Study on Characteristics of Decomposition and Fluorescence Emission of PAHs in Soil by Pulsed Ultraviolet Laser
2020-07-08HUANGYaoZHAONanjingMENGDeshuoZUOZhaoluCHENYunanCHENXiaoweiYINGaofang
HUANG Yao, ZHAO Nan-jing, MENG De-shuo, ZUO Zhao-lu, CHEN Yu-nan, CHEN Xiao-wei, YIN Gao-fang
1. Key Laboratory of Optics and Technology, Anhui Institute of Optics and Fine Mechanics,Chinese Academy of Sciences, Hefei 230031, China 2. University of Science and Technology of China, Hefei 230026, China 3. Key Laboratory of Optical Monitoring Technology for Environment of Anhui Province, Hefei 230031, China
Abstract Polycyclic aromatic hydrocarbons (PAHs) are a group of persistent organic pollutants (POPs) which are mutagenic, carcinogenic and teratogenic. They are widely distributed in air, water and soil. Once PAHs enter the soil, they remain in the soil for a long time. PAHs are concentrated in the soil. They can enter the human body in many ways and pose a threat to human health. Therefore, it is necessary to monitor PAHs in soil. Now, traditional detection methods are cumbersome and time-consuming, which is not conducive to the widely rapid detection of PAHs in the contaminated sites. The method based on laser-induced fluorescence spectroscopy can quickly identify and detect organic pollutants in the soil. However, PAHs are volatile and can be degraded by ultraviolet light, so the selection of UV laser energy is very important. In this work,a 266nm laser-induced fluorescence system is established in the laboratory. Anthracene, pyrene, and phenanthrene are used to investigate the decomposition and fluorescence spectra of PAHs under different laser energies. The results showed that when the energy density of the laser changed, the peak positions of the fluorescence center did not shift, but the relative standard deviations of the maximum intensity at the fluorescence peaks of three PAHs decreased firstly and increased then. When the energy density was 8.54 mJ·cm-2, the relative standard deviations of the three PAHs in 10 spectral measurements were the largest, and the relative standard deviations of the fluorescence peak intensities of anthracene, pyrene and phenanthrene reach the minimum value at 1.72, 1.00 and 1.47 mJ·cm-2. The decomposition rates were 59.3%, 69.8% and 63.6% for anthracene, pyrene and phenanthrene at 100 s, respectively. At higher energies, three PAHs decompose rapidly. Compared with the other two PAHs, pyrene was more prone to photodegradation and thermal decomposition, and the relative standard deviation of fluorescence peak intensity was also higher than that of anthracene and phenanthrene. For anthracene, when the laser energy density was 1.72 mJ·cm-2, the decomposition rate was close to 0 at 10 s and 12.8% at 100 s, and the relative standard deviation of the fluorescence peak intensity was the lowest. When the laser energy density was reduced to 0.88 mJ·cm-2, the decomposition of anthracene in 100s was almost negligible. For pyrene, when the laser energy density dropped below 1.00 mJ·cm-2, the decomposition tended to be consistent, and the decomposition rate was 47.3%~47.4% at 100 s. For phenanthrene, when the energy density of the laser was lower than 1.47 mJ·cm-2, the decomposition rates no longer decreased, and the decomposition rates were 36.8%~38.6%. Pyrene and phenanthrene still decompose in low energy density.
Keywords Laser-induced fluorescence; Soil; Polycyclic aromatic hydrocarbons; Energy density
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are characterized by refractory, carcinogenic, and teratogenic. They are widely distributed in the air, soil and water, and more than ten of them have been listed as pollutants under priority monitoring and controlled by many countries and regions[1-3]. Traditional PAHs monitoring methods have the advantages of good selectivity and high sensitivity, but processes are cumbersome, time-consuming and laborious[4-5]. It cannot accurately reflect the spatial and temporal distribution characteristics of PAHs in the environment, let alone achieve rapid detection of large areas[6].
