A study of flames with millimeter-wave radiation
2020-07-02JingranXuRuPengChenguangZhuXiaoXieChenguangYan
Jing-ran Xu, Ru Peng, Chen-guang Zhu, Xiao Xie, Chen-guang Yan
Department of Applied Chemistry, School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing, Jiangsu, China
Keywords:Combustion Flame Pyrotechnics Millimeter-wave radiation
ABSTRACT It is a valuable issue to explore whether a flame can radiate microwaves, in spite of the electric field formed in the flame. Presented herein is an experimental study on a series of flames with millimeterwave radiation in the combustion of pyrotechnic films. The pyrotechnic films were composed of ultrafine red phosphorus (P), sodium nitrate (NaNO3), Polyvinyl Alcohol (PVA) and some additives such as chopped carbon fibers (CFs) and aluminized glass fibers (GFs). The combustion temperatures and millimeter-wave radiation signals of the flames were measured, the millimeter-wave emissivity and spectral radiant exitance were calculated to describe the millimeter-wave radiation intensity.The results demonstrate that the flame of the pyrotechnic films based on P/NaNO3/CFs can radiate millimeter waves,and different materials and their proportion have a great effect on the millimeter-wave radiation intensity.
1. Introduction
Millimeter wave guidance technology has developed rapidly and been widely used in different missiles and terminal-sensitive projectiles. The propagation of millimeter waves has the advantages of strong anti-interference ability, high penetration in the atmosphere and good weather adaptability. Millimeter wave guided ammunition with these obvious advantages pose a great threat to facilities and troops on the battlefield. Therefore, it is significant to study on millimeter wave active jamming technology applied in decoys for protecting targets from millimeter wave guided ammunition. Flames that can radiate millimeter waves are well worth studying as an important method of millimeter wave active jamming technology.
The millimeter waves can be radiated by the interaction between the electronic spin and alternating magnetic field, and the alternating magnetic field can be formed by alternating electric field [1]. Magnetron technology, which was principally directed towards the production of the shortest wavelengths in the millimeter region, was reported by Columbia Radiation Laboratory [2].Kompfner obtained millimeter wave radiation utilizing backward traveling spatial harmonic components of an electromagnetic in Backward-Wave Tubes,and gave a simple theory of backward gain and of oscillation starting conditions [3]. Christensen described a program to extend helix backward-wave oscillator techniques to the 4.5-6 mm range [4]. Motz introduced a series of experiments on millimeter wave and light generation by means of an undulator[5].So far,the technology of obtaining millimeter wave radiation by flames has not been reported yet. Although the theories of obtaining millimeter wave radiation in different devices are different, a conclusion could be drawn that moving electrons and alternating electric fields are necessary for the generation of millimeter wave radiation.Therefore,in order for flames to radiate millimeter waves, there must be electric fields formed by charged particles in flames.
There is no doubt that the combustion processes are accompanied by intense ionization [6]. For instance, a gas-phase cloud of charged ions, such as TiO+and SiO+, were observed during the process of synthesizing Ti-C,Ti-N2,and Mo-Si at high temperature in some binary systems [7]. An electric field was formed around a burning magnesium particle by thermionic radiation,electron with thermal radiation was the negative potential field and the charged particles (MgO+) on MgO are positive potential fields [8]. The combustion of metal or nonmetal particles can form a temporal electrical field due to a difference in the electrochemical potential across the growing oxide shell, which usually is a mixed ionic electronic conductor. A strong electric field formed during the initial stage of the combustion of single Zr, Ti, Fe, and Ni particles,the electric field lasted for 20-400 ms [9]. In the study of the electric field formed in the combustion of metal particles(Mg,Al,Ti,Zr,Hf,Cr,Mn,Fe, Co and Ni),the oscillation voltage appears in the combustion process of metal particles,and the voltage curves of the congeners are similar [10,11].
Pyrotechnic composition is a kind of solid fuels composition that is composed of combustible agent, oxidizer, binder and additives,and shows multiphase and size effect [12]. It is important to note that large amounts of burning particles can be brought into the flame in the combustion process [13]. In the meantime, physical changes and chemical reactions of particles happen in the boundary of different phases.The reactions will generate an abundance of ions and free electrons between the particle surfaces and interfaces.These ions and free electrons provide a possibility of forming electric fields.The movement velocities and mobility of the burning particles are different,these particles disperse when they enter the flame and lead to disturbance of the neutrality of the burning particle flame,with the development of local electric fields[14-16].The flow field of a flame can be divided into several regions,so the number of burning particles varies in different regions. Each burning particle is an independent system with its own small-scale temperature field, semi closed mass flow field, and nearly completely closed combustion system [17]. According to the Thomson Effect, the temperature difference potential appears because the reaction temperatures of different regions are different[18].The charged particles in the flame move directionally to form ionic currents because of the temperature difference potential.With the development of combustion reaction, the ion currents in the different regions of the flame changes constantly, resulting in the formation of an alternating electric field in the flame. In summary, it is theoretically possible for pyrotechnic composition to radiate millimeter waves in combustion.
