Dissecting the underlying pharmaceutical mechanism of Danggui Buxue decoction acting on idiopathic pulmonary fibrosis with network pharmacology
2020-06-29CaiPingZhaoHangLiXiaoHongLiuShuangLiangXueLeiLiuXinRongLiYiLuoMeiLingZhu
Cai-Ping Zhao, Hang Li, Xiao-Hong Liu, Shuang Liang, Xue-Lei Liu, Xin-Rong Li, Yi Luo, Mei-Ling Zhu*
Dissecting the underlying pharmaceutical mechanism of Danggui Buxue decoction acting on idiopathic pulmonary fibrosis with network pharmacology
Cai-Ping Zhao1#, Hang Li1#, Xiao-Hong Liu2, Shuang Liang1, Xue-Lei Liu1, Xin-Rong Li1, Yi Luo1, Mei-Ling Zhu1*
1Baoan Hospital of Traditional Chinese Medicine Affiliated to Guangzhou University of Traditional Chinese Medicine, Shenzhen 518133, China.2Guangzhou University of Chinese Medicine, Guangzhou 510006, China.
: Danggui Buxue decoction (DBD), a classical prescription in traditional Chinese medicine, has been found to have protective effect on bleomycin-induced pulmonary fibrosis in rats by reducing alveolar inflammation and fibrosis. However, the biological activity of individual chemical components and mechanism of action of whole formula are not clear.: Potential targets of active ingredients of DBD were collected through Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform and SymMap database. Target genes related to idiopathic pulmonary fibrosis were obtained from the Online Mendelian Inheritance in Man database, Therapeutic Targets Database and Gkb database. Then, the common targets were obtained by overlapping the potential targets of active ingredients in DBD and diseases related targets. The selected targets were subjected to Kyoto Encyclopedia of Genes and Genomes signaling pathway and Gene Ontology analysis, and the network map of active component-target-pathway was established using Cytoscape 3.7.1 software. The active components of DBD with most targets were selected for fibrosis-related marker verification. The mRNA and protein expression of fibrosis markers, α-smooth muscle actin, collagen 1 and fibronectin, were detected in TGF-β1-induced fibroblast cell line after treatment with the active components.: The 14 active ingredients, such as quercetin and kaempferol, were screened from DBD. It acts on 26 targets like estrogen receptor 2 and prostaglandin-endoperoxide synthase 2, and mainly involves 38 signaling pathways such as cell inflammation and autophagy. Kaempferol and quercetin are the two compounds with the highest network regulation, which can inhibit the transformation of fibroblasts into myofibroblasts and reduce the expression of fibrosis markers α-smooth muscle actin, collagen 1 and fibronectin.: The integration mode of multi-component, multi-target, multi-channel and mechanism of DBD in the treatment of idiopathic pulmonary fibrosis are predicted by means of network pharmacology. Our study could indicate the direction of further anti-fibrotic mechanism research.
Danggui Buxue decoction, Pulmonary fibrosis, Network pharmacology, Myofibroblast, Chinese medicine formula, Mechanism of action
Kaempferol and quercetin are found to be the two main compounds of classical prescription of traditional Chinese medicine named Danggui Buxue decoction with the highest network regulation, which can inhibit the transformation of fibroblasts into myofibroblasts and reduce the expression of fibrosis markers α-smooth muscle actin, collagen 1, fibronectin.
Danggui Buxue decoction (DBD), a classical prescription in traditional Chinese medicine composed of Huangqi () and Danggui () in a ratio of 5:1, originates from the Chinese medicine ancient book entitled Neiwai Shangbian Huolun written by the famous medical scientist Li Dongyuan in 1247 C.E. At present, there are many researchers who try to study the biological activity of individual chemical components in DBD and the mechanism of action of whole formula. Some studies have shown that DBD has effect on fibrosis of heart, liver and kidney and has protective effect on bleomycin-induced pulmonary fibrosis in rats by reducing alveolar inflammation and fibrosis.

Background
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, fibrotic interstitial lung disease that occurs in the middle-aged and elderly population. The disease is mostly sporadic. According to statistics, the prevalence rate in the whole population is about (2-29)/100,000 per year, and it is gradually increasing. As a chronic interstitial lung disease, IPF’s onset is concealed, and patients’ condition gradually becomes worsen. In addition, it often manifested as acute exacerbation. The average survival after IPF diagnosis is only 2.8 years [1], and the mortality rate is higher than most tumors, so it is called a tumor-like disease. Currently, the drugs the FDA recommends for IPF are only pirfenidone and nintedanib [2]. However, they are only effective in some patients and have some serious side effects. Therefore, there is still an urgent need to further develop new strategies for the treatment of this refractory respiratory disease.
In traditional Chinese medicine (TCM), IPF is classified into the category of Fei Bi or Fei Wei [3]. Its main clinical manifestations are dyspnea, dry cough and other symptoms, and eventually leading to respiratory failure [4-6]. At present, there are many experimental and clinical studies on the treatment of IPF with TCM therapy and in some ways, they have achieved good results. For example, studies have shown that the classical prescription of TCM named Renshen Pingfei decoction can reduce the degree of early lung injury and fibrosis and improve lung function in IPF model rats by down-regulating TGF-β1/Smad3 signaling pathway [7]. Wu Zhihuang shows that the prescription of experience Bufei Huoxue decoction has good curative effect on IPF, and it is safe and has few side effects [8]. However, the mechanism of action of TCM for treating pulmonary fibrosis is still at an exploratory stage.
Danggui Buxue decoction (DBD) is from the ancient book of Chinese medicine entitled Neiwai Shangbian Huolun, written by the famous medical scientist Li Dongyuan in 1247 C.E., composed of Huangqi () and Danggui () in a ratio of 5:1 [9]. Studies have shown that DBD has effect on fibrosis of heart, liver and kidney, which can alleviate peroxidative damage [10], reduce collagen content, improve capillary function [11], and reduce the degree of fibrosis [12]. The study also found that DBD acted on bleomycin-induced pulmonary fibrosis in rats, which could significantly reduce alveolar inflammation and fibrosis, and had protective effect on pulmonary fibrosis rats [13, 14]. At present, there are many researchers studying the biological activity of individual chemical components in Huangqi () and Danggui (), but the target and mechanism of action of whole formula are not clear.