Soil is the ultimate destination of PAHs in the air and water[7-8]. The concentration and characteristic distribution of PAHs directly affect human health[9]. Among them, anthracene, pyrene and phenanthrene are among the 16 PAHs under the priority control of the US Environmental Protection Agency. Currently, the detection of anthracene, pyrene and phenanthrene in the soil is still mainly based on solid-liquid extraction, concentration, purification and chromatographic separation[10-11], but some scholars have begun to try to use more convenient,less chemical reagent and lower cost methods. Laser-induced fluorescence (LIF) is one of the rapid, non-destructive and in situ detection of PAHs in soil. Laser-induced fluorescence spectroscopy is a highly sensitive detection technology for organic pollutants. It utilizes the strong fluorescent signal emitted by the excited orbital electrons of aromatic hydrocarbons to identify and quantify PAHs. Some have carried out studies on the detection of polycyclic aromatic hydrocarbons in soil by LIF technology: Yang et al.[12]established a linear relationship between the concentration of anthracene in the standard soil and the fluorescence intensity. He Jun et al.[13]established a laser-induced detection based on 250 nm laser to improve the detection limit and linear relationship of PAHs in soil. In recent years, a small number of on-site fluorescence spectroscopy techniques were reported for the detection of organic pollutants in soil. For example, the US Environmental Protection Agency adoptedUVF-3100A (Sitelab Corp., MA,USA) to detect total hydrocarbon contaminants with a detection limit of 3.4 mg·kg-1; The accuracy of total hydrocarbons in the soil reached 89.2%±5.4%, and the detection limit reached 5 mg·kg-1of ROST LIF by Aldstadlt et al[14].
The numbers of fluorescent photons are proportional to the incident light intensity within a certain range; that is, the intensity of the laser induced fluorescence peak is positively correlated with laser energy[15]. Since PAHs are closed conjugated structures with multiple benzene rings, their absorption peaks are mostly located in the ultraviolet bands, and they have high fluorescence efficiency when irradiated with ultraviolet light[16]. Therefore, UV lasersare usually selected as the excitation light source[17]. However, PAHs are volatile organic compounds, which may undergo photolysis and thermal decomposition, thus reducing the stability of the samples[18-20].When the laser energy is weak and acquisition time is short, exciting fluorescence intensity is low, which is not conducive to the identification and quantification of polycyclic aromatic hydrocarbons in the soil. However, when the energy is higher and acquisition time is long, it is easy to break the sample, even to inspire plasma, so choosing the appropriate laser power and test time are the key to the rapid identification and detection of PAHs in soil. Unfortunately, few amounts of research have been reported. In this work, anthracene, pyrene, phenanthrene was selected as an object, characteristics of the fluorescence emission spectrum of PAHs were discussed. The decomposition characteristics of different polycyclic aromatic hydrocarbons under laser irradiation were studied. The suitable laser pulse energy and pulse accumulation times, experimental data support were provided, which provided a reference for the selection of excitation sources for on-site and fast LIF devices.
1 Materials and methods
1.1 Experimental system
The fluorescence peaks of organic matters are broad, and the spectrometer with a resolution of 0.5~1.1 nm can meet the detection requirements. Therefore, Maya2000 pro from the Ocean Company of the US was adopted with the integraltime of 1 000 ms, the detection range of 200~1 100 nm, resolution of 1.5 nm, high sensitivity and response speed, and obvious advantages in signal acquisition speed. Quantel Nd∶YAG solid state laser with a wavelength of 266 nm and frequency of 10 Hz was adopted. The laser was reflected by the 266 nm total reflector mirror and expanded by the beam expander, then incident on the surface of the soil sample on the sample stage. This can excite the fluorescence of PAHs in the soil in a large area, and on the other hand, can avoid laser from concentrating on the soil surface and thus damaging the chemical structure of organic matters. The excited fluorescent signal was focused by a focusing lens, coupled into the optical fiber, and transmitted to the spectrometer to complete the detection analysis. The angle between the incident light and the optical fiber was always maintained at 90°.
