三维无溶剂含能Ag-MOF的制备、热分解动力学及爆炸性能
2020-06-30乔成芳吕磊许文风夏正强周春生陈三平高胜利
乔成芳,吕磊,许文风,夏正强,周春生,陈三平,*,高胜利,
1商洛学院化学工程与现代材料学院,陕西省尾矿资源综合利用重点实验室,陕西 商洛 726000
2西北大学化学与材料科学学院,教育部合成与天然功能分子化学重点实验室,西安 710127
3延安大学化学与化工学院,陕西省化学反应工程重点实验室,陕西 延安 716000
1 Introduction
The development of new energetic materials with high energy,low sensitivity, and good thermal stability is always desirable1.Nowadays the main attention has been paid on the design and synthesis of high energy density single compound explosive,which is formed by combining the high-energy or explosive groups (―NO2, ―ONO2, ―N3,etc.) and energetic skeletons(such as the nitrogen-rich heterocycles: furazan, tetrazole,triazole, tetrazine,etc.) in one molecule2-4. However, the sensitivity of materials rapidly increases with the rising energy density, resulting in the restriction of mass production and extensive application5,6. Recently, energetic metal-organic frameworks (EMOFs) constructed by high-energy ligands bridging metal ions have been demonstrated to be one of the most acceptable strategies to harmonize the above-mentioned con flict between energy and safety performance of energetic materials7,8. On one hand, abundant high-energy ligands are highly aggregated and regularly distributed within limited space by robust coordination bonds to improve the energy density of material and give remarkable heats of detonation9. On the other hand, rich supramolecular interactions (such as H-bonds andππstacking) among the multiple framework components are very beneficial to enhance thermostability and reduce insensitivity10,11. Meanwhile, compared to the 1D and 2D EMOFs, 3D EMOFs generally provide higher structural stability and more superior energetic property owing to their more complicated coordination modes and reinforced structures12.
However, the assembly process of EMOFs is often controlled by thermodynamics and kinetics, and the resulting structures are always unpredictable. The solvent molecules can randomly diffuse into the channels of EMOFs, occupy the lattice or coordinate to metal centers13. Their existence will undoubtedly reduce the energy density of EMOFs, leading to decreased heats of detonation14. And at the same time, the heat-release of the solvent molecules will easily cause pressure at low temperature region and further reduce the stability of EMOFs15. Therefore,the synthesis of solvent-free EMOF is one of the most efficient ways to obtain energetic materials with excellent performances.
The latest research of our group has successfully synthesized some solvent-free EMOFs with superior detonation performances and low sensitivity16,17, in which the orthobistetrazole ligands featuring large steric hindrance effect and strong chelation coordination ability play an important role in hindering the solvents to be incorporated in EMOFs. So a nitrogen-rich heterocyclic ligand, 2,3-di(1H-tetrazol-5-yl)pyrazine (H2DTPZ), is designed and synthesized to construct the EMOFs based on the following three reasons: i) a high nitrogen content of 65% provides the energy source of EMOF;ii) the ten nitrogen atoms act as strong multidentate coordination sites to occupy the coordination sphere of metal center and prevent the coordination of solvent molecules; iii) the noncoplanar torsions between pyrazine and tetrazole rings are beneficial to produce large steric hindrance or interpenetration for reducing the available void and impeding lattice solvents.Herein, a 3D solvent-free EMOF, [Ag2(DTPZ)]n(1), is synthesized under hydrothermal conditions by the reaction of H2DTPZ ligand and silver(I) ions. The crystal structure, thermal stability, non-isothermal kinetics analysis of the decomposition process, and corresponding thermodynamic parameter of 1 are discussed in detail. Additionally, the detonation and safety performance show that 1 is insensitive to impact and friction, and the detonation performance is superior to that of TNT. The results suggest that the 3D solvent-free EMOFs are promising high energy density materials and could be used in the field of explosives and propellants.
2 Experimental section
2.1 Materials and instruments
Caution! H2DTPZ and compound 1 are hazardous materials,explosions of which may occur in certain conditions.Appropriate safety precautions such as the use of safety glasses,face shields and plastic spatulas should be taken during the experiments, especially when the compounds are prepared on a large scale.