In this work, a series of pyrotechnic films in different formulations were designed according to the principle of millimeter-wave radiation and the combustion characteristics of pyrotechnic compositions. The combustion temperatures and millimeter-wave radiation signals of the flame were measured, and the millimeterwave emissivity and spectral radiant exitance were calculated,then the effects of each component of the pyrotechnic films on these parameters were analyzed.
2. Experiments
2.1. Materials selection
On the purpose of radiating millimeter waves, the main principles should be followed in the materials selection were lower combustion temperature and higher possibility to form electric fields. The millimeter-wave radiation of the flame follows the Wien’s displacement law to some extent, the radiation curve for different temperatures peak at a wavelength inversely proportional to the temperature [19]. Therefore, the combustion temperature should be controlled in a lower range in the combustion process.It is important to use a kind of appropriate additives that can contribute to form electric fields in the flame.
Combustible agent is one of the main components of pyrotechnic composition. There are three major types of combustible agents for pyrotechnic composition:metal combustible agents(Mg,Al, Zn, Ti, etc.); non-metallic combustible agents (P, S, C, etc.);organic combustible agents (resin, carbohydrates, oils and other high polymer compounds).Based on the purpose of this study,the combustible agents should be helpful to distribute elective field.Most of the ions and free electrons appear on the surface of the combustion particles, so solid and granular combustible materials are suitable to use in the experiment.If metal particles are used as the combustible agent, a large amount of metallic oxide particles will be generated, these nonflammable particles may reduce the millimeter-wave radiation intensity in flames. Therefore, nonmetallic combustible agent was the best type in this study. Ultrafine red phosphorus is a common flammable non-metallic combustible agent with low melting point, low boiling point, low burning temperature, and high-density electron [20]. In addition,the combustion products do not affect the millimeter-wave radiation in flames, because P2O5is gaseous at high temperature.Considering the above factors, ultra-fine red phosphorus (P) was selected to be used as the combustible agent in the experiment.
Oxidizer is the main oxygen source in pyrotechnic composition,the oxidation of pyrotechnic composition mostly depends on the oxygen contained in the oxidizer.There are many types of common oxidizer materials such as perchlorate, nitrate, chlorate, sulfate,chromates, peroxides, metal oxides, and so on. In this study, an appropriate oxidizer should be selected on the basis of the purpose of this study and the properties of materials. Most of the combustion products of chromate and sulfate may cause serious environmental pollution, so they are rarely used in experiments.Considering that red phosphorus was selected to be used as the combustible agent, chlorate and perchlorate are not suitable because they easily cause burning or explosion when they are being mixed with red phosphorus. Peroxide was not selected because of its poor safety.The temperature of flame should not be too high in this experiment, so the metal oxides were excluded. Therefor nitrate may be the most suitable oxidizer [21]. Y. Kim introduces a sodium ion battery, using the amorphous phosphorus/carbon as the anode material of batteries [22]. In conclusion, NaNO3was selected as the oxidizer in this study.
Binder is an optional ingredient in pyrotechnic composition that can enhance the bonding force between combustible agent particles and oxidizer particles in pyrotechnic composition,improve the physical and chemical stability of pyrotechnic composition, make the burning rate stable, and prevent combustible agent from reacting with O2/H2O in the air. The binders in pyrotechnic compositions are mainly organics or polymeric materials.According to the selected combustible agent (ultra-fine red phosphorus) and oxidizer (sodium nitrate), it is more suitable to use Polyvinyl Alcohol as the binder [23]. Generally, the amount of binder in pyrotechnic composition should not be too much,because the excess binder will affect the oxygen balance of pyrotechnic composition.Thus 1%-2.5% PVA was used as the binder in this experiment.
In order to generate spin electrons and form alternating magnetic field in flames,an appropriate additive is necessary.Filament of conductor or semiconductor is easy to gather the charge in flames, just like antennas, and the electric potential is formed on antennas.As a result,the alternating electric field can be formed in flames. Additives should be resistant to high temperature and difficult to burn. Therefore, chopped carbon fibers (CFs) and aluminized glass fibers (GFs) were used as the additives in this study. According to the resistivity of the materials, CFs are semiconductors,and GFs are conductors.In addition,in order to obtain remarkable antenna effect, the content of additives should not be less than 1%, but too much additives will hinder the combustion,thus the total proportion of additives is 1%-2%. The main parameters of the materials used in the experiment are shown in Table 1.
2.2. Determination of pyrotechnic formulations
The chemical reaction equations of each component are as follows.