1.2 Sample preparation
The natural uncontaminated soil used in the experiment was collected from Dongpu Island of Hefei City. Anthrance, pyrene and phenanthrene were weighed and dissolved in dichloromethane, then quickly added to the soil. The remaining dichloromethane was stored in the fume cupboard for 12 hours, and the simulated soil with a corresponding concentration of polycyclic aromatic hydrocarbons was prepared for LIF determination.
2 Results and discussion
Figure 1 showed laser induced fluorescence spectrum of soils contaminated with anthracene, pyrene and phenanthrene. Under the excitation of 266 nm laser, when the energy density of the laser changed, the position of the central fluorescence peak did not shift. The fluorescence peaks of anthracenewere located near 418 and 444 nn, the fluorescence peak of pyrene was located near 478 nm, and the fluorescence peaks of phenanthrene were located near 386, 407 and 433 nm. Therefore, the fluorescence peaks of 418 nm of anthracene, 478nm of anthracene and 408 nm of phenanthrene were selected as the reference basis for analyzing the influence of laser energy and test time.
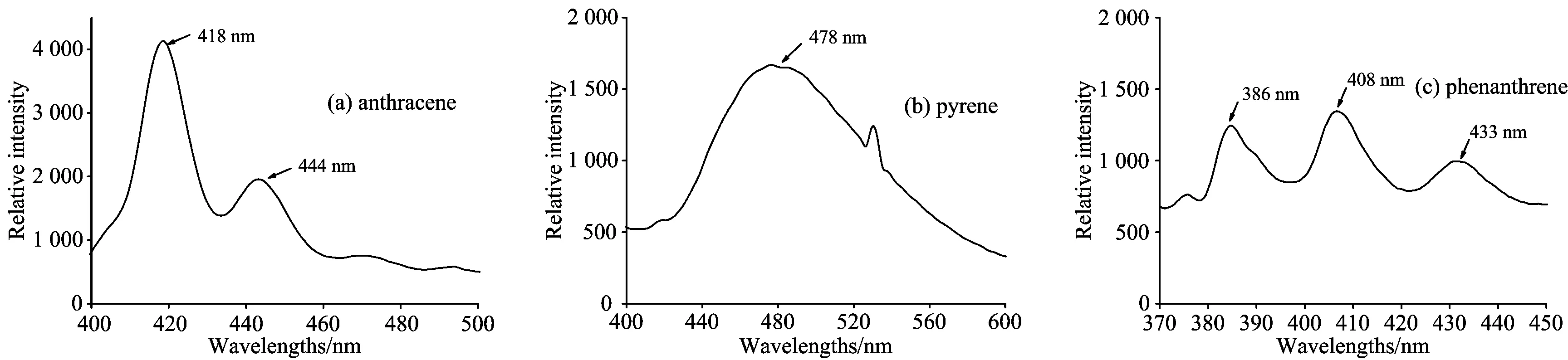
Fig.1 The laser-induced fluorescence spectra of anthracene in soil
13 sets of laser energy densities were obtained by changing the laser energy. As could be seen from Table 1, the laser energy density was stable, and the relative standard deviations of the 100 sets of energy densities did not exceed 2.19%.
