Early treatment efficacy of S-adenosylmethionine in patients with intrahepatic cholestasis:A systematic review
2020-06-28
Mazen Noureddin,Division of Digestive and Liver Diseases,Comprehensive Transplant Center,Cedars-Sinai Medical Center,Los Angeles,CA 90048,United States
Suntje Sander-Struckmeier,Gastroenterology/Hepatology,Abbott Laboratories GmbH,Hannover 30173,Germany
José M Mato,CIC bioGUNE,Basque Research and Technology Alliance (BRTA),CIBERehd,Derio 48160,Bizkaia,Spain
Abstract
Key words:S-adenosylmethionine;Intrahepatic cholestasis;Chronic liver disease;Liver enzymes;Symptoms of cholestasis
INTRODUCTION
Intrahepatic cholestasis (IHC) is the impairment of bile formation or bile flow resulting from hepatocellular functional defects or obstructive lesions of the intrahepatic biliary tract[1].IHC is a feature of several chronic liver diseases including alcoholic liver disease (ALD),later stages of non-alcoholic fatty liver disease(NAFLD),drug-induced liver injury (DILI) and others[2,3].Chronic liver diseases are associated with substantial mortality and morbidity,and are a significant healthcare burden[1,4,5].
The clinical signs and symptoms of IHC include pruritus,jaundice,and fatigue,which may subsequently be associated with depression,autonomic dysfunction,and sleep disturbances[1].Liver enzymes such as alanine aminotransferase (ALT),aspartate aminotransferase (AST),alkaline phosphatase (ALP),and gamma-glutamyl transferase (γGT) may be elevated in patients with IHC,and changes in their levels are important for guiding diagnosis and assessing response to treatment[1,6].For example,ALP is an early marker that is often increased in asymptomatic patients,and levels 1.5 times the upper limit of normal have been proposed as a threshold for further diagnostic analysis[1].Indeed,improvement in ALP is considered a biomarker of disease response in cholestatic liver diseases[7-9].In addition,improvement in ALT has recently been proposed to predict treatment response in patients with NAFLD[10].Furthermore,a sustained > 10-fold rise in liver transaminases is indicative of a higher risk of mortality in patients with liver disease[6].
S-adenosylmethionine (AdoMet,also abbreviated as SAMe and SAM) is a molecule that participates in multiple cellular reactions (transmethylation,transsulfuration,and aminopropylation;Figure1),as the precursor for the synthesis of glutathione;it is the principal methyl donor in methyltransferase reactions that modify DNA,RNA,histones,and other proteins[11-14].The synthesis of AdoMet is known to be reduced in chronic liver diseases,and depletion of AdoMet promotes increased cellular proliferation and growth,which may be deleterious in chronic conditions[11,13-15].
Preclinical studies support a potential role for AdoMet in the treatment of chronic liver diseases[16-21].In mouse models of NAFLD,progression to the more severe nonalcoholic steatohepatitis (NASH) is associated with AdoMet depletion[18],while AdoMet supplementation reduces the severity of NASH and improves liver function[17,20].In a rat model of cholestasisviabile duct ligation,animals treated with AdoMet before ligation showed less oxidative stress and a reduced ratio of oxidized to total glutathione,as well as improvements in biochemical liver parameters[16].The molecular mechanisms by which AdoMet attenuates downregulation of glutathione synthetic enzymes and increases glutathione levels during bile duct ligation have recently been delineated,and they appear to involve the induction of nuclear factorerythroid 2related factor 2 and suppression of Maf proteins[19,21].
Pharmacokinetic studies in healthy volunteers have shown that AdoMet has a short terminal half-life [81 min following a 100 mg intravenous (iv) dose;101 min following a 500 mg iv dose] and is rapidly cleared (3.7 mL/min/kg and 3.1 mL/min/kg for 100 mg and 500 mg doses,respectively)[22].Time to maximum concentration (Tmax) is 3-5 h after single oral doses of 400-1000 mg,with concentrations declining to baseline levels within 24 h[13].This is in agreement with previous measurements of labile methyl balance in healthy volunteers,which indicated that the synthesis and catabolism of AdoMet was very rapid[23].Due to a significant first-pass effect[24],AdoMet is readily bioavailable in the liver,i.e.,the target organ.The rapid hepatic metabolism associated with oral administration means that the bioavailability of AdoMet is increased with parenteral administration[13].
