Glycogen synthase kinase 3b biology in bone and soft tissue sarcomas
2020-07-20KensakuAbeShingoShimozakiTakahiroDomotoNorioYamamotoHiroyukiTsuchiyaToshinariMinamoto
Kensaku Abe, Shingo Shimozaki, Takahiro Domoto, Norio Yamamoto, Hiroyuki Tsuchiya, Toshinari Minamoto
1Department of Orthopaedic Surgery, Graduate School of Medical Sciences, Kanazawa University, Kanazawa 920-8641, Japan.
2Division of Translational and Clinical Oncology, Cancer Research Institute, Kanazawa University, Kanazawa 920-0934, Japan.
3Shimozaki Orthopaedic Hospital, Hakusan, Ishikawa 924-0802, Japan.
#Authors contributed equally.
Abstract Bone and soft tissue sarcomas are malignant neoplasms probably originating from musculoskeletal and mesenchymal progenitor cells. More than 80 different histopathological subtypes are encountered in orthopedics. The standard of care for sarcoma patients involves a multidisciplinary combination of surgery, anthracycline-based multiagent chemotherapy and radiation. Unfortunately, these are associated with adverse events and occasionally disappointing outcomes. Various genomic-, biologically-, and immunologically-based therapies are still under evaluation in earlyphase clinical trials. However, there are strong barriers to the development and clinical translation of new therapeutic modalities. This is due to the rarity of these diseases, the broad spectrum of tumor subtypes with genetic and biological heterogeneity, and the wide variability in clinical manifestation, response to treatment and prognosis. A potential approach toward overcoming this barrier is to identify therapeutic targets that cover multiple sarcoma types. Glycogen synthase kinase 3b (GSK3b) has emerged as a common therapeutic target in more than 25 different cancer types. Here we review the evidence for tumor-promoting roles of GSK3b in the major types of bone and soft tissue sarcomas including osteosarcoma, rhabdomyosarcoma, synovial sarcoma, and fibrosarcoma. In this review, we describe the therapeutic effects of inhibiting GSK3b in these sarcoma types, while also protecting healthy cells and tissues from detrimental effects associated with conventional therapies, such as doxorubicin-induced cardiotoxicity. Consequently, we highlight GSK3b as a potential therapeutic target spanning multiple sarcoma types.
Keywords: Osteosarcoma, rhabdomyosarcoma, synovial sarcoma, fibrosarcoma, glycogen synthase kinase 3b
INTRODUCTION
Sarcomas are rare malignant neoplasms of putative mesenchymal resident (progenitor) cell origin that comprise 20% of all bone sarcomas and 80% of all soft tissue sarcomas[1,2]. They account for almost 1% of newly diagnosed malignancies and deaths from the disease[3]. Sarcomas can arise at almost any anatomical site and occur most often in children, adolescents, and young adults. More than 80 histopathological subtypes of sarcomas are defined by the updated WHO classification[4], with wide variability in their clinical manifestation, response to treatment and prognosis. Accurate and differential diagnosis of the respective sarcoma types is challenging because of their similarity and overlapping morphological features. Several new approaches for cytogenetic, molecular, and immunohistochemical testing methods have been combined with clinical and histopathological evaluation[5,6]. The wide variety of tumor subtypes with a difficult histopathologic diagnosis and the occurrence of tumors at many possible anatomical sites complicate the overall biological and clinical understanding of bone and soft tissue sarcomas. Hence, the current clinical practice guidelines for bone and soft tissue sarcomas[7,8]do not adequately cover patient management for all sarcoma types.
Surgery remains the mainstay of treatment for most sarcoma patients with localized tumor. This is often combined with chemotherapy and/or radiation in neoadjuvant and adjuvant settings. Patients with metastasis, at initial diagnosis or after curative surgery, undergo chemotherapy and radiation, either alone or in combination[7,8]. Currently, anthracycline-based chemotherapy is widely accepted as the first-line therapy for most patients with advanced sarcoma. Doxorubicin remains a pivotal agent and is prescribed in various combinations with other chemotherapeutics including ifosfamide, dacarbazine, gemcitabine, and docetaxel. However, the empirical cytotoxic chemotherapies are associated with disappointing patient outcomes and inevitable adverse effects, even when combined with new generation anticancer agents such as eribulin and trabectedin[9,10].
Recently, major efforts to decipher the genomic, epigenomic, and other biological properties of various sarcoma types have identified several actionable molecular targets with potential therapeutic application. Some of the molecular alterations found in sarcomas include activation of mutations in the c-kit and B-raf genes, gene translocation involving growth factors such as platelet-derived growth factor (PDGF) receptor (PDGFR) and colony stimulating factor 1 receptor, gene translocation involving transcription factors such as vascular endothelial growth factor receptor (VEGFR), inactivation of tumor suppressor genes (TSC1/2 and PTEN) leading to activation of mechanistic target of rapamycin (mTOR), and overexpression of PDGFR and VEGFR[11-15]. Several agents developed against these actionable targets have been tested in clinical trials of advanced sarcoma patients and preliminary results shown improved survival. However, most clinical trials for sarcoma remain in the early stages (phase I or phase II)[11,16,17]. The rarity of sarcoma, the wide variety of histological subtypes and the lack of predictive biomarkers are major hurdles in the clinical evaluation of available targeted agents. Consequently, ongoing clinical trials have yet to show a significant survival benefit of the targeted agents over conventional chemotherapy. Identification of therapeutic targets that span multiple sarcoma subtypes is therefore required to break the current deadlock in developing innovative sarcoma therapies. This review focuses on glycogen synthase kinase 3b (GSK3b) as an emerging and common therapeutic target in major sarcoma types including osteosarcoma, rhabdomyosarcoma, synovial sarcoma, and fibrosarcoma that are frequently encountered in orthopedics.