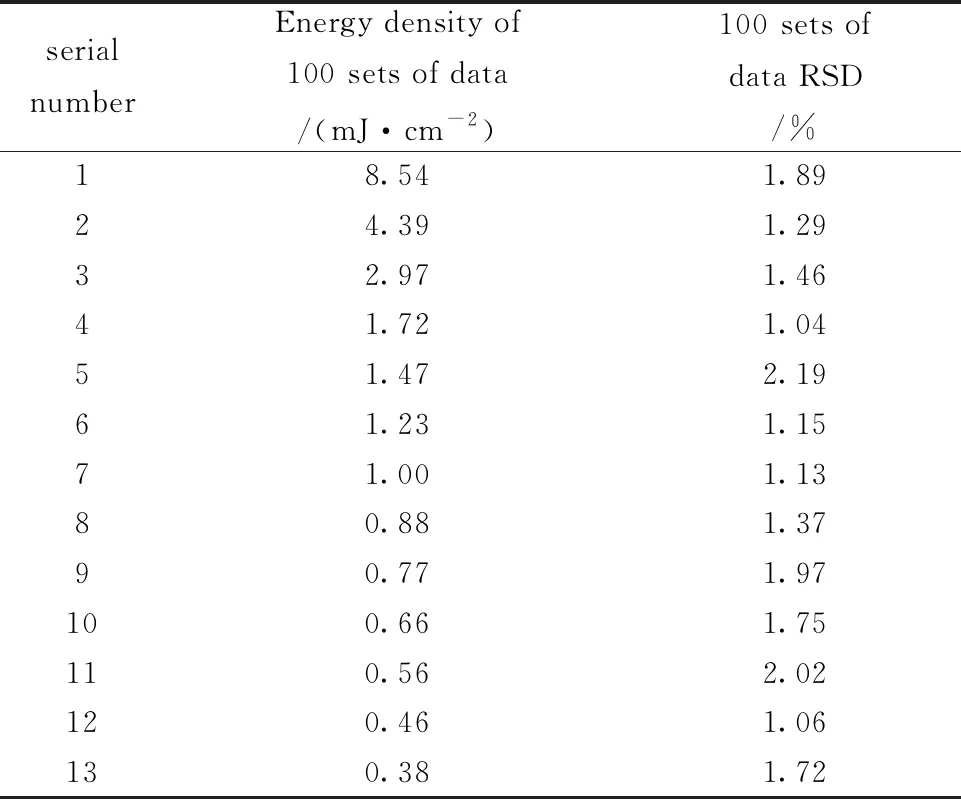
Table 1 Different laser energy densitiesand RSD
The intensity of the emission spectrum of the fluorescent substance was positively correlated with the concentration of the fluorescent substance. Therefore, the intensity of the fluorescent substance can reflect the concentration of the fluorescent substance to a certain extent.The ratio of the difference between the!fluorescence intensity at a certain point of time and the initial fluorescence intensity to the initial fluorescence intensity could be defined as the decomposition rate or rate of decline. The intensity of the fluorescence peaks of anthracene, pyrene and phenanthrene under different pulsed laser energies was shown in Fig.2. With the decrease of laser energy, the intensity of PAHs decreased significantly; that is, the concentration of organic matter decreased. At the same time, the fluorescence intensities of anthracene, pyrene and phenanthrene collected at 10 s were lower than the initial value, indicating that PAHs in the soil did undergo a series of light and thermal decomposition under the continuous action of the laser. As a result, the concentration of the substance was lowered, so the fluorescence intensity of the PAHs at 10 s was significantly lower than the initial value. It was worth noting that for anthracene when the energy density of the pulsed laser reached 1.72 mJ·cm-2, the fluorescence intensity of the soil anthracene was almost the same as the initial value at 10 s, and the difference in intensity gradually decreased and approached zero. For pyrene and phenanthrene, even laser energy continuously reduced, the fluorescence intensity at 10 s was still different from the initial value. Although the difference in strength was decreasing, it never reached zero. As the laser energy decreased, the decompositions of three PAHs were gradually weakened, especially anthracene. When the energy density was lower than 1.72 mJ·cm-2, the decomposition almost stopped, and the decomposition of phenanthrene and pyreneremained.
