes carry damage-associated molecular patterns (DAMPs) such as DNA and RNA to myeloid cells as shown by Kurywchaket al.[18]: “Cancer cells release exosomes that carry DAMPs such as DNA and RNA to myeloid cells which activate the intracellular virus-sensing pathways cyclic GMP-AMP synthase - stimulator of interferon genes, retinoic acid-inducible gene I, and absent in melanoma 2 (AIM-2), and stimulate the production of inflammatory cytokines such as interleukin (IL)-6, TNF-α, IL-8, and IL-1β.”[18].
Additionally, cancer exosomes have the characteristic to generate new vessel formation, which allows an influx of oxygen and nutrients and waste removal and impact the metabolic reprogramming of cancercells[19].Exosome features such as the capability to modulate the host immune response, communication network of exosomes with the tumour microenvironment and involvement of exosomes in tumor progression and metastasis show that they play an important role in the tumor microenvironment[19].
Tumor hypoxia is considered a negative predictive factor in HNSCC[20,21].Hypoxic cancer cells are considered to be radioresistant.Interestingly, hypoxia-derived exosomes have been described to express a different phenotype, and consequently, profile change of exosomes toward hypoxia could be used as a biomarker to guide further therapy[22].
The aspects of exosomes mentioned above given their use as diagnostic tools are summarized in the following Figure 1.
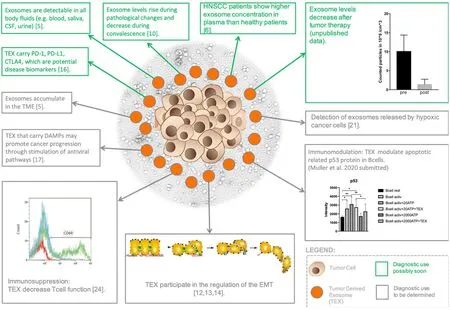
Figure 1.Exosomes and some of their properties with their potential use as diagnostic tools:this figure illustrates tumor cells centrally surrounded by exosomes, including tumor-derived exosomes (TEX) and some of their properties as prescribed above applicable in the clinical routine soon.It also shows the diagnostic value and applicability in the clinical routine not yet determined.For example,TEX participate in the regulation of the epithelial-to-mesenchymal transition (EMT), TEX carrying damage-associated molecular patterns (DAMPs) may promote cancer progression through stimulation of antiviral pathways, exosomes accumulate in the tumor microenvironment (TME), and TEX promote immunomodulation.Some typical exosome analyses have been included: TEX cause a decrease in CD69 in T-cells by flow cytometry (lower left), nanoparticle analyses by Zetaviewer before and after primary radiochemotherapy in head and neck squamous cell carcinoma (HNSCC) (upper right) and the influence of TEX on the protein expression profile of p53 in B-cells by dot blots (lower right)
PROS AND CONS OF EXOSOME USE IN LIQUID BIOPSY
For the assessment of tumor profiling, a tissue biopsy is one of the methods standardly used in which a sample of tissue is taken from the body by using specific needles or surgery.This procedure is not only an invasive method, but it also shows the disadvantage that the anatomical location of some oncogenic mutations is not always accessible by tissue biopsy.Another disadvantage is that the tissue extraction maycause an expansion of the metastatic lesion[23].In addition, it is time-consuming since several surgeries are needed to follow up on tumor progression, making it more expensive[9].Exosomes are steady in the circulation and are found in almost every body fluid which makes them easy to use as a diagnostic tool.In comparison to tissue biopsy, liquid biopsy has the advantage of being a non-invasive method, which allows real-time monitoring of disease progression as well as detection of any tumor in any anatomical location with no risk of augmenting the metastatic lesion.Moreover, it improves the chance of an early diagnosis of cancer.Exosomes are presently emerging as promising non-invasive biomarkers of tumor progression and promotion of malignancy.
In the circulatory system of a patient with HNSCC, circulating tumor DNA (ctDNA, from tumor cells) is detected[4,24].ctDNA may be detected in various body fluids[25].During the formation of a tumor, circulating tumor cells (CTC) can be detected in the bloodstream through different techniques, which allows CTC lines to be used for drug sensitivity analysis and extraction of information at the cellular level[4,26].Therefore,CTC and ctDNA can be used as a diagnostic tool for real-time monitoring of tumor progression.However,it requires fresh samples, demands larger amounts of blood and requires the processing of samples within hours.
The advantage of exosomes is that one can use not only fresh samples but frozen samples as well.Thus,we were able to conduct exosome analyses on long-term frozen samples from a vaccination trial.It was possible to show the antitumor response and correlate the results with the patients’ survival[27].Exosomes among other cancer-related particles are present in higher concentrations and are detectable earlier in metastatic disease than CTCs[28].As a result, another considerable advantage of exosomes is the small amount of blood needed to detect exosomes.Around one milliliter of blood may be enough depending on the extent of the analyses planned.Exosomes are more profuse and more specific since they contain highly informative protein content and genetic cargo such as DNA and mRNA[12].Besides, exosomes reveal specific markers such as HSP70 and Alix.They can reveal the original cell markers by imagining particular surface proteins and their target cells, making the isolation easy for tissue and target cell-specific exosomes[29].The content of nucleic acids in exosomes is a qualified source for cancer analysis.The DNA content of exosomes can reveal the mutational status of the original cell[29].