A systematic review and meta-analysis of clinical studies assessing the efficacy and safety of AdoMet for the treatment of chronic liver diseases demonstrated that AdoMet treatment was associated with significant improvements in some biochemical liver parameters (total bilirubin and AST);however,the efficacy of AdoMet in the early weeks of treatment was not specifically evaluated[25].While sustained treatment efficacy is crucial in patients with IHC,early onset of efficacy may also be a key consideration to facilitate a rapid improvement in liver function and,subsequently,a reduction in the debilitating symptoms of cholestasis.The primary objective of this systematic review was to evaluate the efficacy of AdoMet in improving biochemical liver parameters (ALT,AST,ALP,and γGT) within 8 wk of initiating treatment in adult patients with IHC.The secondary objective was to analyze the efficacy of AdoMet in improving clinical symptoms of cholestasis in these patients during this timeframe.
MATERIALS AND METHODS
Protocol registration
The protocol for this systematic review is registered with the international prospective register of systematic reviews (PROSPERO;registration number CRD42018090936)and can be viewed at https://www.crd.york.ac.uk/PROSPERO/.This study followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement[26].
Eligibility criteria
Published clinical trials reporting the efficacy of AdoMet within 8 wk of treatment initiation were considered for inclusion in this systematic review.
The inclusion criteria were:(1) Full-text articles published between January 1,1990,and June 10,2019 reporting prospective,randomized,open-label,and observational studies;(2) Studies including male and female adults with IHC;(3) Studies reporting the efficacy of iv,intramuscular (im),or oral forms of AdoMet in terms of changes in biochemical liver parameters (ALT,AST,ALP,and γGT);and (4) Studies reporting the efficacy of AdoMet within the first 8 wk of treatment compared with control(comparator or placebo) or baseline values.
Studies of IHC in pregnancy were not eligible for inclusion,due to differences in underlying pathology between patients with IHC of pregnancy compared with those with nonpregnancyrelated IHC[27].Review articles,retrospective studies,and animal studies were also excluded.Shortlisted full-text articles published in non-English languages were translated to English.
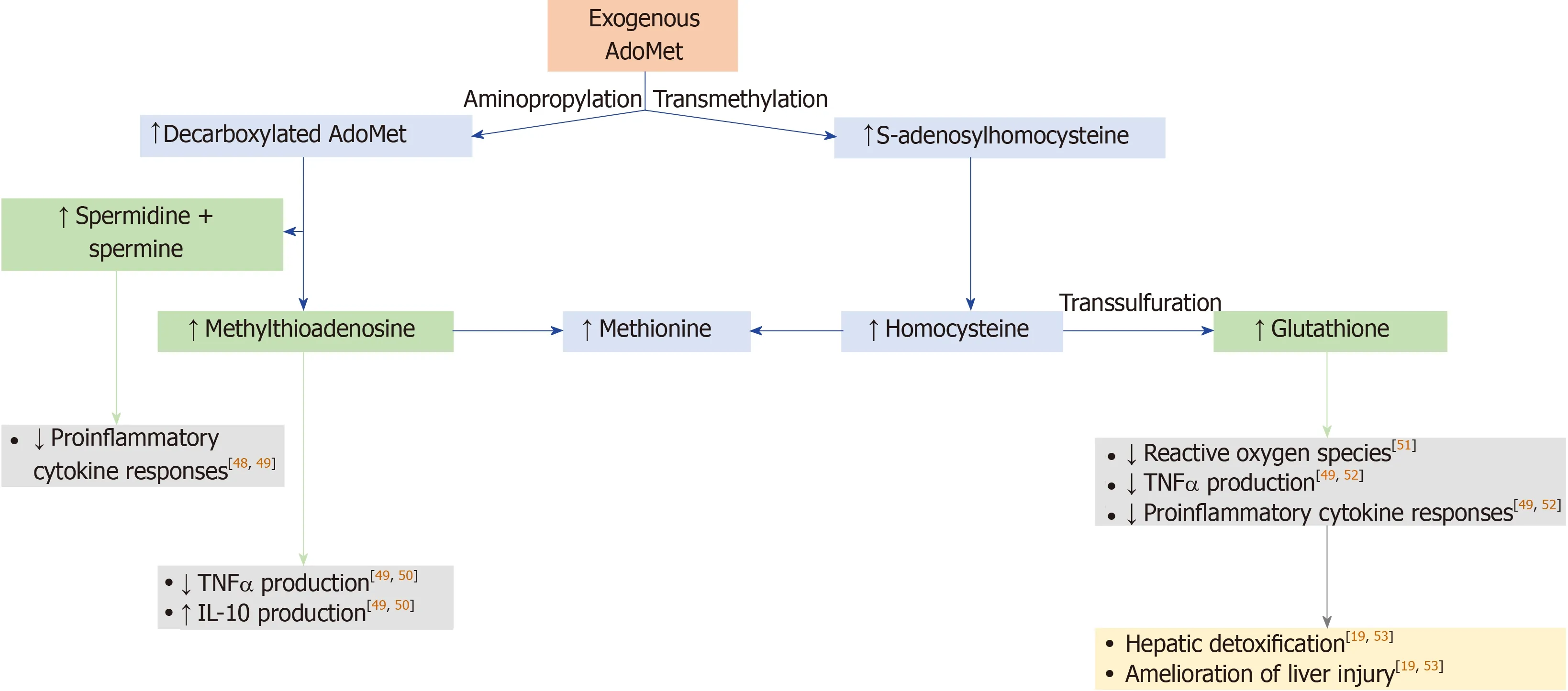
Figure1 Mechanism of action of S-adenosylmethionine[11,13,47].S-adenosylmethionine (AdoMet)-dependent methylation reactions yield S-adenosylhomocysteine as a by-product,and S-adenosylhomocysteine is cleaved into adenosine and homocysteine by S-adenosylhomocysteine hydrolase.Remethylation of homocysteine to form methionine occurs by methionine synthase and betaine homocysteine methyltransferase.In the transsulfuration pathway,homocysteine is converted to cysteine,which is the rate-limiting precursor for glutathione,via