Network pharmacology is a new technology combines multi-disciplines, such as system biology, multi-directional pharmacology, and network analysis. By constructing an interactive network such as disease-target-component, it uses professional software to analyze the association between disease-protein targets, protein targets-drugs, and then systematically reveals the interaction and mechanism between drugs and organisms. The holistic and multi-component, multi-channel and multi-target characteristics of TCM are consistent with the network pharmacology characteristics [15]. In summary, this study used network pharmacology to study and explore the mechanism of action of DBD in the treatment of IPF by constructing an effective network between the active constituents of DBD and IPF related targets.
Materials and methods
The active ingredients screening and target prediction of DBD
DBD consists of two Chinese herbal medicines-Huangqi () and Danggui (). The compounds of these two Chinese herbal medicines were collected using the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) (http://lsp.nwu.edu.cn/tcmsp.php) and the SymMap database (http://www.symmap.org/). The pharmacokinetic properties of the drug are collected, including oral bioavailability (OB), intestinal epithelial permeability, blood brain barrier, and water solubility. OB > 30% and drug-like (DL) > 0.18 were used as indicators for screening compounds. There are 14 active ingredients in DBD showed good bioavailability and DL properties through oral availability and DL analysis. OB is the fraction of an oral administered drug that reaches systemic circulation. This is essential for determining whether a chemical component of TCM has pharmacological activity. In the early development stage of the drug, accurate evaluation of the compound’s DL can help to screen out the excellent compounds and improve the hit rate of drug candidates. Compounds with a DL greater than 0.18 are considered to have higher DL. Therefore, bioavailability and DL are selected to screen for candidate components. The target proteins corresponding to the candidate components were searched using the TCMSP, Therapeutic Targets Database (TTD) (https://db.idrblab.org/ttd/), Gkb (https://www.pharmgkb.org/) and Drug Bank (https://www.drugbank.ca/) database. The gene name of the target protein is queried by the Unitprot database (http://www.Unitprot.org/).
IPF target collection and target interaction
The IPF disease-associated phenotype was searched by querying the Online Mendelian Inheritance in Man (OMIM) (https://www.omim.org/) database, and disease-related proteins and gene targets were collected using OMIM, TTD, and Drug Bank databases and literature research. The DBD and IPF targets were imported into the Bioinformatics & Evolutionary Genomics database for Venn map analysis, and the common targets in the two sets of data were selected as potential targets.
Construction of compound-target-pathway network
In order to reflect the relationship between drug molecules and diseases, this study used the Database for Annotation,Visualization and Integrated Discovery (DAVID) (https://david.ncifcrf.gov/) to analyze the potential targets of Kyoto Encyclopedia of Genes and Genomes (KEGG) signaling pathways and screen out the signal pathways with higher target numbers. Targets, compounds, and pathways are used as nodes in the network, and the interaction between the two is the edge of the network. The Cytoscape 3.7.1 software is introduced to construct a compound-target-pathway network. At the same time, the network analyzer plug-in is used for network interaction analysis, and the degree of each target protein are calculated. Functional characterization and Gene Ontology (GO) enrichment analysis of target proteins were performed using the DAVID database. Thevalue reflects the significance of the biological function of the protein. The specific operation process is shown in Figure 1. By constructing a network to study the multi-component, multi-target and multi-channel modes of DBD, the mechanism of DBD acting on IPF can be understood from different perspectives, which can provide reference for Chinese medicine therapy of IPF.

Figure 1 Danggui Buxue decoction acts on IPF network pharmacology flow chart
Note: DBD,Danggui Buxue decoction; IPF,Idiopathic pulmonary fibrosis; TCMSP, Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform; OMIM, Online Mendelian Inheritance in Man; TTD, Therapeutic Targets Database; PCR, Polymerase chain reaction; WB, Western blot; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Experiment
Reagents and instruments. Mouse embryonic fibroblasts (NIH/3T3) established by the National Institutes of Health were purchased from Shanghai Academy of TCM and routinely cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 1% streptomycin/penicillin in a 5% carbon dioxide, 37 °C incubator.
Quercetin (B20527) and kaempferol (B21126) were purchased from Yuanye (Shanghai, China), TGF-β1 (7666-MB-005) was purchased from R&D (Minneapolis, MN, USA), MTS (G3581) was purchased from Promega (Madison, WI, USA), RNA extraction kit (DP419) was purchased from Tiangen (Beijing, China), RNA reverse transcription kit (AT341) and qPCR kit (AQ601) were purchased from Quanshijin (Beijing, China), rabbit-derived collagen1 antibody (84336) and horseradish peroxidase-labeled secondary antibody (7074) were purchased from CST (Danvers, MA, USA), rabbit-derived fibronectin antibody (ab32419) was purchased from Abcam (Cambridge, MA, USA). Multi-function microplate reader was purchased from Biotek (Biotek Winooski, Vermont, USA), Bio-Rad vertical electrophoresis system and T100 Thermal cycler gradient PCR instrument were purchased from Bio-Rad (Hercules, CA, USA), Nanodrop One nucleic acid quantitation instrument was purchased from Thermo (Waltham, MA, USA), LC 480II fluorescence quantitative PCR instrument was purchased from Roche (Basel, Switzerland), FluorChem E chemiluminescence gel imager was purchased from ProteinSimple (Silicon Valley, CA, USA).
Effect of quercetin and kaempferol on cell viability of NIH/3T3 cell line by MTS assay. NIH/3T3 cells were seeded in 96-well plates at a concentration of 1.5 × 104cells/well, and cultured overnight at 37°C. The medium was removed, and 100 μL/well of the medium containing different concentrations of drugs was added to treat the cells, and the medium was used as a control well. The cells were cultured in a 5% CO2, 37°C incubator for 24 h. The MTS solution was added, and the mixture was incubated at 37°C for 1 h in the dark, and the absorbance was measured by a multi-function microplate reader.