Table 1 The material parameters.

In the combustion process of pyrotechnic films, the fuel is mostly oxidized by the oxygen element of the oxidizer, also, the oxygen in the air is involved in the reaction.The oxygen balance is the negative oxygen balance in this case.According to the chemical reaction (1-3) and the principle of negative oxygen balance, the percentage of each component was determined.
The pyrotechnic compositions were prepared into circular films with 50 mm in diameter and 0.70 mm in thickness.
The formulation A~H were designed,and the proportion of each component in the formulation were presented in Tables 2-9.
2.3. Materials preparation
All the raw materials were analytical reagents with the purity of more than 99%. According to the designed formulations, a certain amount of CFs and GFs were pre-dispersed evenly by ultrasound vibration in a beaker of dispersant (0.6% cationic polyacrylamide solution) for 30 min. Next, PVA dissolved completely in thedispersant at 363 K. Then the suspension had formed after P and NaNO3were added into the dispersant. The suspension was dispersed again for 30 min in the ultrasonic dispersing system,and the final slurry was obtained. The slurry was kept at 363 K to eliminate H2O until H2O evaporated completely. The film could be removed from the beaker by some ethanol. The pyrotechnic film,which contained P, NaNO3, PVA, CFs or GFs, was prepared.
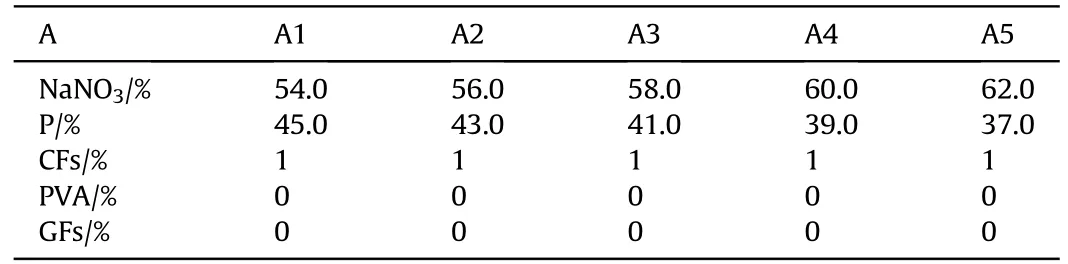
Table 2 Formulation A.

Table 3 Formulation B.

Table 4 Formulation C.

Table 5 Formulation D.

Table 6 Formulation E.

Table 7 Formulation F.

Table 8 Formulation G.

Table 9 Formulation H.
2.4. Experimental methods and setup
As shown in Fig.1,the combustion of nonmetal-oxide mixtures was initiated by ignition powder along the front of the sample.The flame of the pyrotechnic film was observed by a high speed camera connected with the computer 1 at a distance of 1.5 m. The combustion temperatures were measured with the non-contact infrared temperature measurement instrument (Range:623-2773 K, IMPAC, IGA-140) connected to the computer 2 at a distance of 1 m. And the millimeter-wave radiation was measured with 3 mm band DC radiometer(Produced by Nanjing University of Science and Technology) at a distance of 1.2 m, the voltage signals were recorded by a data acquisition system (Omega Eng. Inc.,Stanford, CT)) connected to the computer 2.

Fig.1. Experimental methods and setup.
2.5. Combustion of the pyrotechnic films
The images of combustion interface were recorded by the high speed camera as shown in the Fig. 2. The combustion reaction of each component in the pyrotechnic film started in flames, which contains several CF.
2.6. The theory of alternating electric field formation
Combustion reactions occurred in a thin layer of the pyrotechnic film near the flame.There were many burning particles in the flame of pyrotechnic films, these burning particles were elementary particles and composite particles containing unburned ultra-fine red phosphorus and NaNO3. NaNO3absorbed heat and decomposed into Na++,further decomposed into+ O. A part of ultra-fine red phosphorus (P) coated by NaNO3were oxidized into P2O5by O and much heat was released at this stage.Next,decomposed into N2+, a large amount of gases were generated. Na+originally existed in the form of Na2O2because Na2O was not stable at temperature higher than 900 K, so the O2-absorbed two electrons and converted into. Then, as the reaction moved away from the high temperature region,Na2O2lost O and converted into Na2O (→O2-+ O). Finally, the remaining non-oxidized P particles reacted with O. The whole reaction process is shown in reaction (4)[24].It could be seen that there were many ions and electrons in the pyrotechnic flame, such as Na+,,,,, O2-, e-.