an enzymatic process catalyzed by cystathionine β-synthase and cystathionase.To synthesize polyamines,AdoMet is decarboxylated in a reaction catalyzed by AdoMet decarboxylase.The predominant polyamines in mammalian cells are spermidine and spermine,which are made by sequential addition of aminopropyl groups from AdoMet decarboxylase;methylthioadenosine is a by-product of these reactions.These metabolites of AdoMet have biological effects[48-52],which may improve hepatic detoxification and amelioration of liver injury[19,53].AdoMet:S-adenosylmethionine;IL:Interleukin;TNF:Tumor necrosis factor.
Information sources
PubMed and Embase databases were searched for relevant articles that met the inclusion criteria summarized above.Additional articles that were not available through PubMed or Embase were identified via searches of reference lists and contact with authors from this study.
The following terms were used to search PubMed for articles published between January 1,1990 and June 10,2019:AdoMet OR samyr OR transmetil OR heptral OR ademetionine OR Sadenosyl-L-methionine OR S-adenosyl-L-methionine 1,4-butanedisulfonate OR toluenesulfonate OR tosylate OR tosylate disulfate OR disulfate monooleate AND (intrahepatic cholestasis OR alcoholic liver disease OR nonalcoholic liver disease OR non-alcoholic steatohepatitis OR drug-induced liver injury OR chronic liver disease) NOT pregnancy.These terms were adapted for Embase searches.
Study selection
The initial results from the literature searches were screened against the preestablished criteria for inclusion to remove articles,using their titles and abstracts.Excluded articles included those reporting studies in children,studies that did not report the prespecified outcome measures of interest,and conference abstracts.At this initial screening stage,all studies reporting outcomes in chronic liver diseases were included to ensure that any studies involving patients with IHC were not incorrectly excluded.The full texts of the remaining articles were then examined in detail to determine their suitability for inclusion in the review;studies reporting outcomes in chronic liver diseases that did not include patients with IHC were removed at this point.At least two review team members assessed studies for eligibility and disagreements were resolved by discussion.
Data extraction
Data were extracted from the included articles and findings were discussed between authors.Queries regarding data reported in the articles were resolvedviaconsultation with statisticians and correspondence with study investigators.
Information extracted from the selected articles included reference citation,country in which the research was carried out,study design,patient population,details of study interventions,number of patients in each treatment group,AdoMet treatment protocol,duration of study treatment,and data relating to the prespecified outcome measures (biochemical parameters and symptoms of cholestasis).