HNSCC patients during an active disease state show a significant rise in circulating exosomes with exceptionally high levels of TEX and immunosuppressive factors[7,30].Not only TEX but also T-cell derived exosomes bear comparatively high levels of immunosuppressive factors in HNSCC patients[31].In case of advanced disease (stage III/IV), T-cell derived exosomes conveyed much higher levels of CD15s, a functional Treg marker, than in patients in the first stage[31].This is another indicator that plasma-derived exosomes of HNSCC patients could function as an easily accessible, non-invasive “liquid biopsy” of disease progression.
There is a way to transport drugs to cells since exosomes are taken up by various cells.Packaging paclitaxel in exosomes facilitates the allocation of the pharmacological agent[32].Many more studies are needed regarding this issue, and time will reveal which of these approaches will lead to a better outcome in patient care.
The disadvantage might be that the enhancement of exosomes by ultracentrifugation, ultrafiltration, or density gradient centrifugation is not only time-consuming but also requires considerable equipment, thus being cost-intensive[29].In the future, faster isolation methods should be developed for clinical application and standardization of results and diagnostic reference levels as well.
TECHNIQUES FOR EXOSOME CHARACTERIZATION
There are various isolation procedures of exosomes from body fluids and cell culture supernatants,including immunoaffinity capture, polyethylene glycol-mediated precipitation, ultracentrifugation, and microfluidics.To be able to use exosomes as a tumor biomarker, it is crucial to preserve their origin,structural integrity, molecular and genetic substance, and their functional properties as well to be suitable for DNA, RNA, and protein analysis.
Nevertheless, the simultaneous purification of exosomes is essential since plasma-derived exosomes have a high abundance of proteins[33].From current data, the mini-SEC method so far seems to be a favorable method compared to other isolation methods due to its better recovery and consistency of vesicles, because there is a risk of protein complex aggregation and loss of vesicles and biological functions as a result of ultracentrifugation.
In the following section, we give an overview of commonly used exosome characterization procedures.
Electron microscopy
Electron microscopy (EM) is the gold standard for exosome identification and verification of exosome preparation quality since it was one of the first methods used.It is also the only technique for direct exosome visualization.However, this method is difficult to use on an everyday basis.One of the reasons is that EM results in exosome loss during dehydration and embedding[34].Other variations of this technique may improve the results such as scanning EM and cryoEM, but further quantification of exosomes is a bigger issue.
Nanoparticle tracking analysis
This method provides an assembled analysis of Brownian motion via light dispersion to rank and size nanoparticles in a liquid suspension[35-38].Based on Brownian motion, analyzing over time light scatter produced by a laser beam that hits the particles yields a size distribution and concentration[39].Although good and regular calibration is essential, it is one of the more favorable methods used because it does not depend on detecting a definite marker and also due to the suspension of exosomes in a broad range of solutions[39].
Tunable resistive pulse sensing
Tunable resistive pulse sensingdiscovers the passage of distinct particles through a membrane’s pores.This happens when temporary electrical resistance is measured.It correlates with the amount and size of particles present.Pore size can be modified to improve the measurement.The analysis gives a concentration and particle distribution similar to nanoparticle tracking analysis[39].
Developing a concordance between techniques for quantification of exosomes plays an important role in establishing the future of exosome biomarkers in clinical use[39].
lmmunodetection methods
These are analyses that based on the detection of mono- or polyclonal antibody bound to the antigen in the sample.
Western blots are used for the study of exosome cargo, and specifically, the semi-quantitative densitometry analysis of protein bands is insightful for disease progression[12].It also reveals that PD-L1 or TGF-β/LAP are significantly linked with disease progression[12].
Flow cytometry, another valuable quantitative analysis of exosome cargo, is used for exosome capture on streptavidin-coated magnetic beads.It was also demonstrated for the collection of CD63+ exosomes utilizing biotin-labelled anti-CD63 Ab, starting with fraction number four of exosomes separated by mini-SEC[40].This method demonstrates that the levels of PD-1 and PD-L1 in exosomes obtained from HNSCC patients’ plasma is significantly associated with pathological changes such as disease progression[40].
PD-L1 is a ligand with the potential of inducing immune suppression in activated T cells that express PD-1.When PD-L1-bearing exosomes were obtained with CD3 and CD69 T lymphocytes, the downregulation of CD69 expression was blocked.Anti-PD1 antibodies inactivate this inhibition.Likewise,when plasma-derived exosomes are obtained with activated CD8 T-cells, fast programmed cell death of T cells takes place.This reveals that TEX modulate immune cell activity and are able to function as immune biomarkers[30,40,41].