OVERVIEW OF GSK3b BIOLOGY AND DISEASES
GSK3b was initially identified as an isoform of the GSK3 family of protein kinases. In addition to its primary function of phosphorylating and thus inactivating glycogen synthase, GSK3b phosphorylates serine and threonine residues in various functional and structural proteins, thereby serving multipurpose roles in pivotal cellular pathways. GSK3b is constitutively active in cells upon tyrosine 216 phosphorylation (pGSK3bY216). Negative regulation of its activity via serine 9 phosphorylation (pGSK3bS9) occurs to control vital activities and homeostasis in normal cells in response to endogenous and exogenous stimuli[18,19]. Aberrant expression and activation of GSK3b contribute to the pathogenesis and progression of common diseases including glucose intolerance, neurodegenerative disorders with cognitive disturbance, and chronic inflammatory diseases[20,21]. Such differential functions in healthy and diseased cells have highlighted GSK3b as a potential drug target in various diseases and have stimulated the development of pharmacological GSK3b inhibitors[22-24].
In the field of oncology, GSK3b has long been hypothesized to suppress tumorigenesis. This is based on its inactivation, as indicated by pGSK3bS9, in major pro-oncogenic pathways mediated by Wnt/b-catenin, hedgehog (Hh), notch, and c-Myc signaling, as well as in the process of epithelial-to-mesenchymal transition (EMT)[25,26]. However, few studies have shown that active GSK3b suppresses tumor development and progression by disrupting these pro-oncogenic pathways. In contrast to the hypothesis that GSK3b is a tumor suppressor, a growing number of experimental studies over the past 15 years have demonstrated that deregulated expression and activity of GSK3b contributes to the pathogenesis and progression of various cancer types. The notion that GSK3b has pro-tumorigenic properties is supported by observations that tumor cells depend mechanistically on GSK3b for their survival, proliferation, and invasion, and that GSK3b renders them unresponsive to chemotherapy, radiation, and some molecular targeted agents in refractory cancer types. A tumor-promoting role for GSK3b is also supported by evidence of specific and strong therapeutic effects of various GSK3b inhibitors against at least 25 different cancer types, while sparing the normal cells and tissues[27-30]. This increasing experimental evidence supports the notion of GSK3b as a promising therapeutic target in cancer, thereby encouraging the screening and identification of GSK3b-specific inhibitors for treatment of cancer[24,31,32].
GSK3b INVOLVEMENT IN BONE AND SOFT TISSUE SARCOMAS
Among the many bone and soft tissue sarcoma types, the tumor-promoting role of GSK3b has been reported in osteosarcoma, rhabdomyosarcoma (alveolar and embryonal types), synovial sarcoma, and fibrosarcoma [Table 1].
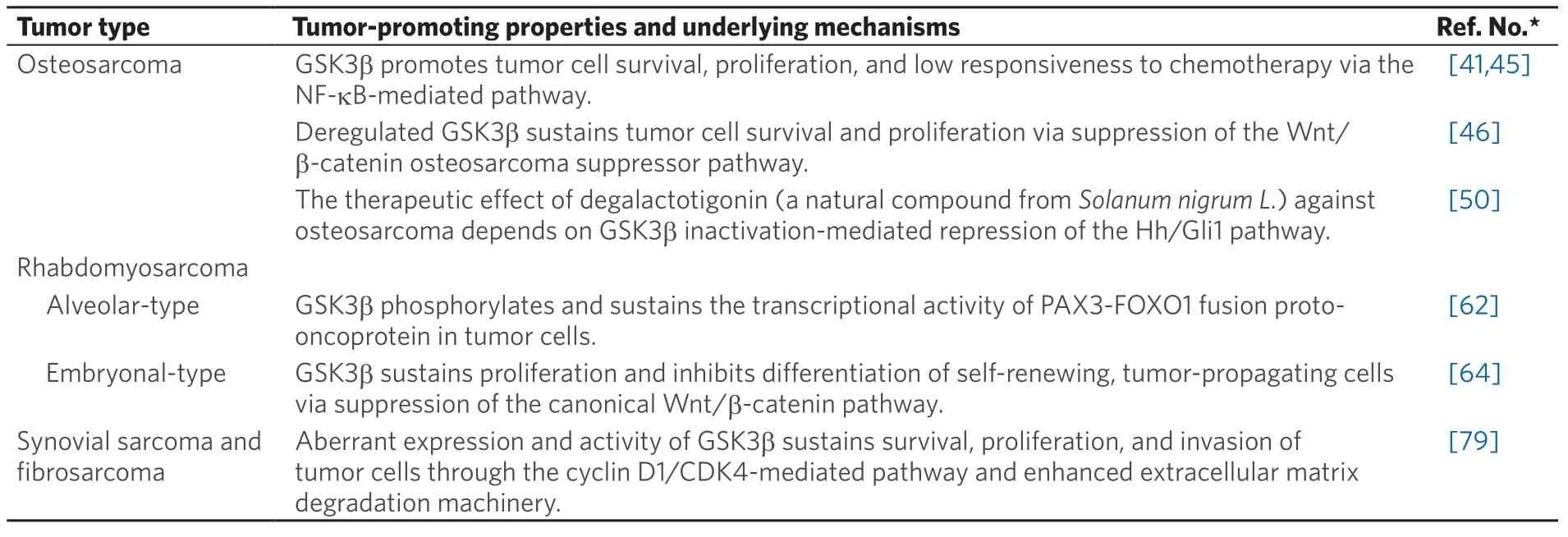
Table 1. Tumor-promoting properties of GSK3b reported in bone and soft tissue sarcomas
Osteosarcoma
Although rare, osteosarcoma is the most prevalent primary malignant bone tumor, followed by chondrosarcoma and Ewing sarcoma. It typically affects the long bone of the limbs in children, adolescents, and young adults[33]. The anatomical site of the primary tumor, clinical characteristics, treatment response, and patient prognosis distinguish high-grade osteosarcoma (accounting for 80% to 90% of cases) from low/moderate-grade osteosarcoma (10% to 20%)[34]. The current standard of care for the treatment of patients with no detectable metastasis at initial diagnosis (accounting for 80% to 85% of cases) sequentially combines surgery with pre-operative (neoadjuvant) and post-operative (adjuvant) chemotherapy. The remaining 15% to 20% of patients have metastasis at diagnosis and undergo multi-agent chemotherapy. The most effective chemotherapy regimen combines high-dose methotrexate, doxorubicin, and cisplatin[7,33,35,36]. Beginning in the 1970s, the use of multi-agent chemotherapy in patients with localized disease increased their survival rate from less than 20% to almost 70%[33]. However, no further substantial improvement has been achieved over the past 25 years. In contrast to the survival benefits obtained with chemotherapy for localized primary tumors, little improvement has been achieved in the 5-year survival rate for patients with concurrent metastasis or post-operative recurrence. This highlights the need for new therapeutic approaches against metastatic progression in osteosarcoma[37,38].