Summary measures
Mean serum levels of ALT,AST,ALP,and γGT following AdoMet treatmentvsplacebo,comparator,or baseline were summarized to assess the efficacy of AdoMet within the first 8 wk of treatment.Additionally,mean scores of patient-reported fatigue and symptoms of depressionvsplacebo,comparator,or baseline were summarized to determine differences in the clinical symptoms of cholestasis following treatment with AdoMet.The results of this research were described using subjective evaluations.
Risk of bias
The risk of bias across studies was limited by ensuring comprehensive searches for all eligible published studies,and by independent assessment of studies identified from these searches by review team members.
Both randomized and non-randomized studies were eligible for inclusion in this review.As the domains that may be subject to bias vary between randomized and non-randomized studies,separate risk-of-bias analyses were performed for the two types of study.The risk of bias in individual randomized studies was determined by assessment of the following domains in accordance with the Cochrane tool for assessing risk of bias in randomized studies[28]:(1) The adequacy of sequence generation;(2) The adequacy of allocation concealment;(3) The adequacy of blinding;(4) The handling of incomplete outcome data;(5) Selective reporting of outcomes;and(6) Any other source of bias.The risk of bias in individual non-randomized studies was determined by assessment of the following domains in accordance with the riskof-bias assessment tool for non-randomized studies (RoBANS)[29]:(1) The adequacy of the selection of participants;(2) The adequacy of consideration of confounding variables;(3) The adequacy of measurement of exposure;(4) The adequacy of blinding outcome assessments;(5) The handling of incomplete outcome data;and (6) Selective reporting of outcomes.
RESULTS
Study selection
The study selection process is summarized in Figure2.In total,115 abstracts were retrieved from the database searches (28 from PubMed,87 from Embase).Ten further references were identified by searching reference lists and through contact with the authors of this study.After 12 duplicate records were removed,113 abstracts remained and were screened against the eligibility criteria;of these,87 were excluded.Full-text articles were assessed for the remaining 26 studies,and a further 17 were excluded.In total,9 studies were determined to be eligible for inclusion in the systematic review.
Study characteristics
The characteristics of the included studies are provided in Table1.
Three randomized studies and six non-randomized (observational) studies of patients with IHC were included.In terms of liver conditions,two studies included patients with IHC and DILI[30,31],three reported IHC and chronic liver diseases from various etiologies[32-34],one reported IHC with ALD[35],and one reported IHC with NAFLD[36].The two remaining studies included patients with IHC and viral hepatitis[37]and IHC due to acute hepatitis or chronic liver disease[38].
In two of the three randomized studies,the intervention was AdoMetvsplacebo[33,38].The remaining randomized study evaluated AdoMetvspotassium magnesium aspartate[39-41].No comparators were used in the observational studies.
AdoMet was administered intravenously,intramuscularly,or orally;iv doses were mostly in the range of 400-1000 mg/d;im doses were 400-800 mg/d;and oral doses were 800-1600 mg/d.Several studies initiated AdoMet treatment with iv or im dosing,then switched to oral dosing after 2-4 wk.The total duration of AdoMet treatment varied from 2 to 8 wk (8 wk was the predefined upper limit of inclusion).
All of the included studies reported biochemical liver parameters and six also provided data on the prespecified symptoms of cholestasis.Overall,data from 1791 patients were collected for this review,of whom,1503 received AdoMet,273 received placebo,and 15 received a comparator (potassium magnesium aspartate).
Risk of bias

Figure2 Flow diagram of the study selection process.1This study evaluated the use of AdoMet in psoriasis and included no data on chronic liver disease.
Randomized studies:A summary of the risk of bias within each of the three randomized studies is provided in Table2.The included domains adhere to the Cochrane tool for assessing risk of bias in randomized studies[28].None of the studies reported information on allocation concealment.The blinding scheme was unclear in one study,and the same study used an active comparator in its control group,giving rise to possible bias[37].This study was also very small,with only 15 patients in each treatment group,and the study endpoints were not clearly defined in the methodology,potentially introducing additional bias.Therefore,greater weighting was assigned to the two placebo-controlled studies throughout the systematic review.