Detection of mRNA expression levels of α-smooth muscle actin, collagen1 and fibronectin in fibroblasts induced by TGF-β1 by RT-PCR. The relative expression of α-smooth muscle actin (α-SMA), collagen1 and fibronectin mRNA was determined by Real-time PCR with GAPDH as an internal reference. NIH/3T3 cells were seeded in six-well plates and incubated in a constant temperature incubator overnight. Quercetin and kaempferol at a final concentration of 0, 5, 10, and 20 μM/L were added to each well. Normal control group was also established. After 2 h, 5ng/mL of TGF-β1 was added to each well and culture was continued for 12 h. The mRNA was extracted: the cells were washed twice with pre-cooled PBS, the lysate was added, and the sample was beaten several times until the solution was transparent. Then 200 μL of chloroform was added and shaken vigorously for 15 secs. Centrifuged at 12,000 rpm for 10 min at 4°C, transferred the aqueous phase to a new tube, slowly added 0.5 volumes of absolute ethanol, and mixed. Centrifuged at 12,000 rpm at 4°C for 30 secs and discarded the waste. Five hundred μL of deproteinized liquid RD was added to the adsorption column CR3, and the waste liquid was centrifuged. Five hundred μL of the rinse liquid RW was added to the adsorption column CR3, and the waste liquid was centrifuged, and the residual liquid was removed once. Add 30 μL of RNase-Free ddH2O, let stand for 2 min at room temperature, and centrifuged at 12,000 rpm for 2 min at 4°C to obtain total mRNA. The mRNA was quantified using a nucleic acid quantitation instrument, and then mRNA, gDNA Remover, H2O, and All-in-One No-RT Control SuperMix were mixed into a 20 μL system, incubated at 42°C for 15 min, and incubated at 85°C for 5 secs to obtain cDNA. The Top Green qPCR SuperMix, template, primer and water were mixed into a 20 μL system, and qPCR amplification was performed to obtain a sample Ct value. The primer sequences are shown in Table 1. The sample Ct value was analyzed and quantified by the 2-ΔΔCTmethod in relative quantification.
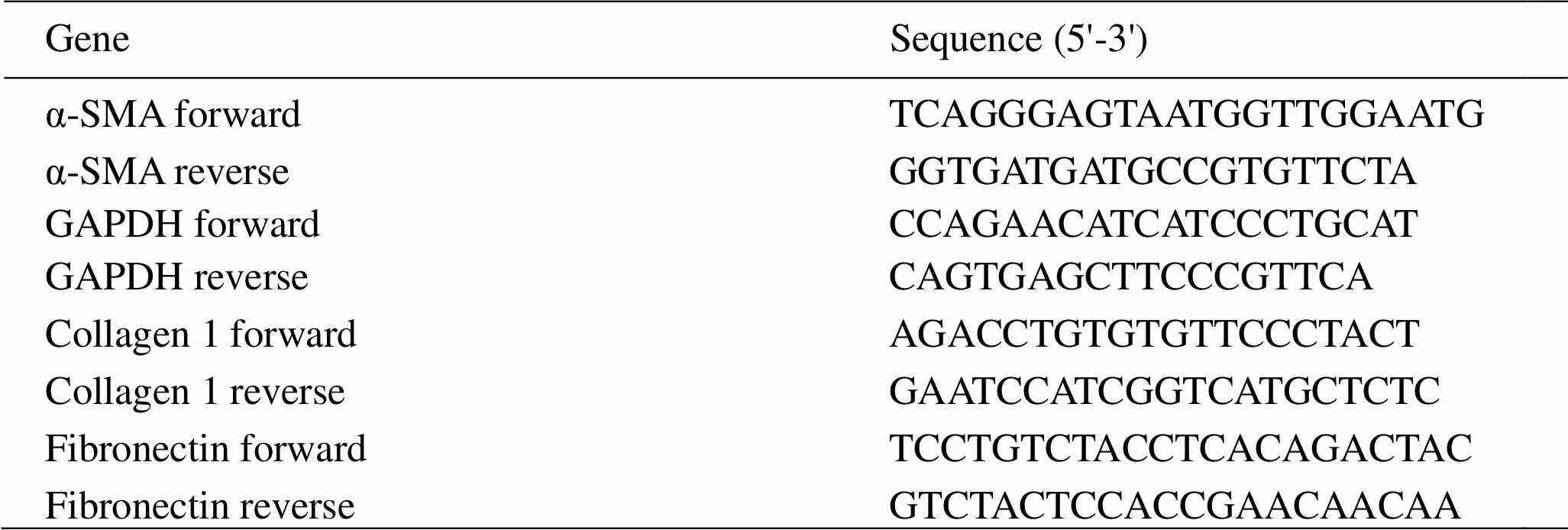
Table 1 Primer sequence
Detection of TGF-β1-induced expression of α-SMA, collagen 1 and fibronectin in fibroblasts by western blot. Western blot was used to detect the effects of quercetin and kaempferol on lung fibrosis-associated proteins induced by TGF-β1 in NIH/3T3 cells were examined. Logarithmic growth phase cells were seeded in six-well plates, and the cells were incubated overnight for adherent administration. The drug groups were divided into different concentrations: 0, 5, 10, 20 μM/L. At the same time, a normal control group was established. After 2 hours, 5ng/mL of TGF-β1 was administered and cultured for 24 hours to induce fibrosis. Total cellular protein was extracted. The quantification and denaturation of protein were performed. An 8% SDS-PAGE gel was prepared, and the sample was electrophoresed and transferred to a PVDF membrane. The 5% skim milk powder was used to blocked protein for 2 h. The membranes were exposed to primary antibody: anti-fibronectin, anti-collagen1, anti-α-SMA antibodies at dilutions of 1:1000 respectively, and incubated overnight at 4°C. This was followed by incubation with the secondary antibody for 2 h at room temperature. The strip was developed using a chemiluminometer. The protein band was analyzed, and the relative expression amount was calculated from the ratio of the gray value of the target protein to the gray value of the internal reference Tubulin.
Statistical analysis. Data analysis was performed using SPSS 20.0 statistical software. The data were expressed as mean ± standard deviation (`± s). The comparison between the data of multiple groups was analyzed by one-way ANOVA.< 0.05 was considered statistically significant.
Result
Collection of active ingredients in Danggui Buxue decoction and predicted targets
Through the search of above databases, DBD collected a total of 212 active ingredients, of which 125 active ingredients related to Danggui () and 87 active ingredients related to Huangqi ().AfterOBandDLscreening,22 active ingredients were obtained. The data in Table 2 show the names of the active ingredients, OB values and DL values. The target proteins corresponding to the screened compounds were obtained and a total of 354 active component’s targets were collected. Table 3 shows the targets number of nodes ≥ 6, including Uniprot number, target name, gene name, number of nodes.