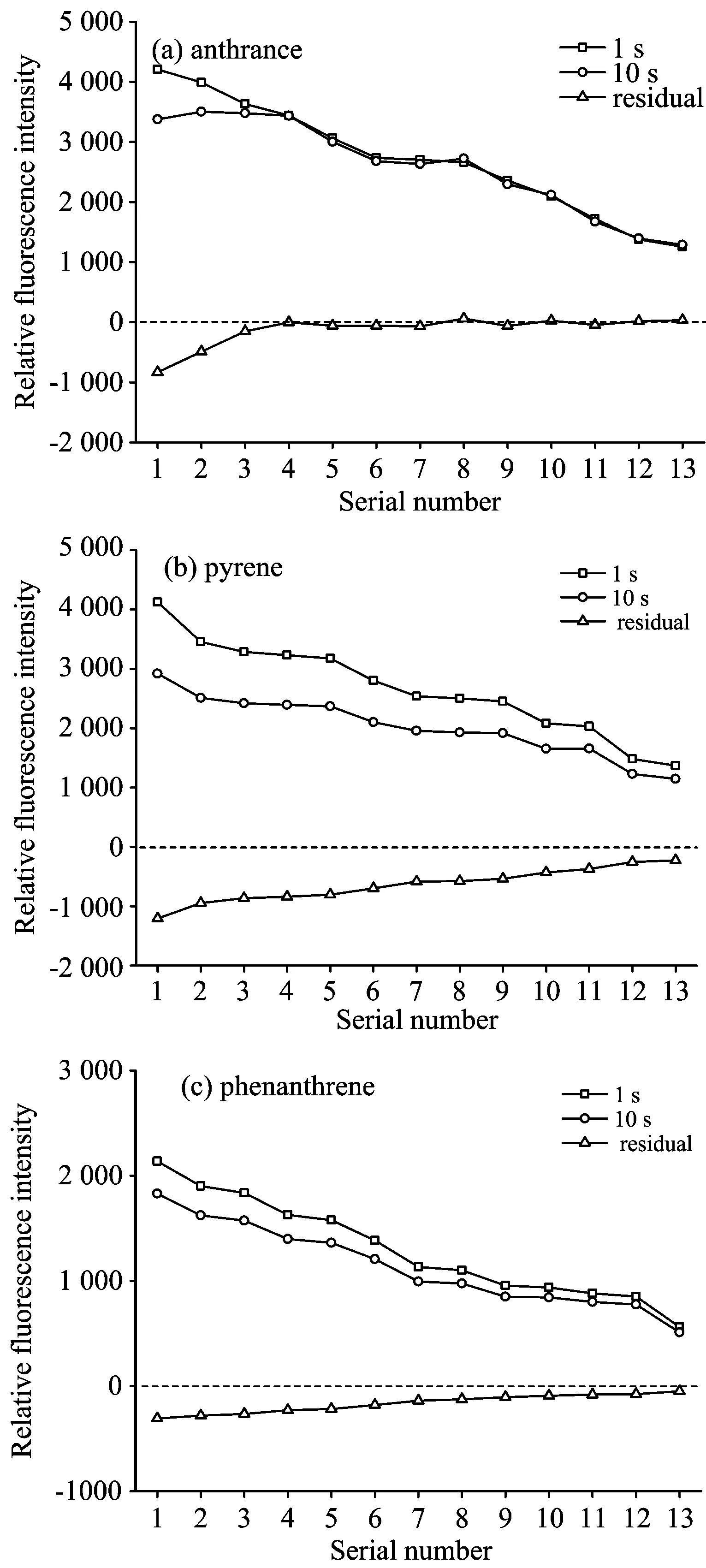
Fig.2 Fluorescence intensity of PAHs in the soil at 1 and 10 s
Under the action of the laser, the concentrations of PAHs decreased continuously, and the fluorescence intensities also decreased. The stability of the fluorescence intensity was worthy of discussion cause the output of the laser itself fluctuated especially when multiple sets of pulse data were accumulated or averaged. The RSD values of the fluorescence peak intensities of PAIs in soil under different laser energy densities were shown in Fig.3. It could be seen from the figure that the relative standard deviation of the maximum intensity at the fluorescence peaks of anthracene, pyrene and phenanthrene tended to decrease firstly and then increased with the decrease of laser energy density. When the energy density was 8.54 mJ·cm-2, the relative standard deviations of the 10 sets of spectral measurements of the three substances in 10 s were the largest, exceeding 8%, and the maximum intensity at the fluorescent peak appeared to decrease significantly. In the higher energy density, PAHs in the soil underwent a series of chemical reactions such as thermal decomposition and photolysis. However, with the laser energy density decreased, the relative standard deviation of the fluorescence peak intensity decreased. It could be seen that the decomposition was weakened and the spectral changes tended to be stable with the decrease of laser energy. The RSD of anthracene, pyrene and phenanthrene reached a minimum at 1.72, 1.00 and 1.47 mJ·cm-2, respectively. However, the relative standard deviation of the maximum intensity at the fluorescence peaks of the three substances increased to some extentwith the increase of laser energy density. It was speculated that the fluorescence intensity decreased due to the decrease in energy density. Under the weak energy density, the fluorescence value was low, and data was unstable. The RSD value of