Non-randomized studies:A summary of the risk of bias within each of the six nonrandomized studies is provided in Table3.The included domains adhere to the RoBANS tool for assessing risk of bias in non-randomized studies[29].Confounding variables were not reported in most of the studies,but one did highlight differences in baseline clinical characteristics between treatment groups[32].Five studies included patient-reported outcomes and,therefore,were judged to be at a high risk of measurement bias[30,31,34-36].Two studies appeared to have incomplete outcome data:Virukalpattigopalratnamet al[36]reported data for 244 patients at baseline and 243 patients at Visit 1,with no explanation for the missing patient;and Larionovaet al[30]reported data for 99,95,and 73 patients at Baseline,Day 14,and Day 42,respectively,with no explanation for the missing data.
Early treatment efficacy of AdoMet:Liver parameters
Efficacy within 2 wk:The two randomized,double-blind,placebo-controlled studies reported significant reductions in two or more of the four liver parameters studied within 2 wk of starting AdoMet treatment (Table4)[33,38].Frezzaet al[33]demonstrated significant reductions in ALT at Week 2 (P< 0.05),and ALP at Week 1 (P< 0.05) and Week 2 (P< 0.01),but did not observe significant changes in AST or γGT at either of these timepoints.Manzilloet al[38]demonstrated significant reductions in ALT,AST,and γGT (P< 0.01 toP< 0.05) at Week 2,but did not observe significant changes in ALP.
In contrast,the small comparator-controlled study did not report significant reductions in ALT,AST,or ALPvspotassium magnesium aspartate at Week 2[37].
All of the four non-randomized studies that investigated changes in ALT,AST,ALP,and/or γGT within 2 wk of AdoMet treatment initiation reported significant reductions in at least two of these parameters (Table5)[30-32,35].Fiorelli[32]and Perlamutrovet al[31]reported significant reductions in ALT,AST,ALP,and γGT within this timeframe (P <0.01 toP <0.05).Larionovaet al[30]reported significant reductions in ALT and AST at Week 2 (bothP <0.001) and Ivashkinet al[35]reported significant reductions in ALP and γGT at Week 2 (P< 0.0001 for both parameters).