Table 2 The active ingredients of Danggui Buxue decoction after screening
TCMSP, Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform;OB, Oral bioavailability;DL, Drug-like.

Table 3 Part of the target of Danggui Buxue decoction component (nodes ≥ 6)

Figure 2 Compound-target-pathway network
Note: The protein is a blue diamond frame, the active ingredient is a red circular frame, and the pathway is a yellow square frame. The more nodes, the larger the font.
Target collection and potential target prediction of IPF disease
The 5,980 IPF-related phenotypes and targets were obtained by database search, and 401 disease-related targets were obtained after combined descreening. After mapping 401 targets of IPF and 354 targets of DBD by Venn, the common potential targets numbers were 26.
Analysis of compound-target-pathway network
Fourteen active components, 26 target proteins, and signal pathways with higher nodes are shown on the compound-target-pathway network map, as shown in Figure 2. The proteins, active components and pathways with a large number of nodes are shown as prominent fonts. Use different shapes to represent different nodes. The protein is a diamond-shaped frame, the active component is a circular frame, and the passage is a square frame. The use of different colors and shapes to process the image indicates the corresponding effects of multiple active components, multiple targets and multiple channels. Ten targets are shown in Table 4 including numbers, abbreviations, degrees and mediators. The active ingredient information is shown in Table 5.
Pathway analysis and GO analysis
The above 26 targets were subjected to KEGG pathway enrichment analysis and GO analysis using the DAVID database. Through the KEGG pathway enrichment analysis, a total of 12 signaling pathways were obtained (< 0.05). Among them, the phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway [16], the mitogen-activated protein kinase (MAPK) signaling pathway [17], the HIF-1 signaling pathway [18], the T cell receptor signaling pathway [19], and the tumor necrosis factor (TNF) signaling pathway [20], Ras signaling pathway [21], FoxO signaling pathway [22], Toll-like receptor signaling pathway [23], TGF-β signaling pathway [24] are closely related with pulmonary fibrotic inflammatory immune response, angiogenesis, apoptosis muscle fibroblast migration and phenotypic transformation, cell matrix metabolism abnormalities and other aspects. Through the GO analysis, biological process, cellular compenent and molecular function were obtained. In the chart of biological process categories, response to stimulus and biological regulation account for 24 genes. The extracellular space, membrane and endomembrane system are the first three factors in the chart of cellular compenent categories. In the chart of molecular function categories, the gene numbers of protein linding and iron binding are 23 and 12, respectively. This suggests that DBD can prevent and treat pulmonary fibrosis by improving the above aspects. The number and percentage of pathway targets andvalues are shown in Table 6. A compound-pathway network map is drawn for the active components of DBD and the predicted signaling pathways, see Figure 3. The GO analysis result is shown in Figure 4. Kaempferol and quercetin are the active constituents of Huangqi () and Danggui (), respectively. They have the highest number of nodes in the compound-target-pathway network, indicating that they may be the main component of DBD. Then the next step is to verify the drug's effectiveness through a myofibroblast model to verify the accuracy of the network.
Verification of the effects of quercetin and kaempferol on myofibroblasts
Effect of quercetin and kaempferol on cell viability of NIH/3T3 cell line. The results showed that there is no significant change in cell viability in the quercetin and kaempferol treatment groups compared with the normal control group, and there is no statistical difference(Figure 5). It indicates that the concentration used for quercetin and kaempferol have no toxic effects on cells.
Effects of quercetin and kaempferol on the mRNA expression of α-SMA, collagen1 and fibronectin induced by TGF-β1 in fibroblasts. The levels of α-SMA, collagen1 and fibronectin mRNA in the TGF-β1 group are significantly higher than those in the normal group. Compared with the model group, kaempferol and quercetin can significantly inhibit the increase of α-SMA, collagen1 and fibronectin mRNA levels in NIH/3T3 cells. The mRNA levels of α-SMA and collagen1 are dose-dependent in kaempferol groups, and the levels of α-SMA, collagen1 and fibronectin mRNA of quercetin groups are dose-dependent, as shown in Figure 6.
Effects of quercetin and kaempferol on TGF-β1-induced protein expression of collagen-1 and fibronectin in fibroblasts. Compared with the normal group, the levels of collagen1 and fibronectin protein in the TGF-β1 group are significantly higher. Compared with the TGF-β1 group, medium (10 μM/L) and high (20 μM/L) dose of kaempferol reduce the increased collagen1 and fibronectin expression induced by TGF-β1, as shown in Figure 7A. Different doses of quercetin reduce collagen1 and fibronectin protein expression, as shown in Figure 7B.

Table 4 Network and mediators of ten targets
Note: PTGS2, Prostaglandin-endoperoxide synthase 2; PRSS1, Recombinant protease, serine 1; AR, Androgen receptor; ESR2, Estrogen receptor 2; SCN5A,Sodium voltage-gated channel alpha subunit 5; AHSA1,Activator of HSP90 ATPase activity 1; CYP3A4, Cytochrome P450 family 3 subfamily a member 4; GSTM1, Glutathione s-transferase, MU-1; GSTP1, Glutathione s-transferase, P1.

Table 5 The node and median of Danggui Buxue decoction active ingredients in the network
TCMSP, Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform.