All chemicals were of analytical grade, purchased from commercial sources and used without further purification.H2DTPZ was synthesized according to the published procedures18. Elemental analyses of C, H and N were performed on a Vario EL III analyzer (Elementar, Germany). Infrared (IR)spectra were recorded on a Tensor 27 spectrometer (Bruker Optics, Ettlingen, Germany) with KBr pellets (4000-400 cm−1).Powder X-ray diffraction (PXRD) measurements were performed on a Rigaku RU200 diffractometer (Rigaku Corporation, Japan) (CuKα,λ= 0.15406 nm).Thermogravimetric analysis (TGA) was conducted on a Netzsch STA 449C instrument (Germany) under a N2atmosphere with a heating rate of 10 °C·min−1from ambient temperature up to 800 °C. The differential scanning calorimetry (DSC) experiment was performed on a CDR-4P thermal analyzer of Shanghai Balance Instrument factory (calibrated by standard pure indium and zinc) from 30 to 500 °C in a nitrogen flow. The sensitivity to impact stimuli was determined by fall hammer apparatus applying standard staircase method using a 2 kg drop weight and the results were reported in terms of height for 50% probability of explosion (h50%)19. The friction sensitivity was determined on a Julius Peter’s apparatus by following the BAM method20. The constant-volume combustion energy of the compound was determined using a precise rotating-bomb calorimeter (RBC-type II, Mianyang Zhongwu Thermal Analysis Instrument Co.LTD, China)21.
2.2 Synthesis of [Ag2(DTPZ)]n (1)
A mixture of H2DTPZ (5.4 mg, 0.025 mmol), AgNO3 (8.5 mg,0.05 mmol) in H2O (8 mL) was sealed in a 15 mL Teflon-liner stainless autoclave and heated at 160 °C for 72 h. After cooling to room temperature at a rate of 5 °C·h−1, yellow rod-shaped crystals were obtained (yield: 51%, based on AgI). Anal. Calcd.for AgC3HN5(%): C, 16.76; H, 0.47; N, 32.58 Found: C, 16.69;H, 0.89; N, 32.78. IR (KBr, cm−1): 3493s, 2270w, 1720w, 1630s,1481w, 1150m, 1020w, 752w, 691m, 514w. The phase purity of the bulk sample of 1 was verified by the PXRD patterns (Fig. S1,in Supporting Information).
2.3 X-ray crystallography

Table 1 Crystallographic data for the compound 1.
Single-crystal XRD data were collected on a Bruker Smart Apex CCD diffractometer equipped with graphite monochromatized MoKαradiation source (λ= 0.071073 nm)usingωandφscan mode. The crystal structure was solved by direct methods and refined with full-matrix least-squares refinements based onF2using SHELXS-97 and SHELXL-9722,23.All non-hydrogen atoms were located using subsequent Fourierdifference methods and refined anisotropically. Hydrogen atoms were placed in calculated positions. The Crystallographic data of compound 1 are summarized in Table 1, selected bond lengths and angles are shown in Table 2. CCDC 1544023 contains the supplementary crystallographic data of 1. These data can be obtained free of charge from The Cambridge Crystallographic Data Centreviawww.ccdc.cam.ac.uk/data_request/cif; e-mail:deposit@ccdc.cam.ac.uk.
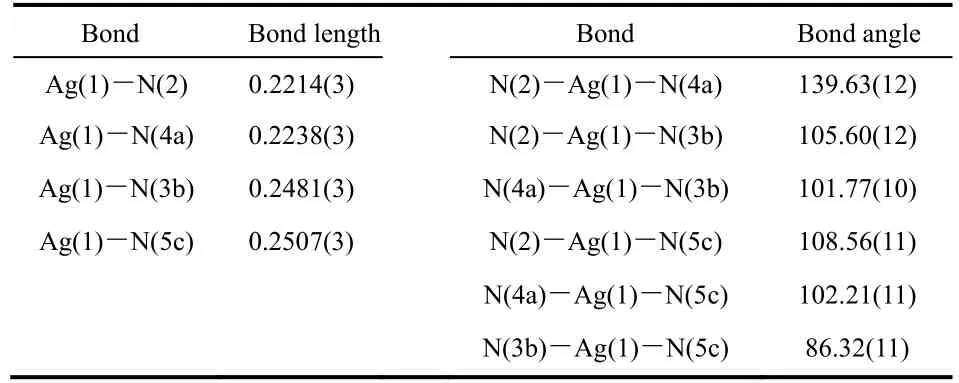
Table 2 Selected bond lengths (nm) and bond angles (º) for 1.