the rising fluorescence peak intensity still did not exceed the RSD value of the fluorescence peak intensity at the previous high energy density. Therefore, when the laser energy density was too high, the maximum intensity of the PAHs in the soil decreased due to the decomposition of organic matter, and the stability was deteriorated. When the laser energy density was too low, the fluorescence intensity was low, and the RSD value started to rise again.
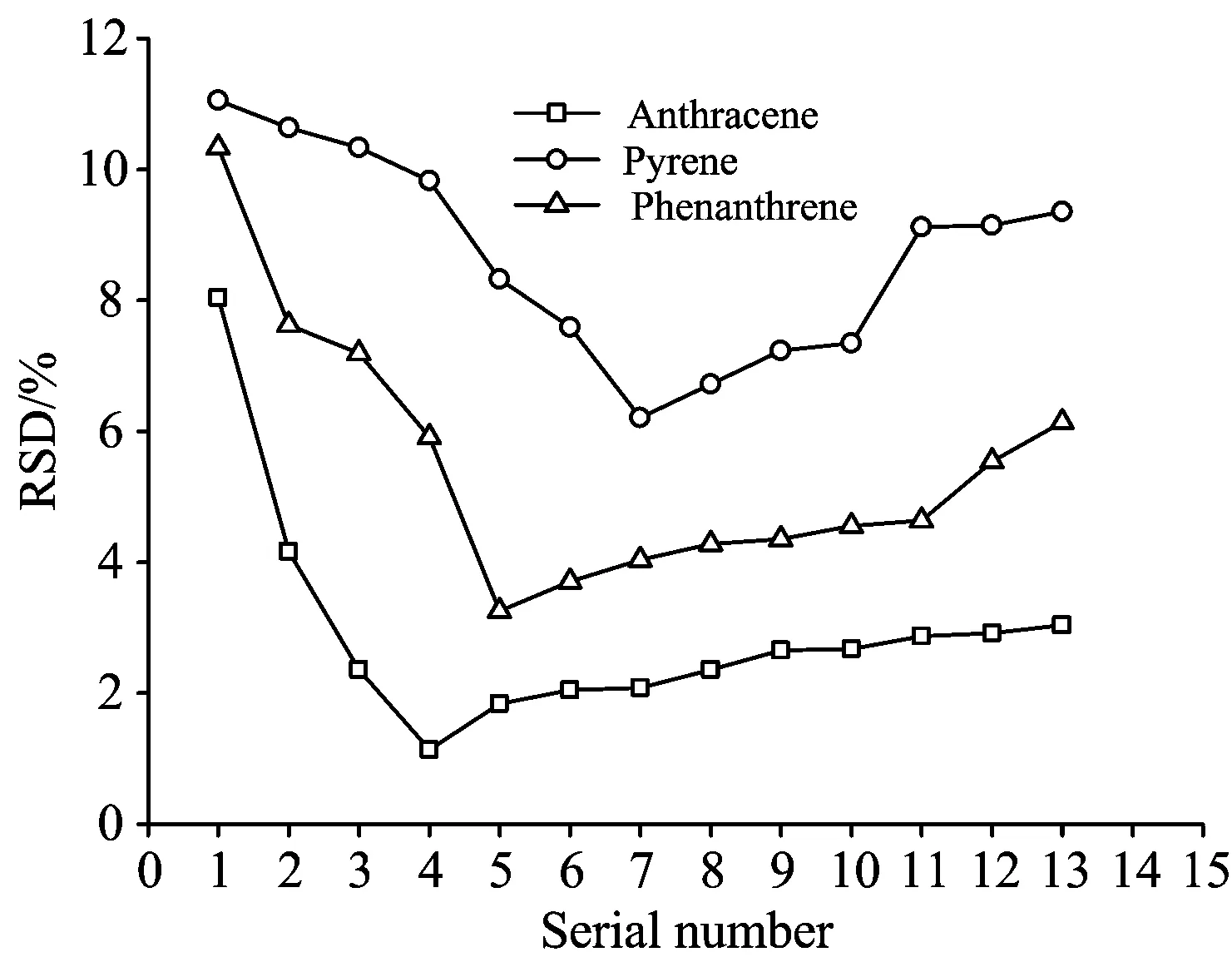
Fig.3 RSD of the fluorescence peak intensity of PAHs at different energy densities
In order to further explore the decomposition of polycyclic aromatic hydrocarbons at different laser energy densities, laser-induced fluorescence spectra of anthracene, pyrene and phenanthrene in 100 s were collected. The ratio of the maximum intensity at the peak of fluorescence was taken as the decomposition rate, and figure 3 was obtained. As could be seen from Fig.3, when the laser energy density was 8.54 mJ·cm-2, the decomposition rates of anthracene, pyrene and phenanthrene were the highest. At 100 s, the decomposition rates were 59.3%, 69.8% and 63.6%, respectively, indicating anthracene, pyrene and phenanthrene decomposed faster at higher energy. Under different energy densities, the decomposition rate of 100 s was anthracene>pyrene>phenanthrene, indicating that pyrenewas more likely to undergo photodegradation and thermal decomposition than other two PAHs. Therefore, the RSD of pyrene in Fig.2 was higher than that of anthracene and phenanthrene. It was worth noting that when the laser energy density was 1.72 mJ·cm-2, the decomposition rate at 10 s was close to 0, and the decomposition rate was only 12.8% at 100 s. Therefore, the RSD at this energy was the lowest. When the laser energy density was reduced to 0.88 mJ·cm-2, the decomposition of anthracene within 100 s was almost negligible, that is, the decomposition would no longer occur. For pyrene, when