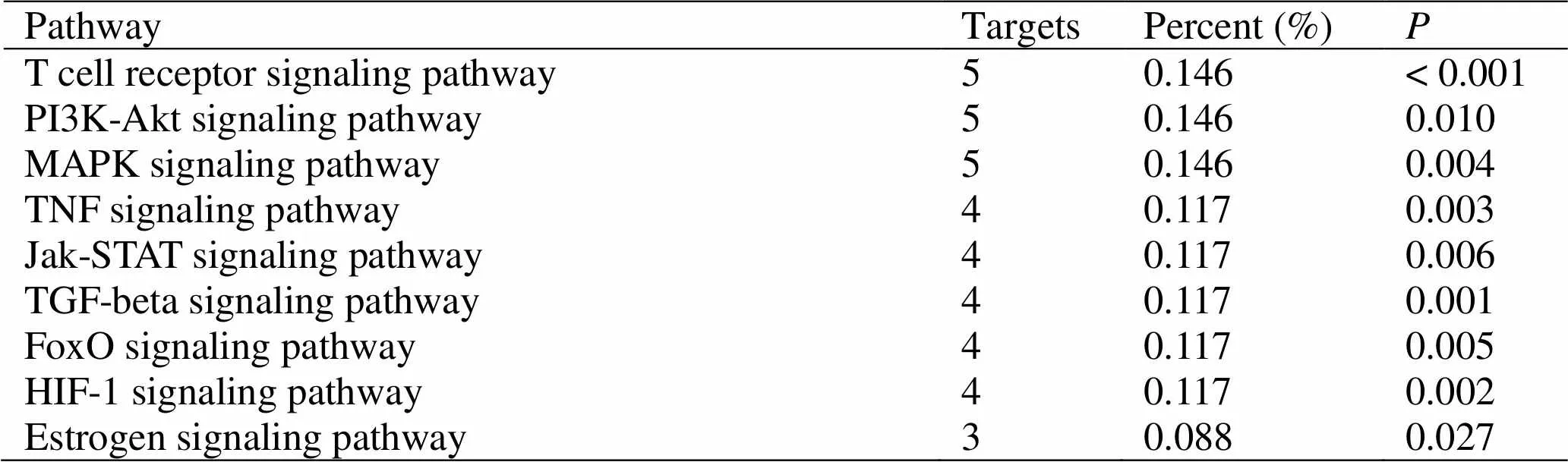
Table 6 KEGG metabolic pathway statistics of Danggui Buxue decoction target
MAPK, Mitogen-activated protein kinase; KEGG, Kyoto Encyclopedia of Genes and Genomes; PI3K, Phosphatidylinositol 3-kinase.

Figure 3 Compound-pathway network
Figure 4 Gene Ontology analysis

Figure 5 Effect of quercetin and kaempferol on cell viability of NIH/3T3 cell line
Figure 6 Effect of quercetin and kaempferol on mRNA of α-SMA and collagen 1 (`± s, n = 3)
Note: Compared with the normal control group,a< 0.01,b< 0.001; compared with the model group,c< 0.05,d< 0.01,e< 0.001.
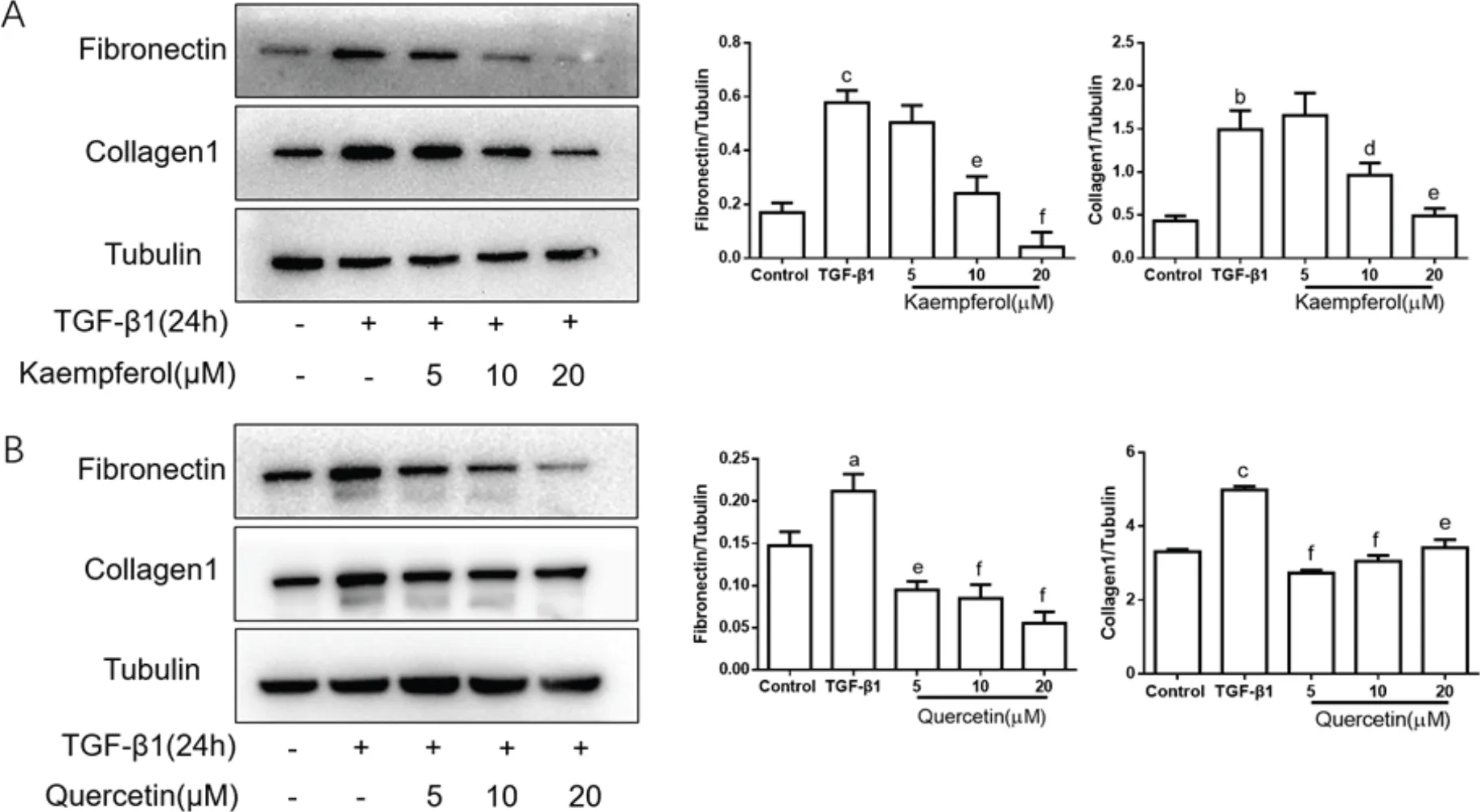
Figure 7 Effect of quercetin and kaempferol on protein of collagen-1 and fibronectin (`± s, n = 3)
Note: Compared with the normal control group,a< 0.05,b< 0.01,c< 0.001; compared with the model group,d< 0.05,e< 0.01,f< 0.001.