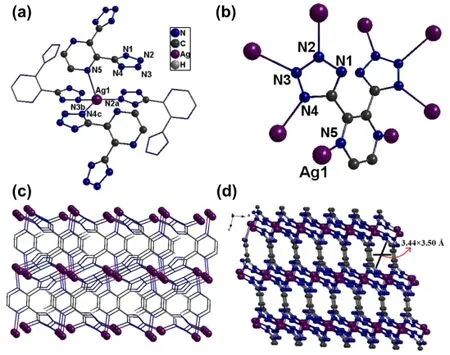
Fig. 1 (a) Coordination environment of AgI ions.(b) Coordination model of the DTPZ2− ligand. 3D frameworks of compound 1 viewed along the a axis (c) and b axis (d).
3 Results and discussion
3.1 Structural description
Singe-crystal X-ray diffraction reveals that compound 1 crystallizes in the monoclinic space groupC2/c. The asymmetric unit consists of one crystallographically independent AgIcenter and half deprotonated DTPZ2−anion, and there is no solvent molecule in the structure. As shown in Fig. 1a, each AgIion is coordinated by three N atoms of three independent tetrazole rings from three different DTPZ2−ligands and one N atom of pyrazine ring from another DTPZ2−ligand to furnish a distorted tetrahedral geometry. The Ag-N distances are ranging from 0.2214(3) to 0.2507(3) nm, and the coordination angles around AgIcenters vary from 86.32(11)° to 139.63(12)° (Table 2),which are both within normal ranges and comparable with those observed in the reported AgI-tetrazole compounds24,25.Moreover, the configuration of DTPZ2−twists heavily, and the dihedral angle between two tetrazole rings is 46.7°, the dihedral angle between tetrazole ring and pyrazine plane is 45.5°. Each DTPZ2−ligand links eight AgIcenters with an octadentate mode(Fig. 1b) extending in all three dimensions to form a 3D microporous EMOFs (Fig. 1c). Notably, the strongπ-πstacking interactions [centroid-centroid distance = 0.34461(1) nm]between the parallel distributed tetrazole rings (C1N1N2N3N4)from different DTPZ2−ligands can be observed (Fig. S2), which could benefit the stability of framework. Along theb-axis, 1D rhombus channels with the window size of 3.44 Å × 3.50 Å can be observed. PLATON analysis shows that the effective free volume of EMOFs is 7.8% of the crystal volume26. Such a low porosity value could be interpreted by the flexible torsion of DTPZ2−, and the large steric hindrance occupies the most effective void of 1.
3.2 Thermal stability
Thermal stability is an important evaluation index for energetic materials. The thermal decomposition behavior of 1 was investigated by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) technology. As shown in Fig. 2a, the solvent-free framework remains stable up to 345.9 °C and then undergoes one-step of fast weight loss within the short temperature range of 346-426 °C, suggesting good thermal stability and potential explosive characteristic of 1. The rapid weight loss can be attributed to the decomposition of the organic ligand and the collapse of framework. The remaining substance still experiences continuous very slow mass losses and does not stop until 800 °C. The high thermal stability of 1 may be due to the solvent-free characteristics of the system and abundant robust coordination bonds and strongπ-πstacking interactions within the 3D framework.

Fig. 2 The TG (a) and DSC (b) curves of 1.
For the DSC curve of 1, an intense exothermic process occuring at 345.5 °C and ends at 422.2 °C with a peak temperature of 385.5 °C represents the sharp energy release of the energetic components in 1, which is almost identical to the phenomenon observed on the TG curve (Fig. 2b). The sharp exothermic peak at 385.5 °C on DSC curve corresponds to the fastest decomposition process.
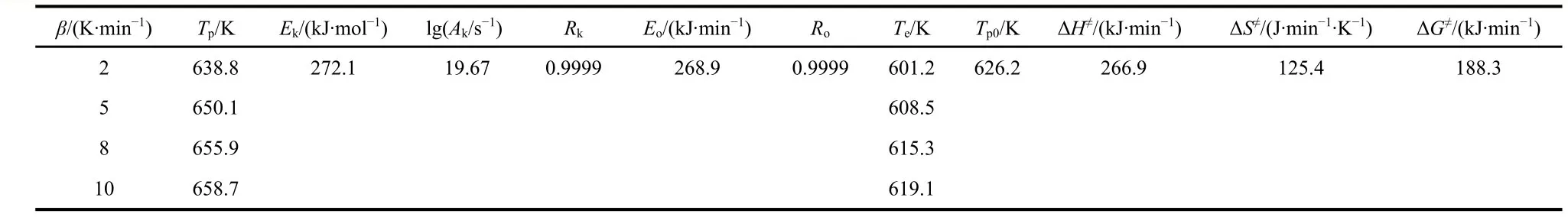
Table 3 Thermokinetic and apparent thermodynamic parameters of the exothermic decomposition reaction of 1.