At the same time,there were some CFs in a flame.In the reaction interface of pyrotechnic film, ions and electrons interact with CFs,CFs can be regarded as a conductive electrode [25]. The thermoelectromotive force appears because the temperatures in different regions are different[18].The generated electrons form current by moving in CFs from high temperature region to low temperature region [26,27], and transit to the adjacent CFs by the tunnel effect[28]. Fig. 3 describes this formation process of alternating electric field.
2.7. Calculation of the millimeter-wave radiation
The millimeter-wave radiation signals were measured by the 3 mm band DC radiometer, and the output signals were voltage values.The millimeter-wave emissivity ε can be calculated with the voltage by Eq. (5).

Where the εiis the millimeter-wave emissivity, Uiis the output voltage value of each flame, V, Ui0is the output voltage value of a metal plate (ε=0) at the same temperature as the flame, V, Uimis the output voltage value of a black body (ε=1) at the same temperature as the flame,V.
According to the Planck’s law and the Lambert’s cosine law,the spectral radiant exitance of a black body around a given wavelength can be calculated by Eq. (6) [29]:

Where the h is the Planck constant,λ is the wavelength,m,K is the Boltzmann constant,c is the speed of light in the medium,m/s,T is the temperature of the object, K.
Based on the definition of emissivity,ε is given bywhere the Me,λ is the spectral radiant exitance of millimeter-wave radiated flame at the maximum temperature, W/m3, Moe,λ is the spectral radiant exitance of a black body at the same temperature, W/m3.Therefore, the spectral radiant exitance of millimeter-wave radiation of the flame can be represented as Eq. (7):

Fig. 2. The image of combustion interface.

Fig. 3. The formation process of alternating electric field.

The spectral radiant exitance Me,λ are always used to describe the radiation intensity of electromagnetic waves.
The combustion temperature of the flames varies with time during the process of the combustion. In this paper, temperatures of the whole combustion process were measured (the response time of the instrument is 1 ms),the maximum temperature of each combustion was recorded as Tmaxfor analysis.The spectral radiant exitance of the black body()were calculated with the Tmax,the millimeter-wave emissivities ε were calculated by the signal voltage values. Therefore, the spectral radiant exitance of the flames could be obtained byand ε.
3. Results and discussion
3.1. The millimeter-wave radiation of P/NaNO3/CFs formulations
The pyrotechnic films in formulation A, B and C only contains the main components P/NaNO3/CFs. These three figures illustrate that the combustion temperature and the millimeter-wave radiation intensity are hardly affected by the amount of NaNO3except formulation A. Because of the low CFs ratio in formulation A, the combustion performance(such as flame temperature,burning rate and completeness of reaction)of P/NaNO3is more closely related to their ratio. When the percentage of NaNO3is 60%, the oxygen balance is closest to zero in air atmosphere, so the reaction under this ratio is more complete, faster and the flame temperature is higher.As shown in Fig.4,with the increase of CFs,the combustion temperature decreases slightly. However, this trend is not seen when A is compared with either B or C. When the percentage of NaNO3was 54% and 62%, the combustion temperature is lower,because the ratio of P/NaNO3deviates from zero oxygen balance seriously. The formation of electric field is the main factor to generate millimeter-wave radiation,and the electric field is formed by the electric potential difference(voltage)on CFs that can gather the charge in flames. Therefore, the CFs in the formulation is essential for the formation of millimeter-wave radiation. In previous experiments, the millimeter-wave radiation could not be detected in pyrotechnic films without any additives. However, the millimeter-wave emissivity and spectral radiant exitance decreases with the increase of CFs. Because that the non-combustible CFs hindered combustion, resulting in a lower number of burning particles in the flame at one time. In this case, the number of moving electrons and ions decreased, and the electric potential energy decreased.It indicates that the amount of CFs should not be too much, excessive CFs will lead to the reduction of millimeterwave radiation intensity, although CFs is the basic condition for the formation of electric.

Fig. 4. The maximum combustion temperature, millimeter-wave emissivity and millimeter-wave spectral radiant exitance in 3 mm band of pyrotechnic films in formulation A, B and C.