Discussion
The cause of IPF is unknown, and its pathogenesis is complicated. TCM has the characteristics of multi-component and multi-target, and it has unique advantages by forming the component-target network to treat pulmonary fibrosis. Finding effective anti-pulmonary fibrosis herbal medicine, formula or small molecule compounds from TCM is of great significance for improving the treatment of IPF [25]. In this study, 14 active ingredients and their corresponding 354 targets were screened from the Chinese medicine classical prescription DBD using a network pharmacology tool. At the same time, 401 disease-related targets were screened from the human disease database, and 26 pairs of active components-targets were determined after target interaction. After functional KEGG, and GO analysis, a compound-target-pathway network was constructed. Then, the active compounds with higher nodes were tested by fibrosis related indicators. Further clarify the mechanism of DBD in the treatment of IPF.
To be sure, candidate compounds with a higher degree and centrality are very important in DBD. In Table 2, 7 of the 22 key compounds are known active compounds. For example, formononetin is an effective component of DBD, which can be used to reduce related indicators such as pulmonary edema and lung cancer [26]. β-sitosterol has been shown to act on lung inflammation and edema through the NF-κB pathway, thereby alleviating acute lung injury [27]. It also includes two well-recognized molecules with high bioactivity-kaempferol and quercetin. Studies have shown that kaempferol and quercetin have anti-infection, anti-inflammation and anti-cancer effects. However, the main components of Huangqi (), such as astragaloside, have relatively low oral availability. It may be caused by four reasons: consumption before entering the organism’s circulation, metabolism of the gastrointestinal tract, strong efflux and low membrane permeability. Among these reasons, membrane permeability may be a key factor in determining the entry of drugs into the organism. In addition, in future research, we should pay attention to the compounds of DBD, such as hederagenin and isolaflavanone, which may be potential new drugs for the treatment of pulmonary fibrosis.
Through network analysis, it was found that the top three candidate active molecules were kaempferol, quercetin and fordononetin, and the top three targets were prostaglandin-endoperoxide synthase 2 (PTGS2), recombinant protease, serine 1 (PRSS1) and androgen receptor. These active ingredients and targets may play a key role in the decoction. The targets of the active ingredients in DBD are participate in signal transduction, anti-apoptosis, inflammatory reaction and other processes, and undergoes molecular reactions such as protein binding, zinc ion binding and ATP binding, and exerts its pharmacological action in the nuclear, plasma membrane and cytoplasmic parts. In addition, there are 26 common targets for DBD and IPF. Although the number of common targets is small, it can also play a role in pulmonary fibrosis. PTGS2 is a key regulatory enzyme in the synthesis of prostaglandin E2 (PGE2) and prostaglandin D2 [28]. Since PGE2 can inhibit fibroblast proliferation, collagen synthesis, migration and differentiation into myofibroblasts and induce fibroblast apoptosis, it is considered to be an anti-fibrotic molecule [29]. Fibroblast studies from IPF lungs showed a relative deficiency production and reduced reactivity in PGE2. Several studies have shown that bleomycin-induced fibrosis is enhanced when PTGS2-derived PGE2 is low or absent [30, 31]. Furthermore, downregulating the expression of TGF-β1 in fibrotic tissue is a major cause of ESR mitigation. Estrogen can significantly inhibit cell proliferation and activation, reduce the number of ER significantly, decrease the secretion of TGF-β1, interrupt its own positive feedback, and reduce fibrosis [32].
According to the compound-target-pathway network of this study, DBD activates PI3K/Akt and MAPK pathways mainly through targets such as TGF-β1, MMP2 and TNF. In addition, it acts on lipid metabolism, immune and inflammatory reactions, and fibrosis protection to treat IPF. It is basically consistent with the possible mechanism of the effects of Danggui () and Huangqi () on IPF. In this process, the TGF-β/Smads signaling pathway is activated to regulate the transcription and expression of downstream connective tissue growth factor and other genes. Furthermore, extracellular matrix and epithelial-mesenchymal transition are induced to accelerate the oxidation reaction, and cytokine imbalance is promoted. The other signaling pathways such as MAPK and extracellular regulated protein kinases synergistically affect cell growth, signal transduction, extracellular matrix and negative feedback systems and then causing IPF. As studies have shown that Huangqi () can reduce the activation of TGF-β1/Smad [33], reduce the expression of MMP2 [34], inhibit epithelial-mesenchymal transition, delay or even inhibit the occurrence of pulmonary fibrosis. It has also been confirmed by experiments that both Danggui () and Huangqi () membranaceus can correct the abnormality of extracellular matrix metabolism and act on peroxide to prevent and treat pulmonary fibrosis by regulating the expression of TNF-α and free radical levels. Huangqi () also has an effect on the activation of related pathways. For example, astragaloside can significantly inhibit the activation of TGF-β1/PI3K/Akt [35] and MAPK [6] pathways during fibrosis, reverse epithelial-mesenchymal transition, and alleviate pulmonary fibrosis. This is consistent with the results of this study.
At present, the pathogenesis of pulmonary fibrosis is still unclear. The hypotheses have inflammation, abnormal angiogenesis, apoptosis and so on. Abnormal repair after cell injury plays a decisive role in the pathogenesis of IPF. The basis of pulmonary fibrosis is the synthesis and deposition of collagen. TGF-β1 is a key factor in promoting myofibroblast formation and subsequent collagen production. The α-SMA is an important molecule for various types of cell movement and cytoskeleton maintenance, and it can be inhibited by fibrotic drugs. And myofibroblasts characterized by the expression of α-SMA are the main coordinators of fibrosis in organs such as lung and liver [36]. Myofibroblasts produce large amounts of extracellular matrix leading to pulmonary fibrosis. TGF-β also mediates the synthesis, secretion and assembly of type I collagen corresponding to fibroblast transdifferentiation, which can affect the expression of collagen and fibronectin [37, 38]. This study also further showed that TGF-β1 can stimulate the occurrence of pulmonary fibrosis, and the expression of α-SMA, collagen1 and fibronectin decreased after medicament.