During the last decade, comprehensive genomics studies have revealed the highly heterogeneous nature of genetic alterations in high-grade osteosarcoma. Although several studies suggested candidate genetic biomarkers for future clinical translation, no actionable genes for targeted therapy have yet been identified[13]. Based on studies of the biological and immunological characteristics of osteosarcoma, nearly 30 clinical trials involving osteosarcoma patients have tested several agents that target receptortype tyrosine kinases (e.g., VEGFR, PDGFR, and c-Kit), cyclin-dependent kinases (CDKs; e.g., CDK4 and CDK6), pro-oncogenic signaling pathways (e.g., Hh and mTOR), the bone microenvironment (e.g., osteoclasts), and immune checkpoint systems. Most trials are in early phases (I and/or II) and none of the targeted agents have so far been approved for the treatment of osteosarcoma[13,16,36,39,40].
During the past decade, GSK3b has been proposed as a potential therapeutic target in bone and soft tissue sarcomas including osteosarcoma [Table 1]. An earlier study showed an inverse association between the level of pGSK3bS9(inactive form) and the capacity of tumor formation in human osteosarcoma cells[41]. This study also demonstrated the role of a constitutively active form of GSK3b (artificial transversion of S9 to alanine) in promoting tumor proliferation. Moreover, it was shown that lithium chloride, an ATPnon-competitive and non-specific GSK3b inhibitor[42], attenuated the proliferation of osteosarcoma cells, induced their apoptosis and enhanced the efficacy of doxorubicin and methotrexate against sarcoma cells. The therapeutic effect of lithium against tumor cells was shown to be associated with reduced activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)[41], consistent with studies showing that GSK3b is indispensable for the NF-κB-mediated pro-survival pathway[43,44]. Subsequently, another study showed overexpression of GSK3b in primary osteosarcomas, and induction of apoptosis in sarcoma cells following treatment with a pharmacological GSK3b inhibitor that reduced Bcl-2 expression[45]. Our work has shown that the expression of GSK3b and level of pGSK3bY216(active form) in osteosarcoma cells was higher than in normal osteoblasts, but the level of pGSK3bS9was lower. We also demonstrated that GSK3b-specific inhibitors and RNA interference attenuated the survival and proliferation of tumor cells and induced apoptosis, while sparing normal osteoblasts. The effect of GSK3b inhibition against tumor cells was coincident with reduced phosphorylation of GSK3b-phospho-acceptor sites in b-catenin and with increased b-catenin expression, nuclear translocalization, and co-transcriptional activity[46]. Our results suggest the therapeutic effects of GSK3b inhibition are associated with the activation of b-catenin, a putative tumor suppressor in osteosarcoma[47,48]. However, the role of the Wnt/b-catenin pathway in the development of osteosarcoma remains controversial[49]. A recent study reported that the therapeutic effect of degalactotigonin (a natural compound fromSolanum nigrum L.) against osteosarcoma occurred via GSK3b inactivation-mediated repression of the Hh/Gli1 pathway, thus indirectly suggesting a protumorigenic role for GSK3b[50]. As discussed in FUTURE PERSPECTIVES FOR GSK3b IN SARCOMA BIOLOGY AND THERAPY, the GSK3b/b-catenin axis has opposing roles in normal osteogenesis and in the osteoclastic process. A series of studies described here have helped to understand the biology of GSK3b and identified it as a promising target for the treatment of osteosarcoma [Figure 1]. These advances should facilitate the development of new GSK3b inhibitors for this refractory disease[51].