Clinical analyses need to be simple, reliable and quick.To meet these criteria, we are currently working on a new isolation technique using immunoaffinity that allows us to have results in flow cytometry within a few hours.This will enable a high-throughput analysis of patient samples.
One interesting method described could be the photoacoustic and fluorescence flow cytometry platform, as described by Nolanet al.[28].This is able to performin vivoflow cytometry on tumor-associated exosomes in mouse models.Also, more research is needed to bring this technique into human diagnostics, where it seems to be a challenging but promising path.
Mass spectrometry
MS is one of the most favorable techniques for proteome characterization, where analytes are converted to gaseous ions and are classified recording to their mass-to-charge ratio.It offers various advantages such as its specificity, throughput, sensitivity and cost-effective testing.It uses three different proteomic MS techniques: digestion by trypsin (bottom-up), digestion by a protein with less-frequent cleavage sites(middle-down), and analysis of native protein (top-down).MS has also been linked with ChIP to quantify histone modifications, transcription factors and other chromatin-associated proteins.
As already mentioned above, exosomes contain a variety of functional proteins and genetic cargo such as DNA, mRNAs, miRNAs and long non-coding RNAs.Tanget al.[42]state: “Sucrose density gradients,ultrafiltration, high performance liquid chromatography-based protocols and immunoaffinity-capture methods, singly or combined with the application of ultracentrifugation, can provide high enrichment and purity of exosomes”.Besides, a number of alternative methods for analysis of exosome RNA extraction methods have been used: phenol-based techniques (TRIzol) and combined phenol and pure column-based techniques.The Total Exosome RNA and Protein Isolation kit (TER) has been particularly selected for RNA isolation[42].
Global proteomics is an identification method using as many proteins as possible within one sample.There are two different strategies: via data dependent acquisition (DDA) or data independent acquisition (DIA),with DDA being used more commonly than DIA[43].
CONCLUSION
Cancer is one of the biggest challenges of medicine, and we still cannot defeat it as a whole.However, liquid biopsy, the analysis of exosomes, ctDNA and CTCs may give us the possibility for non-invasive diagnosis,real-time monitoring of disease progression and developing a treatment according to gene profile as well.The special features of exosomes make them ideal for minimally invasive liquid biopsy.Exosomes are unique; they express not only “dead” particles such as ctDNA but they also show actual biological activitythat helps us study the tumor more accurately.Liquid biopsy is one of the advanced methods that will soon help to diagnose tumors earlier and also to follow their morphological development, making treatment more precise than in the past.It might not replace the X-ray, MRI and CT scan because it is still essential to know where precisely the tumor grows, but once the tumor is visible, it is more likely to be associated with an adverse prognosis.With the help of liquid biopsy, we can diagnose the tumor much earlier and thus decrease the need for chemotherapy, which may be useful for cancer therapy in the advanced stage but may have many side effects for the patient.Thus, we could achieve a better result with the help of a liquid biopsy.
Every tumor is individually different.The liquid biopsy helps us track down its morphological development earlier and, in this way, helps us to control it better and enables us to develop a treatment according to its genetic profile.This is what we call precision medicine.Exosomes can be released by both immune cells and non-immune cells.They have immunomodulatory properties.EX lead to signalling in T-cells and in other immune cells.The primary mechanism is the uptake of exosomes and the release of non-coding RNAs,especially microRNAs which play a part in the regulation of RNA translation[8].TEX regulate immune cells by the direct interaction between Fas and Fas-ligand, which causes cell death of activated CD8 cytotoxic T-cells[8].Exosomes seem to have multiple effects on the immune system.As such they transport pro- and anti-tumorigenic as well as pro- and anti-inflammatory signals to target cells.Depending on the target cell,for example, dendritic cells, T lymphocytes or B cells, exosomes may send clear signals[8].
Due to their various properties, exosomes are an exciting target for therapeutic and diagnostic aims.Tumor cells are known to produce numerous exosomes.In the plasma of HNSCC patients, exosomes are enormously increased in comparison to healthy patients.Their profile difference makes them attractive to be used in liquid biopsy analysis[8].Cancer treatment such as immunotherapy in HNSCC patients is being conducted in clinical practice currently.First data on using exosomes as tumor biomarkers in HNSCC have been reported[17,30].However, more data are needed on the features of circulating exosomes in HNSCC patients.Many investigations on exosomes in cancer treatment as well as the first keen results have been published[44].Many more studies will be carried out on this topic and time will tell which of these approaches will lead to a better outcome in patient care.
DECLARATIONS
Authors’ contributions
Literature research and writing the paper: Pastor K
Figure design: Benecke L
Study idea in design: Muller L
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by Propatient (pp18-08).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2020.