The transformation of fibroblasts into myofibroblasts leads to the formation of pulmonary scarring, which is one of the main mechanisms of pulmonary fibrosis [39]. In the compound-target-pathway network we constructed, kaempferol and quercetin accounted for the highest degree and median, indicating that they may be the main component of DBD against fibrosis. We validated the efficacy of the network and drugs through myofibroblast model. The results showed that both quercetin and kaempferol could inhibit the activation of myofibroblast cells induced by TGF-β1. They reduced the mRNA and protein expression of fibrosis-related markers such as α-SMA. This is consistent with previous studies. Previous studies have shown that quercetin can significantly inhibit TGF-β, reduce lipid peroxidation by activating NF-κB and MAPK pathways [40], reverse epithelial-mesenchymal transition, and inhibit collagen deposition acts on the fibrosis process [41]. Kaempferol can improve pulmonary fibrosis by inhibiting the expression of MMP-1 and tissue inhibitor of metalloproteinase 1, reversing epithelial-mesenchymal transition [42, 43]. In this study, only molecules with higher oral availability were selected, and some potential active molecules may be missed. Therefore, further in-depth studies can refer to the Angelica and Huangqi () fractions in TCMSP, which contain important pharmacokinetic information within all intact compound molecules, including all oral bioavailability values.
Conclusion
In summary, this study used network pharmacology to explore the mechanism of DBD in the treatment of IPF and explained the role of DBD in the treatment of multi-component, multi-target and multi-channel pulmonary fibrosis. The experimental verification of the active ingredients was carried out, which provided a research basis for further exploring the pharmacological mechanism of DBD.
1. Ballester B, Milara J, Cortijo J. Idiopathic Pulmonary Fibrosis and Lung Cancer: Mechanisms and Molecular Targets. Int J Mol Sci 2019, 20: E593.
2. Raghu G, Rochwerg B, Zhang Y, et alAn Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med 2015,192: e3–19.
3. Li LC, Kan LD. Traditional Chinese medicine for pulmonary fibrosis therapy: Progress and future prospects. J ethnopharmacol 2017, 198: 45–63.
4. Guo SF. Application of Traditional Chinese Medicine Therapy in the Treatment of Idiopathic Pulmonary Fibrosis. Chin J Inform Tradit Chin Med 2008, 15: 90–91. (Chinese)
5. Chen MG. Application of Bufei Yishen Method in the Treatment of Chronic Respiratory Failure Caused by IPF. Chin J Exp Tradit Med Formulae 2012, 18: 327–329. (Chinese)
6. Xu WJ, Shao XH, Xu XP, et al. Astragaloside IV attenuates renal tubulointerstitial fibrosis by inhibiting p38 MAPK signaling. Chin J Nephrol 2014, 30: 770–776.
7. Chen F, Wang PL, Fan XS, et alEffect of Renshen Pingfei Decoction, a traditional Chinese prescription, on IPF induced by Bleomycin in rats and regulation of TGF-β1/Smad3. J Ethnopharmacol 2016, 186: 289–297.
8. Wu ZH, Zhang XX. Clinical Observation of Bufei Huoxue Decoction in Treating Idiopathic Pulmonary Fibrosis. Beijing J Tradit Chin Med 2010, 29: 118–120. (Chinese)
9. Liu YQ. Danggui Buxue Tang. J Beneficial Readines Drug Inf Med Adv 2015: 50. (Chinese)
10. Chen Y, Tao YY, Li FH. Effects of Danggui Buxue Decoction on liver fibrosis and hepatic lipid peroxidation in rats. Chin J Integr Tradit West Med 2008, 28: 39–42. (Chinese)
11. Guo T. Anti-liver fibrosis effect of Danggui Buxue Decoction and its disassembled. In: proceedings of the National Conference on Integrative Digestive System Diseases, 2013.
12. Wei MG, He WM, Liu W, et al. Experimental study on anti-kidney fibrosis of Jiawei Danggui Buxue Decoction. Chin J Basic Med Tradit Chin Med 2014: 904–908. (Chinese)
13. Zhao P, Zhou WC, Li DL, et alTotal glucosides of Danggui Buxue Tang attenuate BLM-induced pulmonary fibrosis via regulating oxidative stress by inhibiting NOX4. Oxid Med Cell Longev 2015, 2015: 645814.
14. Liu Y Li J, Gao J. Experimental Study of Danggui Buxue Decoction on Rat Pulmonary Fibrosis. Acta Univer Medicinalis Anhui 2009: 594–598. (Chinese)
15. Zhang YQ, Li X. Some advances in network pharmacology and modern research of traditional Chinese medicine. Chin J Pharmacol Toxicol 2015, 29: 883–892. (Chinese)
16. Zhang L, Li Y, Liang C, et alCCN5 overexpression inhibits profibrotic phenotypes via the PI3K/Akt signaling pathway in lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis and in an in vivo model of lung fibrosis. Int J Mol Med 2013 ,33: 478–486.
17. Antoniou KM, Margaritopoulos GA, Giannoula S, et alExpression analysis of Akt and MAPK signaling pathways in lung tissue of patients with idiopathic pulmonary fibrosis (IPF). J Recept Signal Transduct Res 2010, 30: 262–269.
18. Gibbons MA, Fitch PM, Macduff A, et alS124 Macrophage deletion of vHL results in alternative activation and enhanced lung fibrosis independent of HIF-1. Thorax 2010, 65: A57.
19. Simonian PL, Roark CL, Diaz del Valle F, et alRegulatory role of gammadelta T cells in the recruitment of CD4+ and CD8+ T cells to lung and subsequent pulmonary fibrosis. J Immunol 2006, 177: 4436–4443.
20. Epstein SG, Brook E, Israeli-shani L, et alFibroblast paracrine TNF-α signaling elevates integrin A5 expression in idiopathic pulmonary fibrosis (IPF). Respir Res 2017,18:122.
21. Watanabe-Takano H, Takano K, Hatano M, et alDA-Raf-Mediated Suppression of the Ras-ERK Pathway Is Essential for TGF-β1-Induced Epithelial-Mesenchymal Transition in Alveolar Epithelial Type 2 Cells. PloS One 2015, 10: e0127888.
22. Nho RS, Im J, Ho YY, et alMicroRNA-96 inhibits FoxO3a function in IPF fibroblasts on type I collagen matrix. Am J Physiol Lung Cell Mol Physiol 2014, 307: 632–642.
23. Ahmed S, Moawad M, Elhefny R, et alIs toll like receptor 4 a common pathway hypothesis for development of lung cancer and idiopathic pulmonary fibrosis? Egypt J Chest Dis Tuberc 2016, 65: 289–294.
24. Saito S, Zhuang Y, Shan B, et alTubastatin ameliorates pulmonary fibrosis by targeting the TGFβ-PI3K-Akt pathway. PloS One 2017, 12: e0186615.