Rhabdomyosarcoma
Rhabdomyosarcoma is the most prevalent pediatric sarcoma and is characterized by tumor cells with a skeletal myoblast-like phenotype, possibly arising from primitive mesenchymal cells[52,53]. The two major subtypes are embryonal (approximately 60% of cases) and alveolar (20%) rhabdomyosarcoma, with the less prevalent subtypes being pleomorphic (10%) and spindle/sclerosing (10%)[54]. While rhabdomyosarcomas can arise at any anatomical site, the embryonal subtype preferentially arises in the head and neck region and in the genitourinary tract of children and young adolescents. This tumor subtype frequently shows loss of heterozygosity at the 11p15 locus that includes the insulin-like growth factor-II gene. The alveolar subtype is notoriously aggressive and affects the trunk (in particular, the perineal and paraspinal areas) and extremities in adolescents and young adults. This subtype is characterized genetically by gene rearrangement of the forkhead box O-subfamily 1 (FOXO1) resulting in t(1;13)(p36;q14) translocation generating the paired box (PAX)3-FOXO1 fusion or t(2;13)(q35;q14) translocation generating the PAX7-FOXO1 fusion proto-oncogene[52-55].
The treatment strategy for rhabdomyosarcoma is based on a risk stratification (low-, intermediate-, and high-risk) of the disease that consists of tumor histological subtype, the tumor stage (equivalent to TNM classification) prior to treatment, and the post-surgery clinical grouping (e.g., extent of residual tumor, presence of lymph node metastasis and of distant metastasis)[52,53,55]. Most rhabdomyosarcoma patients require a multimodal combination of chemotherapy, surgery, and/or radiation therapy. The two standard chemotherapy regimens include the combination of vincristine and actinomycin D with either cyclophosphamide or ifosfamide. Implementation of combined multi-agent chemotherapy has significantly improved patient outcomes. However, the efficacy of treatment for patients with high-risk rhabdomyosarcoma (defined as the presence of distant metastasis[52,55]) has not improved for the past three decades[53,55]. Several clinical trials for rhabdomyosarcoma conducted over the last decade have evaluated molecular targeted agents, immune checkpoints-blocking agents, and cellular immunotherapeutics. Only pazopanib, a multi-kinase inhibitor that targets PDGFR-α, VEGFRs, and c-Kit[56], has been tested in a phase III clinical trial for patients with metastatic soft tissue sarcomas including rhabdomyosarcoma. All the remaining trials have been either phase I or II[57]. Comprehensive whole genome analyses for embryonal and alveolar rhabdomyosarcomas has failed to identify any actionable therapeutic targets[55,58,59], hence the urgent need to identify new therapeutic targets.
A previous study showed that a liposome-protamine-siRNA (LPR) nanoparticle that targets the PAX3-FOXO1 fusion proto-oncogene transcript inhibited the proliferation of alveolar rhabdomyosarcoma cells and their xenograft tumors[60]. Another study demonstrated that entinostat, a class-I histone deacetylase inhibitor, reduced the expression of PAX3-FOXO1 in alveolar rhabdomyosarcoma cells, thereby sensitizing them to chemotherapeutic agents[61]. These studies suggest that PAX3-FOXO1 fusion proto-oncogene and its product are potentially actionable targets in the treatment of alveolar rhabdomyosarcoma. Consistent with this suggestion is an earlier study[62]that screened 160 different kinase inhibitors against alveolar rhabdomyosarcoma cell lines and identified GSK3b inhibitors including TWS119[63]as tumor type-selective inhibitors. This study found that GSK3b phosphorylated the PAX3-FOXO1 fusion protein in tumor cells and that inhibition of GSK3b attenuated the transcriptional activity of this oncoprotein, suggesting a role for GSK3b in sustaining alveolar rhabdomyosarcoma[62][Figure 1]. Subsequently, a large chemical screen directed against self-renewing, tumor-propagating cells (TPCs) in embryonal rhabdomyosarcoma identified GSK3(b) inhibitors (e.g., BIO, CHIR 98014, and CHIR 99021) as potent suppressors of this tumor type via the inhibition of proliferation and the induction of terminal myogenic differentiation of TPCs[64]. The tumor-suppressive effect of GSK3(b) inhibitors was associated with induction of the canonical Wnt/b-catenin pathway, which was underpinned by the finding that recombinant Wnt3A and stabilized b-catenin enhanced the terminal differentiation of rhabdomyosarcoma TPCs [Figure 1]. Collectively, these studies[62,64]suggest that GSK3b is a potential therapeutic target that covers the major subtypes (embryonal and alveolar; in total nearly 80%) of rhabdomyosarcoma.