3.3 Non-isothermal thermoanalysis kinetics
The thermokinetics parameters of the decomposition process of 1 were discussed by the widely used Kissinger and Ozawa-Doyle methods27,28. The Kissinger (Eq. 1) and Ozawa-Doyle(Eq. 2) equations are as follows, respectively:
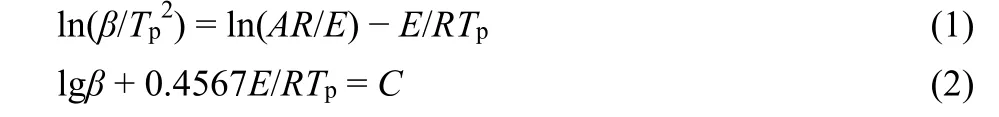
whereEis the apparent activation energy (kJ·mol−1),Ais the pre-exponential factor (s−1),βis the linear heating rate (K·min−1),Tpis the peak temperature (K),Ris the gas constant (8.314 J·mol−1·K−1) andCis a constant. Based on the exothermic peak temperatures measured at four different heating rates of 2, 5, 8,and 10 K·min−1(Fig. S3), the apparent activation energiesEkandEo, the pre-exponential factorAk, and the linear correlation coefficientsRkandRoare listed in Table 3. Apparently, the decomposition peak temperatureTpincreases with the increase of heating rate, and the apparent activation energies calculated by the two methods basically agree with each other and both are within the normal range of deviation allowed. The Arrhenius equation can further be expressed by using the obtainedEa(the average ofEkandEo) and the lgAvalues, as follows: lgk= 19.67− 270.52 × l03/(2.303RT), which can be used to estimate the rate constant of the decomposition process of 1.
The important thermodynamic parameters, the entropy of activation (ΔS≠), the enthalpy of activation (ΔH≠), and the free energy of activation (ΔG≠) of the exothermic decomposition reaction were calculated according to the following Eqs. 3-529.
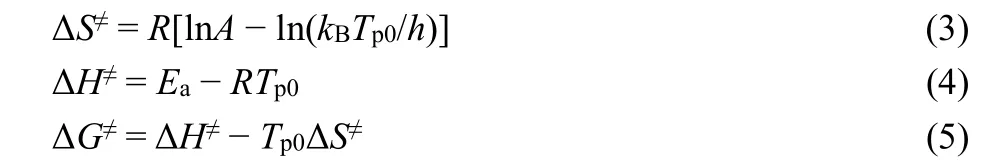
wherekBis the Boltzmann constant,Tp0is the peak temperature point corresponding toβ→ 0,his the Planck constant,Ea=EkandA=Ak. The positive values of ΔG≠and ΔH≠indicate that the thermodecomposition reaction of 1 is a non-spontaneous entropy-driven process (Table 3).
3.4 Critical temperature of thermal explosion
The critical temperature of thermal explosion (Tb) and the selfaccelerating decomposition temperature (TSADT) are important indicators for the thermal safety of energetic materials during storage and operation30. Therefore, based on the values (Te0orTp0) of the initial temperature point corresponding toβ→ 0 obtained from Eq. 6, the Eqs. 7 and 8 are applied to determine the values ofTbandTSADTfor 131:
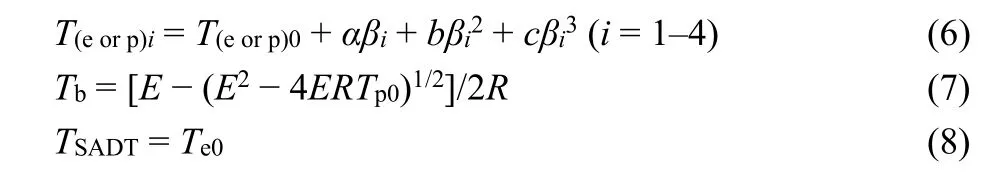
wherea,bandcare coefficients,Te0represents the extrapolated onset temperature corresponding toβ→ 0. The high values(TSADT= 595.8 K andTb= 607.1 K) of 1 indicate that the solventfree Ag-MOF 1 possesses better thermal safety than some common explosives, such as HMX, CL-20 and FOX-7 (Table 4) 29,32-34.