25. Huang H, Peng X, Zhong C. Idiopathic pulmonary fibrosis: The current status of its epidemiology, diagnosis, and treatment in China. Intractable Rare Dis Res 2013, 2: 88–93.
26. Li ZQ Meng XJ. Effects of formononetin on proliferation and apoptosis of human non-small cell lung cancer cells and related mechanisms. Chin J Exp Tradit Med Formulae 2016: 142. (Chinese)
27. Yao F, Zhou QY, Xiong Y. et al. Protective effect of β-sitosterol on lipopolysaccharide-induced acute lung injury in mice. Chin Agricult Sci Bull 2015, 31: 55–61. (Chinese)
28. Smith WL, Langenbach R. Why there are two cyclooxygenase isozymes. J Clin Invest 2001, 107: 1491–1495.
29. Huang SK, White ES, Wettlaufer SH, et alProstaglandin E2 induces fibroblast apoptosis by modulating multiple survival pathways. FASEB J 2009, 23: 4317–4326.
30. Liu F, Mih JD, Shea BS, et alFeedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 2010, 190: 693–706.
31. Hartney JM, Coggins KG, Tilley SL, et alProstaglandin E2 protects lower airways against bronchoconstriction. Am J Physiol Lung Cell Mol Physiol 2006, 290: L105–113.
32. Wang AX Yan XL, Zhao NJ, et alPathological study on the protective effect of sex hormones on acute lung injury and pulmonary fibrosis caused by paraquat poisoning. Chin J Lung Diseases (Electronic Edition), 2013, 6: 20–23. (Chinese)
33. Xu CJ, Wang PF, Huang YW, et alIntervention of total flavonoids of Astragalus membranaceus on miRNA-21, let-7 d and TGF-β/smad signaling in idiopathic pulmonary fibrosis. Chin Archives Tradit Chin Med 2018: 1308–1311. (Chinese)
34. Xin JB Xiang F, Wang SZ, et al. Effects of Astragalus on the expression of MMP-2 and TIMP-1 in rats with pulmonary fibrosis. In: proceedings of the National Academic Conference on Integrated Traditional Chinese and Western Medicine Respiratory Diseases, 2004. (Chinese)
35. Qian W, Cai X, Qian Q, et alAstragaloside IV modulates TGF-β1-dependent epithelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. J Cell Mol Med 2018, 22: 4354–4365.
36. Pechkovsky DV, Hackett TL, An SS, et alHuman lung parenchyma but not proximal bronchi produces fibroblasts with enhanced TGF-beta signaling and alpha-SMA expression. Am J Respir Cell Mol Biol 2010, 43: 641–651.
37. Petrov VV, Fagard RH, Lijnen PJ. Stimulation of collagen production by TGF-beta1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension 2002, 39: 258–263.
38. Hocevar BA, Brown TL, Howe PH. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J 1999, 18: 1345–1356.
39. Coward WR, Saini G, Jenkins G. The pathogenesis of idiopathic pulmonary fibrosis. Ther Adv Respir Dis 2010, 4: 367–388.
40. Biswajit P, Kyoung Seob S, Ho-Yeon S, et alCytoprotective effect of kaempferol on paraquat-exposed BEAS-2B cells via modulating expression of MUC5AC. Biol Pharm Bull 2014, 37: 1486–1494.
41. Gong JH, Cho IH, Shin D, et alInhibition of airway epithelial-to-mesenchymal transition and fibrosis by kaempferol in endotoxin-induced epithelial cells and ovalbumin-sensitized mice. Lab Invest 2014, 94: 297–308.
42. Wang CM, Huang H, Zhang ZX, et alThe protective effects of quercetin on Bleomycin-Induced pulmonary fibrosis in rats. Chin Pharm Bull 2000, 16: 94–96. (Chinese)
43. Zhang X, Cai Y, Zhang W, et alQuercetin ameliorates pulmonary fibrosis by inhibiting SphK1/S1P signaling. Biochem Cell Biol 2018, 96: 742–751.
:
IPF, Idiopathic pulmonary fibrosis;TCM, Traditional Chinese medicine;DBD, Danggui Buxue decoction;TCMSP, Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform; OB, Oral bioavailability;DL, Drug-like;TTD, Therapeutic Targets Database;OMIM, Online Mendelian Inheritance in Man;TNF, Tumor necrosis factor;PTGS2, Prostaglandin-endoperoxide synthase 2;PRSS1, Recombinant protease, serine 1; PGE2, Prostaglandin E2; MAPK, Mitogen-activated protein kinase; GO, Gene Ontology;DAVID, Database for Annotation, Visualization and Integrated Discovery;KEGG, Kyoto Encyclopedia of Genes and Genomes; PI3K, Phosphatidylinositol 3-kinase; α-SMA, α-Smooth muscle actin; AR: Androgen receptor.
:
The authors declare that there is no conflict of interest.
:
Cai-Ping Zhao, Hang Li, Xiao-Hong Liu, et al. Dissecting the underlying pharmaceutical mechanism of Danggui Buxue decoction acting on idiopathic pulmonary fibrosis with network pharmacology.Traditional Medicine Research 2019, 5 (4): 238–251.
:Cui-Hong Zhu, Mathew Goss.
:7 October 2019,
15 November 2019,
: 18 November 2019.
#Cai-Ping Zhao and Hang Li are the co-first authors of this paper.
Mei-Ling Zhu. Baoan Hospital of Traditional Chinese Medicine Affiliated to Guangzhou University of Traditional Chinese Medicine, No. 25, Yu'an Second Road, Xin'an Street, Bao'an District, Shenzhen 518133, Chine. E-mail: 1930896811@qq.com.
10.12032/TMR20191102146
杂志排行
Traditional Medicine Research的其它文章
- Marine natural products with anti-inflammation effects
- Acupuncture and/or moxibustion for the treatment of lumbar disc herniation: quality assessment of systematic reviews
- Can Yin-Chai-Xiao-Du decoction be useful of COVID-19? the mechanism research based on network pharmacology
- Efficacy of Xuebijing injection for the treatment of coronavirus disease 2019 via network pharmacology
- The selection rules of acupoints and meridians of traditional acupuncture for postoperative nausea and vomiting: a data mining-based literature study
- The advances of traditional Chinese medicine in the treatment of liver diseases in 2019