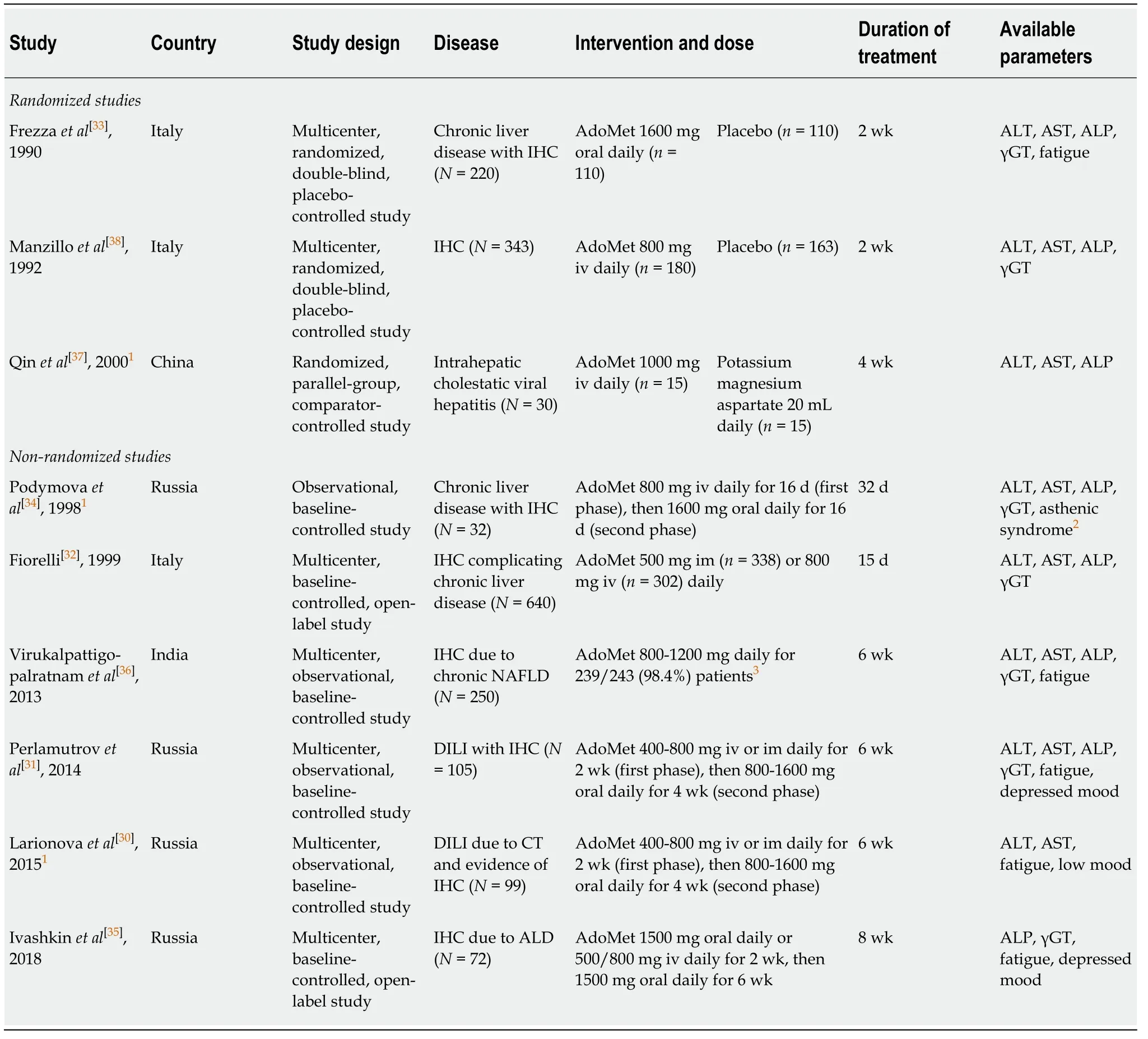
Table1 Overview of the included studies
Efficacy in 2-4 wk:Neither of the two placebo-controlled randomized studies investigated changes in liver parameters between 2 and 4 wk of AdoMet treatment.
The comparator-controlled study reported significant reductions in ALT and AST,but not ALP,vspotassium magnesium aspartate at Week 4 (P< 0.05)[37].
Two non-randomized studies reported outcomes for liver parameters following 2-4 wk of AdoMet treatment (Table5)[32,34].Fiorelli[32]showed significant reductions in ALT,AST,ALP,and γGT at Day 15 (allP <0.01).In contrast,Podymovaet al[34]reported no significant changes in ALT,AST,ALP,or γGT at Day 17.
Efficacy in 4-8 wk:Neither of the two placebo-controlled randomized studies,nor the comparator-controlled study,investigated changes in liver parameters in 4-8 wk of AdoMet treatment.
Four out of five non-randomized studies demonstrated significant reductions in two or more of the four liver parameters in 4-8 wk of AdoMet treatment (Table5)[30,31,35,36].Virukalpattigopalratnamet al[36]and Perlamutrovet al[31]reported significant reductions in ALT,AST,ALP,and γGT at Week 6 (allP <0.05).Larionovaet al[30]observed significant reductions in ALT and AST at Week 6 (bothP <0.001),while Ivashkinet al[35]reported significant reductions in ALP and γGT at Week 8 (P< 0.0001for both parameters).In contrast,Podymovaet al[34]observed no significant changes in ALT,AST,ALP,or γGT at Day 33.

Table2 Risk-of-bias assessments for included randomized studies
Early treatment efficacy of AdoMet:Symptoms and consequences of cholestasis
One of the randomized studies reported the effects of AdoMet treatment on fatigue(Table6)[33];Frezzaet al[33]demonstrated significant reductionsvsplacebo at Weeks 1 and 2 (bothP< 0.01).Of the four non-randomized studies that reported changes in fatigue from baseline,statistically significant reductions were demonstrated by Virukalpattigopalratnamet al[36]at Week 6 (P< 0.0001).Both Perlamutrovet al[31]and Larionovaet al[30]observed reductions in fatigue at Weeks 2 and 6,but statistical analyses were not reported.Similarly,Ivashkinet al[35]reported a reduction in fatigue at Week 8,but statistical analyses were not provided.