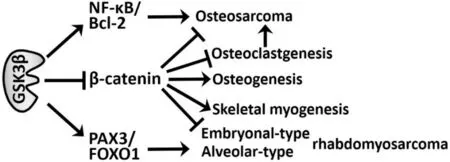
Figure 1. Reported mechanisms by which GSK3b promotes (→) the development of osteosarcoma and rhabdomyosarcoma, while suppressing (—|) osteogenesis and skeletal musculogenesis. →: promoting; —|: suppressive; GSK3b: glycogen synthase kinase 3b; NFκB: nuclear factor kappa-light-chain-enhancer of activated B cells; FOXO1: forkhead box O-subfamily 1; PAX3: paired box 3
Synovial sarcoma and fibrosarcoma
In addition to rhabdomyosarcoma, the major soft tissue sarcoma types include synovial sarcoma, fibrosarcoma, and undifferentiated pleomorphic sarcoma[1,2,65]. Synovial sarcoma accounts for 5%-10% of all soft tissue sarcomas. Although it can arise at almost any anatomical site and at any age, synovial sarcoma frequently affects the extremities, particularly the popliteal fossa, in adolescents and young adults[66]. Histologically, this sarcoma is characterized by biphasic tumor cells comprising epithelioid and spindleshaped cellular components, thereby mimicking synovial tissue. However, unlike its nomenclature, this sarcoma does not arise from synovial tissue and does not express synovial cell markers[67]. Genetically, more than 90% of synovial sarcoma tumors show a pathognomonic t(X;18)(p11.2;q11.2) translocation that generates a fusion of the synovial sarcoma 18 (SS18) and SSX genes, encoding a pro-oncogenic transcription factor[68]. Despite the standard approach of wide radical surgery combined with radiation of the primary tumor[69], local recurrence and distant metastasis are frequently encountered[70], resulting in poor patient outcome[71]. Although synovial sarcoma is relatively chemosensitive compared to other soft tissue sarcomas[72], there is only limited survival benefit from anthracycline-based adjuvant chemotherapy for high-risk patients with metastatic and/or residual tumor[73].
Fibrosarcoma is defined as a malignant neoplasm of fibroblast origin and characterized histologically by a “herringbone” architecture formed by the tumor cells and stromal deposition of collagen within the tumor[4,74]. Fibrosarcomas are divided into the congenital-type that rarely metastasizes, and the adulttype that is highly malignant[75]. The incidence of adult-type fibrosarcoma has declined over the years as the diagnostic criteria has become more strict and other mesenchymal tumors that mimic fibrosarcoma are more accurately defined. Most fibrosarcomas arise from the fascia and tendon of soft tissue, with rare occurrences in the medullary canal and periosteum of bones. Adult-type fibrosarcoma affects the deep soft tissues of the extremities, trunk, head, and neck in middle- and older-aged adults. The mainstay of treatment is surgical removal of the tumor, occasionally followed by radiation for high-grade tumors and cases with insufficient surgical margin. Although chemotherapy is not recommended for the management of fibrosarcoma patients, anthracycline-based chemotherapy is the first-line regimen. However, fibrosarcoma is characterized by low sensitivity to chemotherapy and frequent tumor recurrence. This results in poor overall prognosis, with a 10-year survival rate of 60% and 30% for patients with low- and high-grade tumors, respectively[76].
During the past decade, several targeted agents have been developed for soft tissue sarcomas[14-17], together with a new generation of chemotherapeutic agents (e.g., trabectedin and eribulin)[9,10]. Recently, the multitarget kinase inhibitors pazopanib[56]and anlotinib[77]were approved for multiple soft tissue sarcoma types including synovial sarcoma and fibrosarcoma, followed by the approval of tazemetostat, an inhibitor of enhancer of zeste homolog 2 (EZH2)[78], for advanced or metastatic epithelioid sarcoma[15]. These agents improved the progression-free and overall survival of soft tissue sarcoma patients but showed little improvement over conventional anticancer agents. Therefore, identification of new therapeutic targets is imperative to allow the development of efficient, biologically-based treatments for both sarcoma types.
Recently, we showed the level of pGSK3bY216(active form) was higher in human synovial sarcoma and fibrosarcoma cell lines than in untransformed fibroblast cells, considered to be the normal mesenchymal counterpart cells. Inhibition of the activity or expression of GSK3b suppressed the survival and proliferation of sarcoma cells, attenuated their invasion into collagen gel, and induced their apoptosis. These effects of GSK3b inhibition against sarcoma cells were associated with G0/G1-phase cell cycle arrest and reduced expression of cyclin D1, CDK4, and matrix metalloproteinase 2. Intraperitoneal administration of GSK3bspecific inhibitors attenuated the growth of synovial sarcoma SYO-1 and fibrosarcoma HT1080 cell xenografts in athymic mice, with no obvious side effects. This treatment also suppressed cell proliferation and induced apoptosis in the xenograft tumors. These results indicate that synovial sarcoma and fibrosarcoma depend on deregulated activity of GSK3b to enhance the cyclin D1/CDK4-mediated pathway for cell proliferation and degradation of extracellular matrix for tumor invasion [Table 1]. Our study therefore provides a biological basis for GSK3b as a new and common therapeutic target for these sarcoma types[79]as well as for osteosarcoma[41,45,46]and embryonal/alveolar rhabdomyosarcomas[62,64].