3.5 Oxygen bomb calorimetry
The constant-volume combustion energy of 1 was determined with a precise rotating-oxygen bomb calorimeter (RBC-type II)21.Approximately 200 mg of the samples were pressed with an 800 mg of benzoic acid to form a tablet to ensure better combustion.The recorded data are the average of six single measurements.The calorimeter was calibrated by the combustion of certified benzoic acid (Standard Reference Material, 39i, NIST) in an oxygen atmosphere at a pressure of 3.05 × 106Pa. After six tests,the experimental result for the constant-volume combustion energy (Qv) of 1 is −3509.90 ± 1.22 kJ·mol−1. On the basis of the combustion reaction equation of 1 (Eq. 9), the standard molar enthalpy of combustion (ΔcHmө) of 1 can be calculated to be(−3504.94 ± 1.22) kJ·mol−1according to the following Eq. 10:

where Δng is the change in the number of gas constituents in the reaction process (Δng= 2),T= 298.15 K. Given the known standard molar enthalpies of formation of Ag2O(s) (−31.00 kJ·mol−1), CO2(g) (−393.51 ± 0.13 kJ·mol−1) and H2O(l)(−285.83 ± 0.04 kJ·mol−1)35, the standard enthalpy of formation(ΔfHmө) of 1 can be derived as being (2165.99 ± 0.81) kJ·mol−1by using the Hess law and the Eq. 11.

3.6 Detonation properties
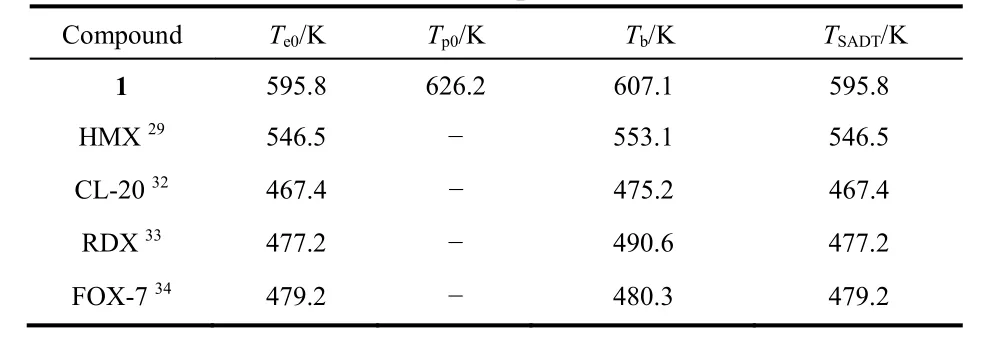
Table 4 The thermal safety parameters of 1 and some common explosives.
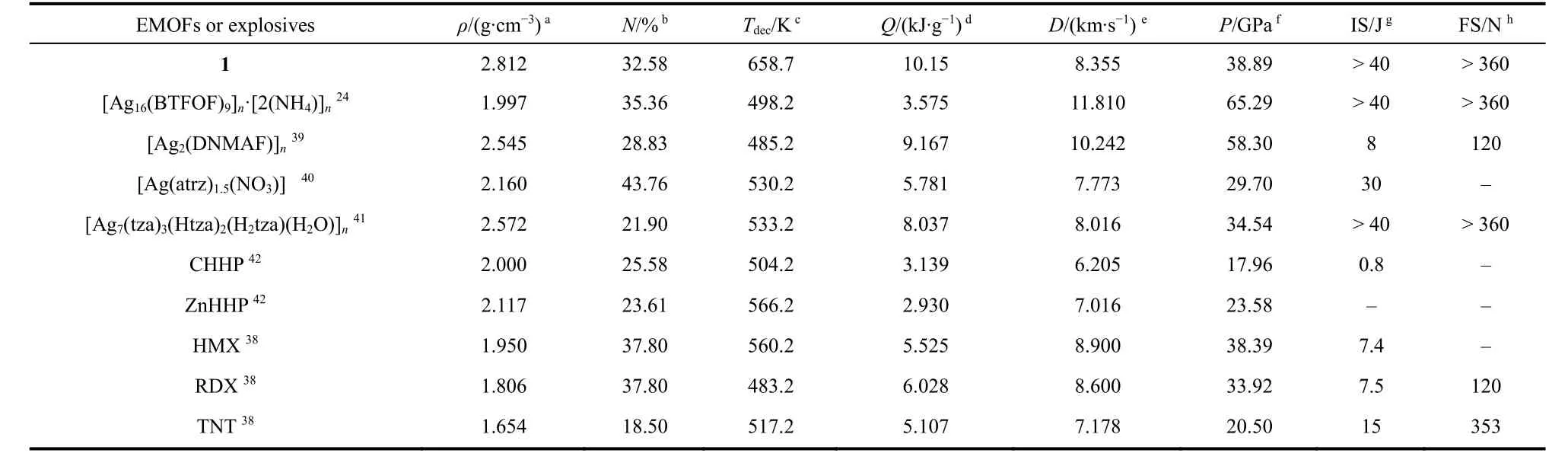
Table 5 Physicochemical properties of 1 and some reported energetic materials.