None of the randomized studies reported changes in symptoms of depression(Table6).Of the non-randomized studies,Perlamutrovet al[31]reported a significant reduction in the number of patients with depressed moodvsbaseline at Weeks 2 and 6 (P< 0.001 for both) and Larionovaet al[30]reported a similar trend for improvement in low mood at Weeks 2 and 6,but did not report any statistical analyses.Similarly,Podymovaet al[34]observed improvements in asthenic syndrome (typically involving irritability,weakness,fatigue,and unstable mood) at Days 17 and 33,but no statistical analyses were reported.Ivashkinet al[35]reported a reduction in depressed mood at Week 8,but statistical analyses were not provided.
Improvements in pruritus and jaundice were also reported in some of the included studies[30,31,33-36].Six studies demonstrated a reduction in pruritus (Table6)[30,31,33-36],with statistically significant reductions reported by Frezzaet al[33]at Weeks 1 and 2 (bothP< 0.01vsplacebo) and Virukalpattigopalratnamet al[36]at Week 6 (P< 0.0001vsbaseline).Reductions in jaundice were observed in five studies (data not shown)[30,31,34-36],although statistical significance was only reported in a nonrandomized study,by Virukalpattigopalratnamet al[36]at Week 6 (P< 0.0001vsbaseline).
DISCUSSION
When treating patients with IHC,the speed of onset of AdoMet efficacy could be an important consideration for clinicians as it may determine the rate of improvement in liver function,as well as help with the debilitating symptoms of cholestasis (such as fatigue and depressed mood).It should be noted,though,that the speed of improvement in liver enzymes may depend on the subtype and severity of underlying chronic liver disease,and that each enzyme may react slightly differently to AdoMet therapy in this context.
The efficacy of AdoMet in the early weeks of treatment in patients with chronic liver diseases has been described in several clinical studies[30-33,35-44].These data have been supported by findings suggesting that its pharmacokinetic parameters may positively affect its speed of clinical efficacy[22].Furthermore,a recent systematic review and meta-analysis demonstrated that AdoMet treatment was associated with significant improvements in some biochemical liver parameters[25];however,the authors did not systematically investigate the early effects of AdoMet within 8 wk of treatment initiation,and did not investigate important symptoms of cholestasis such as fatigue and depression.
The placebo-controlled randomized studies included in this review reportedsignificant reductions in plasma ALT levels in patients treated with AdoMetvsplacebo within 2 wk[33,38].These data are in contrast to the previous meta-analysis,which identified no significant differences in the change in ALT levels between AdoMet and controls[25].The conflicting results may reflect the different study populations:Of the six studies included in the previous meta-analysis,four evaluated pregnant women and one evaluated children,whereas our review only included studies in nonpregnant adults.The