FUTURE PERSPECTIVES FOR GSK3b IN SARCOMA BIOLOGY AND THERAPY
In order to confirm GSK3b as a relevant and potentially valuable therapeutic target in bone and soft tissue sarcomas, it is important to broaden the spectrum of targetable tumor types. Moreover, it is important to explore the mechanistic influence of GSK3b on emerging sarcoma therapies and to clarify the properties of this kinase in normal cells and tissues affected by sarcoma therapy.
Potential involvement of GSK3b in undifferentiated pleomorphic sarcoma (malignant fibrous histiocytoma)
Undifferentiated pleomorphic (UP) sarcoma is currently defined as a subset of the sarcoma type previously designated as malignant fibrous histiocytoma (MFH) that encompassed myxofibrosarcoma, pleiomorphic liposarcoma/rhabdomyosarcoma, and UP sarcoma[4]. UP sarcoma is one of the most prevalent soft tissue sarcomas, accounting for 10% of cases in adults. It frequently affects deep soft tissues in the extremities and trunk, but rarely occurs in superficial regions such as subcutaneous tissue[80,81]. As with most soft tissue sarcomas, the mainstay of curative treatment for UP sarcoma is surgical excision of the tumor and post-surgery irradiation. Optional adjuvant chemotherapy is reported to increase the overall survival of patients[82]. The first-line treatment for metastatic UP sarcoma is doxorubicin-based chemotherapy, occasionally combined with ifosfamide or olaratumab, an anti-PDGF antibody. Although other anti-tumor agents such as trabectedin and pazopanib have shown some efficacy, the outcome of patients with advanced UP sarcoma is worse than for other soft tissue sarcoma types[83].
The genetic profile of UP sarcoma has not been fully elucidated[84], although the inactivation of Rb and loss of p53 function are frequently observed in MFH[85]. A previous study showed the side population cells (hypothetically corresponding to stem-like cells) of UP sarcoma display activation of both Hh- and notchmediated pathways responsible for sarcoma cell self-renewal. This study suggested that UP sarcoma cells share the same molecular pathways as mesenchymal stem cells (MSCs)[86]. Another study demonstrated that human MSCs could be transformed via inhibition of Wnt/b-catenin signaling to form UP sarcomalike tumors in athymic mice, thus suggesting MSCs as the origin of UP sarcoma[87]. GSK3b is a negative regulator of the canonical Wnt/b-catenin pathway[18-20]and of the maintenance of MSCs, as described below. Therefore, GSK3b may potentially play a role in the tumorigenesis and progression of UP sarcoma and could be a therapeutic target in this sarcoma in addition to osteosarcoma[41,45.46,50], rhabdomyosarcoma[62,64], synovial sarcoma, and fibrosarcoma[79], as discussed above.
GSK3b and upfront therapies in bone and soft tissue sarcomas
Immunotherapy
Immunotherapy has recently attracted considerable attention for the treatment of many cancer types[88,89]. It has also emerged as a possible upfront therapy for bone and soft tissue sarcomas[17,90-92]. Currently available cancer immunotherapies are based on innate immune reactions represented by natural killer T (NKT)-cells against cancer cells, adoptive anti-tumor immunity exerted by CD8+memory T cells and genetically engineered chimeric antigen receptor (CAR)-T cells, vaccination with tumor-specific antigens, and finally on the blockade of immune checkpoints with therapeutic antibodies to programmed cell death 1 (PD-1), programmed cell death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)[88,89]. Various regimens consisting of many of these immunotherapeutic cells and agents either alone or in combination with chemotherapy, radiation or other targeted agents have been evaluated in many early phase (I or II) clinical trials. Unfortunately, most have so far resulted in disappointing outcomes[17,90-92].
As reviewed recently by ourselves and others[30,32], inhibition of GSK3b in normal peripheral NKT cells facilitates their maturation and enhances their cytocidal effects against acute myeloid leukemia cells[93,94]. In adoptive tumor immunity, CD8+memory T-cells differentiate into pluripotent memory stem T-cells that are capable of self-renewal and have anti-tumor properties via activation of the Wnt/b-catenin pathway[95]. Consistently, GSK3b inhibition enhances the cytotoxic effect of CD8+memory stem T-cells against gastric cancer cells through the induction of effector T-cell-derived Fas-ligand[96]. Similar to CD8+memory T-cells, inhibition of GSK3b in mouse glioblastoma-specific CAR-T cells increased their survival, proliferation, and generation of memory phenotype, thereby enhancing their cytotoxic capacity[97]. During the blockade of immune checkpoints between tumor cells and CD8+memory T-cells, inactivation of GSK3b suppresses PD-1 expression via upregulation of the transcription factor Tbx21, thereby enhancing CD8+cytotoxic T-cell responses[98,99]. Moreover, GSK3b inhibition reverses the blockade of CD28 by CTLA-4[100]that is required to rescue exhausted CD8+T-cells[101]. These preliminary findings suggest broader roles for GSK3b within the cancer immunosuppressive environment by negatively regulating innate and adoptive antitumor immune reactions and by sustaining the immune checkpoints mediated by the PD-1/PD-L1 axis and by CTLA-4[102]. Consequently, these early studies hold considerable promise for targeting GSK3b during immunotherapy for various cancer types[102]including bone and soft tissue sarcomas [Figure 2].