On the basis of the largest exothermic principle proposed by Kamlet-Jacobs36, an empirical method is employed to investigate the detonation properties of metal-containing explosives37. In such metal explosive systems, the most stable products of detonation reaction were assumed under the constraints of stoichiometrically available oxygen38. Therefore,for 1, nitrogen, carbon, and ammonia were assumed to be the final products of decomposition of the organic part of the framework and the formation of metallic state was assumed to be governed by the deficiency of oxygen36. The complete detonation reaction considered is described by Eq. 12, and the detonation properties are calculated by Kamlet-Jacobs Eqs. 13-16 as follows:

whereDis detonation velocity (km·s−1),Pis detonation pressure(GPa),Nis moles of detonation gases per gram of explosive,Mis average molecular weight of the gases,Qis heat of detonation(kJ·g−1),ρis density of explosive (g·cm−3). According to the known enthalpies of formation of NH3(g) (−46.00 kJ·mol−1) and AgC3HN5(s) (2165.99 kJ·mol−1), the heat of detonation of 1 can be calculated as 10.15 kJ·g−1. Based on the above Eqs. 13-15,the detonation velocity and detonation pressure can be further obtained and listed in Table 524,38-42. The results show that the EMOF 1 exhibits comparable detonation velocity and detonation pressure to TNT and much higher detonation heat than those of the common high explosives and some reported solvent-free Agbasd EMOFs.
3.7 Detonation properties
Impact sensitivity test of 1 was performed on the Fall Hammer Apparatus. 20 mg of 1 was compacted to a copper cap under the press of 39.2 MPa and hit by 2 kg drop hammer. The calculated value ofh50%represents the drop height of 50% initiation probability. The test results show that the EMOF 1 do not fire at the highest point of 200 cm (h50%) corresponding to an impact energy of 40 J, suggesting that 1 has much lower impact sensitivity than that of HMX (7.4 J) (Table 5). Meanwhile, the friction sensitivity of 1 was measured by applying a Julius Peter’s machine using 20 mg sample, and no friction sensitivity was observed up to 360 N. The results reveal that 1 is insensitive to external stimuli, which may be ascribed to the rigid skeleton structure.
4 Conclusions
In summary, a new solvent-free energetic MOF [Ag2(DTPZ)]n(1) was synthesized and structurally characterized. The richnitrogen multidentate ligand DTPZ2−adopting special torsion configuration bridges AgIcenters to form 3D framework, in which no solvent molecules coordinate the metal or occupy the free space of channel. The dense structure endow 1 with high thermostability (Tp= 658.7K) and good thermal safety (TSADT=595.8 K,Tb = 607.1 K). Thermal analysis tests show the typical explosive performance of EMOF 1 based on the abrupt one-step decomposition from TG curve and sharp heat release form DSC curve. The heat of detonation and safety of 1 (Q= 10.15 kJ·g−1,IS > 40 J and FS > 360 N) are superior to those of traditional energetic materials (HMX, RDX and TNT) and many reported Ag(I)-EMOFs, indicating that 1 is a promising insensitivity HEDM and can be applied to explosives and propellants.
Supporting Information: available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