the laser energy density dropped below 1.00 mJ·cm-2, the decomposition was basically consistent, and the decomposition rate was 47.3%~47.4% at 100 s. For phenanthrene, when the energy density of the laser was lower than 1.47 mJ·cm-2, the decomposition rates no longer decreased significantly with the decrease of laser energy density, and the decomposition rates at 100 s were 36.8%~38.6%. For phenanthrene and anthracene, even the laser energy density decreased, the fluorescence intensity of the fluorescence spectrum still decrease significantly, indicating that the decomposition was going.
3 Conclusions
In summary, under the continuous action of the laser, the fluorescence intensity of anthracene, pyrene and phenanthrene in the soil would decrease to some extent. PAHs were prone to decomposition, and the decomposition decreased with the decrease in laser energy density. Under the action of high energy density, three different kinds of PAHs decomposed strongly. The stabilities of the three PAHs under laser action were also different, anthracene>phenanthrene>pyrene. When the laser energy density was reduced to 0.88 mJ·cm-2, anthracene almost did not decompose within 100 s. For the pyrene and phenanthrene, when the laser energy density dropped to 1.00 and 1.47 mJ·cm-2, the decomposition rate changes tended to be consistent, but the decomposition continued. The RSD values of the three PAHs measured within 10 s tended to decrease first and then increased with the decrease of the laser energy density. Therefore, in the selection of energy in the determination of soil organic pollutants, on the one hand, the decomposition of soil organic matter by the laser under high energy should be considered, and on the other hand, the decrease of intensity stability caused by weak or difficult excitation fluorescence under low energy should be considered, so as to avoid the data distortion caused by the accumulation or average of intensity.