Fig.5. The maximum combustion temperature,millimeter-wave emissivity and millimeter-wave spectral radiant exitance in 3 mm band of pyrotechnic films in formulation A,D,E,F and G.
3.2. The millimeter-wave radiation of P/NaNO3/CFs/PVA formulation
Compared with formulation A, PVA was added as the binder in formulation D, E, F and G. As shown in Fig. 5, the maximum combustion temperatures of formulation D,E,F and G were lower than the formulation A.Based on experimental experience and materials properties, the combustion temperature decreased after adding PVA, because that PVA need to absorb enough heat to melt and decompose before it starts to burn and the combustion temperature of PVA is lower than that of P/NaNO3. The results also show that the combustion temperatures of formulation D,E,F and G are similar, it indicates that the combustion temperature were not affected with the further increase of PVA,so the effect of PVA on the reduction to combustion temperature is limited. The millimeterwave radiation intensity of formulation A is similar with formulation D, E, F and G, there is little difference among the millimeterwave radiation intensity of formulation D, E, F and G. A conclusion can be drawn that whether the pyrotechnic film contains PVA does not affect the millimeter-wave radiation intensity, and the amount of PVA in the formulation does not affect the millimeterwave radiation intensity either.
3.3. The millimeter-wave radiation of P/NaNO3/GFs formulations
Compared with formulation C,1%CFs is replaced with 1%GFs in formulation H.As shown in Fig.6,there is no obvious difference in the combustion temperature when 1% CFs is replaced by GFs.Referring to the conclusion given in Fig. 4, the combustion temperature decreases with the increase of CFs,a conclusion can be drawn: the amount of additives rather than the type of additives can affect the combustion temperature because the additives not react in the combustion. It is obvious that the millimeter-wave radiation intensity of formulation H is much less than formulation C. It means the millimeter-wave radiation intensity decreases substantially when some CFs are replaced with GFs. According to the different properties of CFs and GFs, there may be two reasons for this result. Firstly, the electric field is formed by the electric potential difference(voltage)on CFs or GFs,but the resistivity of the aluminized glass fiber is very small,so the voltage is very low when the current is almost constant.Secondly,the aluminum on GFs may react in the combustion, leading to the fact that the GFs without aluminum are no longer conductive and the current cannot be formed. In conclusion,in order to form electric field in flames, the additives should have electrical conductivity and proper resistivity,semiconductor material may be the best type of additives.
4. Conclusions
The main motivation of this study was to explore whether a flame can radiate microwaves, the research emphasis in the microwaves band was the millimeter wave band radiated by flames.In this study, a series of pyrotechnic films with millimeter-wave radiation in different formulations were designed, the combustion temperatures and millimeter-wave radiation signals of the flames were measured, then the millimeter-wave emissivity and spectral radiant exitance were calculated to analyze the millimeter-wave radiation intensity in different formulations. Preliminary results indicate that the solid fuels films in formulations based on P/NaNO3/CFs can radiate millimeter waves in the combustion. With the proportion of the Oxidizer (NaNO3) increased, there was little change in the combustion temperature and the millimeter-wave radiation intensity. The addition of PVA did not affect the millimeter-wave radiation intensity,but decreased the combustion temperature.The amount of CFs need to be limited,the combustion temperature and the millimeter-wave radiation intensity all decreased with the increase of CFs, the most appropriate proportion of CFs was 1%. According to the comparison between the addition of GFs and CFs, it is shown that the semiconductor material is more conducive for pyrotechnic films to radiate millimeter waves. Future research will analyze how each component affects the millimeter-wave radiation more accurately and more detailed,and explore whether a flame of solid fuels can radiate electromagnetic waves in longer wavelength such as centimeter waves.

Fig.6. The maximum combustion temperature,millimeter-wave emissivity and millimeter-wave spectral radiant exitance in 3 mm band of pyrotechnic films in formulation C and H.
Acknowledgement
The support for this work was provided by the National Natural Science Foundation of China (Project No.51676100).
杂志排行
Defence Technology的其它文章
- Analysis of sliding electric contact characteristics in augmented railgun based on the combination of contact resistance and sliding friction coefficient
- Aerodynamics analysis of a hypersonic electromagnetic gun launched projectile
- Synergistic effect of hybrid Himalayan Nettle/Bauhinia-vahlii fibers on physico-mechanical and sliding wear properties of epoxy composites
- Study on dynamic response of multi-degree-of-freedom explosion vessel system under impact load
- An investigation on anti-impact and penetration performance of basalt fiber composites with different weave and lay-up modes
- Modeling and simulation of muzzle flow field of railgun with metal vapor and arc