MSC therapy in bone sarcomas
MSCs are a rare population of non-hematopoietic stromal (stem) cells in the bone marrow and other connective tissues such as adipose tissue. They are capable of self-renewal and of undergoing differentiation into the specific mesenchymal cell types. MSCs have attracted widespread interest in sarcoma research and management as a plausible origin of tumorigenesis and as a component of the tumor-promoting microenvironment[103-105]. Paradoxically, MSCs could also be potential cellular weapons in therapeutic applications[106,107]. Studies have shown that MSCs contribute to osteosarcoma progression through their ability to home into the tumor and induce neovascularization and elicit an immunosuppressive tumor environment, thereby sustaining tumor cell survival and proliferation[105,108]. Conversely, other studies reported that MSCs suppress proliferation and induce apoptosis in tumor cells while altering the properties of stromal cells to induce anti-inflammatory effects, inhibit tumor angiogenesis, and ultimately prevent metastasis[107,108].
Based on the tumor site tropism of MSCs, several recent laboratory studies have genetically engineered MSCs to function as vehicles for the delivery of various anti-tumor agents. These agents include interferons (e.g., IFN-α), interleukins (e.g., IL-12), oncolytic viruses (e.g., coxsackie and adenovirus), tumor necrosis factor (TNF)-α, TNF-related apoptosis-inducing ligand (TRAIL), therapeutic antibodies, and enzyme/prodrug combinations [e.g. cytosine deaminase (CD) combined with 5-fluorocytosine (FC)][108,109]. Recent preclinical studies[109]have evaluated the safety and therapeutic efficacy of transduced MSCs loaded with TRAIL, combined CD/5-FC, IL-12, and osteoprotegerin (OPG)[110]. OPG is a soluble proteoglycan and member of the TNF receptor superfamily that inhibits tumor-promoting osteoclastogenesis and bone resorption by acting as a decoy receptor for the receptor activator of NF-κB ligand (RANKL)-mediated pathway[110]. Previous studies on tissue regeneration and repair have demonstrated the effects of GSK3b inhibitors on sustaining the stemness phenotype and proliferation of MSCs from different origins, as well as inducing their transdifferentiation into mature mesenchymal cells[111-115]. These preliminary observations warrant further investigations to clarify whether GSK3b inhibition enhances the therapeutic efficacy of MSCs against bone sarcomas [Figure 2].

Figure 2. Causative involvement of GSK3b in upfront sarcoma therapies and in the adverse events associated with therapy. →: promoting; —|: suppressive; dotted line: hypothetical effect; NKT: natural killer T; MSCs, mesenchymal stem cells; CAR: chimeric antigen receptor; CNS: central nervous system; GSK3b: glycogen synthase kinase 3b
GSK3b and normal tissue damage associated with sarcoma treatment
Although the mainstay treatments for bone and soft tissue sarcomas remain to be surgery and chemotherapy[7,8], they are inevitably associated with post-surgery tissue defects and adverse events related to the chemotherapeutics, respectively. This section focuses on the beneficial effects that targeting GSK3b has on the undesirable events associated with sarcoma treatment.
Normal tissue defect and repair following surgery
Defects in the constitutive normal tissue adjacent to the tumor are an unavoidable consequence of surgery and are particularly serious for patients with musculoskeletal tumors. As discussed earlier, adjuvant chemotherapy, radiation, and targeted therapies are usually combined with surgery to optimize resection of the tumor and to minimize the resulting defect in tumor-adjacent, healthy tissues. There is strong evidence for a critical role of the Wnt/b-catenin pathway in bone formation and homeostasis through induction of osteoblastogenesis and differentiation of the osteogenic cell lineage[116-120]while also suppressing osteoclastogenesis and the resultant bone resorption[121,122][Figure 1]. Osteoclasts within bone sarcoma lesions have been shown to facilitate the progression of osteosarcoma[40], thereby partially supporting the tumor-suppressive function of the Wnt/b-catenin pathway[47,48]. Moreover, GSK3b inhibition protects skeletal muscle cells from apoptosis, promotes their maturation[123,124], and sustains proliferation and the stemness phenotype (both self-renewal and transdifferentiation capacity) of MSCs from various tissues[111-115]as discussed above. Considering its therapeutic effects against the major sarcoma types [Table 1], the targeting of GSK3b in musculoskeletal sarcomas may have three therapeutic advantages: a direct therapeutic effect against the tumor, reduction of the defect in unaffected tissue following surgical resection of the tumor, and preservation and repair of adjacent healthy tissues [Figures 2 and 3].