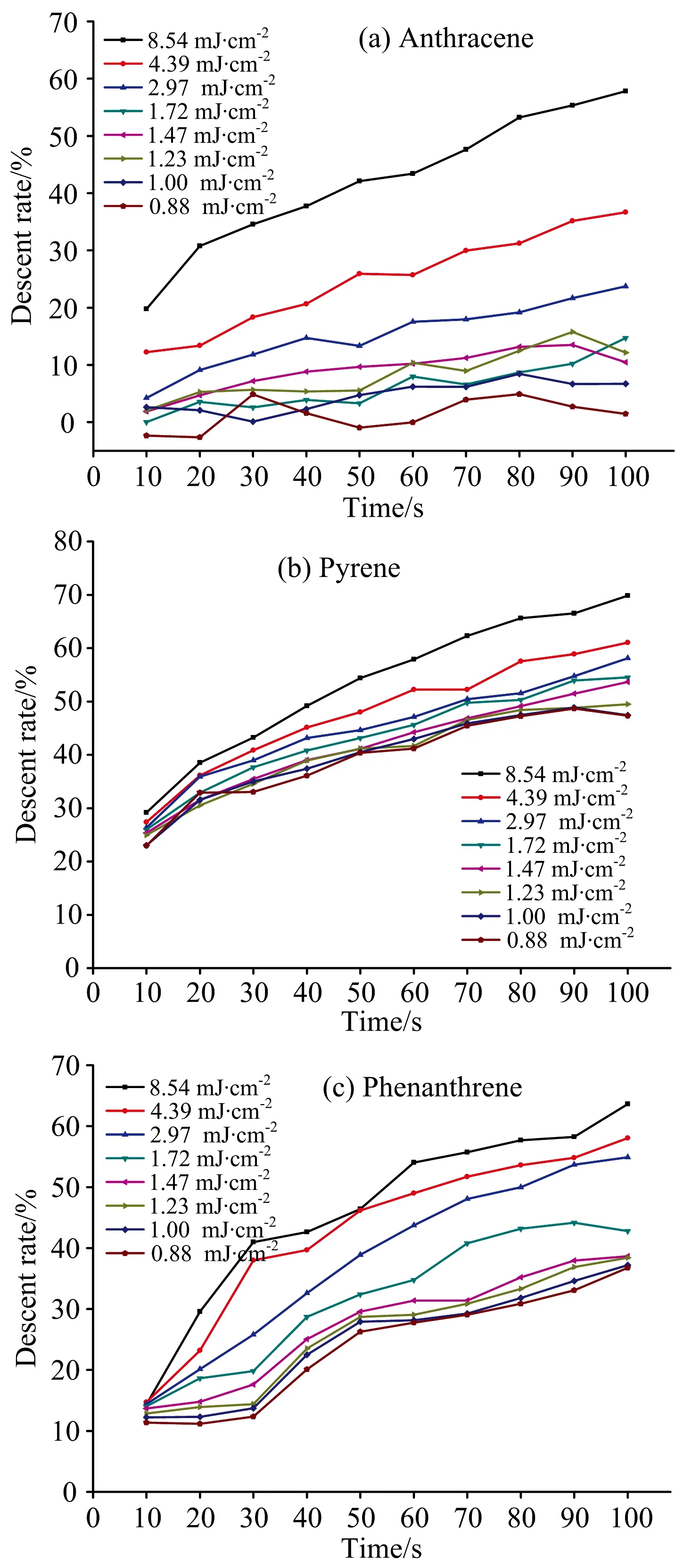
Fig.4 The descent rate of PAHs under different laser energy densities