Figure 3. Therapeutic efficacy of GSK3b inhibitors (AR-A014418 and SB-216763) against human osteosarcoma cell orthograft tumors in the knee joints of mice[46] (left panels) and schematic representation of the hypothetical triple benefits of GSK3b inhibitors: anti-tumor effect, reduction of post-surgery tissue defect, and bone preservation (right panels). Dotted lines in the middle two panels indicate the area of orthograft tumors. GSK3b: glycogen synthase kinase 3b; DMSO: dimethyl sulfoxide (diluent for GSK3b inhibitors)
Doxorubicin-induced cardiotoxicity
Doxorubicin is an anthracycline derivative that comprises the amino sugar daunosamine linked to a hydroxy anthraquinone aglycone[125]. It is the backbone of first line chemotherapy regimens for most bone and soft tissue sarcomas[7,8]. Like all anthracyclines[125], its antitumor effects are mediated by interaction with DNA, generation of oxidative stress, and inhibiting the functions of topoisomerase II in maintaining DNA tangles and supercoils. Resistance to treatment and drug-induced cardiotoxicity are the major concerns with doxorubicin treatment of patients with advanced bone and soft tissue sarcomas. High cumulative dosage of doxorubicin frequently leads to cardiac toxicity and occasionally to irreversible congestive cardiac failure, with younger patients being the most susceptible[126]. This devastating adverse event is associated with disruption of mitochondrial fusion and mitochondrial fragmentation in cardiomyocytes, resulting in impaired mitochondrial function[127]. While the exact molecular mechanism of cardiotoxicity has yet to be clarified, the targeting of impaired mitochondrial dynamics and function is a potential strategy for the prevention and treatment of this adverse cardiac event[127,128]. Many studies have investigated the cardiomyoprotective effect of various compounds derived from phytochemicals such as phenols, terpenoids, quinones, alkaloids, polysaccharides, carotenoids, lignans, and others. Although these phytochemicals are expected to serve as templates for drug development, to date none has yet proven clinically effective in the prevention of doxorubicin-induced cardiotoxicity[128].
The possibility of using GSK3b as a therapeutic target for cardiomyocyte protection has attracted considerable attention[129-132]. Earlier studies reported a causative role for GSK3b in the necrosis and apoptosis of cardiomyocytes[129]and a protective role against cardiac fibrosis[130]. GSK3b inhibition protects cardiomyocytes from necrosis via opening the mitochondrial permeability transition pore. It also protects cardiomyocytes from apoptosis induced by pressure overload or by repeat ischemia and perfusion. This is associated with reduced phosphorylation of p53, heat shock factor-1, and myeloid cell leukemia sequence-1, and inhibition of Bax translocation[129]. Subsequent studies showed the effect of targeting GSK3b on the maintenance of myocardial homeostasis, as well as the therapeutic effects of GSK3b inhibition against diabetes-associated myocardial injury and experimentally induced myocardial infarction[131,132]. A recent study demonstrated that GSK3b inhibition ameliorates triptolide-induced acute cardiac injury in rodents by desensitizing the mitochondrial permeability transition[133]. Another study showed that phosphorylation-mediated inactivation of GSK3b (pGSK3bS9) is associated with the alleviation of doxorubicin-induced inflammation, oxidative stress, and apoptosis in H9c2 rat cardiomyocytes[134]. This was achieved by treatment with Yangxin granules, a Chinese herbal medicine confirmed to possess clinical benefits for the treatment of heart failure. These preliminary studies support the causative involvement of GSK3b in doxorubicin-induced cardiotoxicity and occurrence of congestive heart failure. They also provide new insights into the underlying mechanisms of this fatal cardiac complication and suggest a possible therapy [Figure 2].
Recently, we reviewed the benefits of targeting GSK3b for various cancer therapy-induced adverse events including immunosuppression, hematotoxicity, central, and peripheral neuropathy, and opioid-induced analgesic tolerance and withdrawal syndrome[30][Figure 2]. Increasing evidence has indicated new roles for GSK3b in the repair of DNA base excision and double-strand breaks and in the inhibition of apoptosis via NF-κB activation, thus highlighting the potential of GSK3b inhibitors for inducing chemo- and radiosensitization in various cancer types[135]. In summary, targeting of GSK3b during standard chemotherapy for bone and soft tissue sarcomas is expected to provide the dual benefits of enhancing cytocidal efficacy while reducing the cardiotoxicity of doxorubicin [Figure 2].
CONCLUSION
GSK3b sustains the progression of aggressive bone and soft tissue sarcomas including osteosarcoma, embryonal and alveolar rhabdomyosarcomas, synovial sarcoma, and fibrosarcoma, and potentially also UP sarcoma. Laboratory studies have demonstrated therapeutic effects of GSK3b inhibition against these sarcoma types, as well as against therapy-associated adverse effects including defects in healthy tissues following surgery and doxorubicin-induced cardiotoxicity. The accumulated evidence has provided new insights into the causative role of GSK3b in bone and soft tissue sarcomas, thus reinforcing GSK3b as a potential therapeutic target.
DECLARATIONS
Acknowledgments
We acknowledge Dr. Barry Iacopetta (University of Western Australia) for critical review and editing of the manuscript.
Authors’ contributions
Made substantial contributions to conception and design of this review: Minamoto T Original draft preparation: Abe K, Shimozaki S
Writing, review, and editing of manuscript: Yamamoto N, Tsuchiya H, Minamoto T Performed literature research: Abe K, Shimozaki S, Domoto T
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology-Japan and from the Japan Society for the Promotion of Science (to Abe K, Yamamoto N, Tsuchiya H, and Minamoto T).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2020.
杂志排行
Journal of Cancer Metastasis and Treatment的其它文章
- ROR2 regulates the survival of murine osteosarcoma cells in lung capillaries
- lsoflavone research towards healthcare applications
- Primary malignant tumors of bone surface: a review with emphasis in differential diagnosis
- Current updates in management of relapsed/refractory small cell lung cancer
- Negative effects of tumor cell nitric oxide on antiglioblastoma photodynamic therapy
- Angiogenesis in acute myeloid leukemia