current review also included a third randomized study by Qinet al[37],which reported significant reductions in ALT at Week 4,but not at Week 2.However,this study was not placebo-controlled (the comparator was potassium magnesium aspartate) and the study was small,with only 15 patients in each treatment group giving limited power to detect treatment effects.Therefore,results from this study should be interpreted with caution,and as such,greater weighting was given throughout the review to the two placebo-controlled studies.
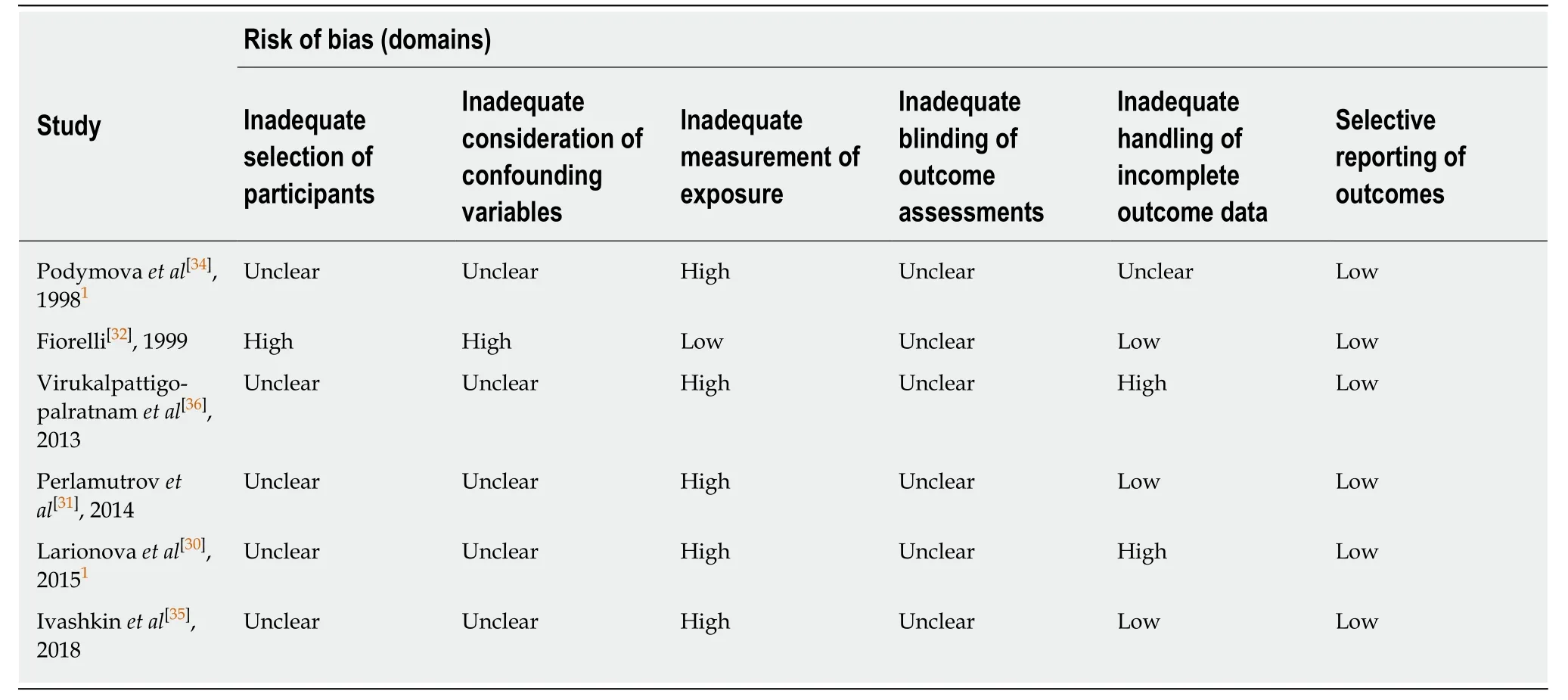
Table3 Risk-of-bias assessments for included non-randomized studies
The previous meta-analysis showed that AST levels were significantly reduced with AdoMetvscontrol[25],and this is partially supported by our findings;one of the two double-blind placebo-controlled randomized studies in the current systematic review reported significant AST reductionsvsplacebo or comparator within 2 wk[38].Of three non-randomized studies that investigated AST levels within 2 wk,two reported significant reductions at Week 2[30,31]and one reported significant reductions at Week 1[32].
The previous meta-analysis did not include ALP or γGT as outcome measures[25].However,we found that one of the two placebo-controlled randomized studies reported a significant reduction in ALP levels at Week 1 and Week 2[33].Furthermore,one of the two placebo-controlled randomized studies reported significant reductions in γGT within 2 wk[38].
Overall,it is notable that both of the double-blind placebo-controlled randomized studies[33,38]and all of the non-randomized studies (4/4)[30-32,35]that investigated changes in ALT,AST,ALP,and/or γGT within 2 wk of AdoMet treatment initiation reported significant reductions in at least two of these parameters.However,significant reductions were not always observed for all four parameters within each study within this short timeframe,possibly because the specific underlying liver diseases may influence the effect and speed of efficacy onset of AdoMet on liver enzymes.
We also identified improvements in the symptoms of cholestasis.Fatigue was improved in five studies,with two studies reporting significant reductions within 6 wk,one of which reported significant reductions within 1 wk[33,36].Symptoms of depression were improved in four studies[30,31,34,35],although statistical significance was only reported in one (non-randomized) study at Week 2 and Week 6[31].Pruritus improved in six studies[30,31,33-36],with statistical significance reported in two of these studies within 6 wk,one of which reported significant reductions within 1 wk[33,36].
Although we did not perform a systematic literature review for changes in bilirubin
levels,both of the double-blind placebo-controlled randomized studies reported significant reductions in serum combined and/or total bilirubin levels with AdoMetvsplacebo or comparator within 2 wk of treatment initiation[33,38].Furthermore,four of the five non-randomized studies that investigated this parameter found that AdoMet significantly reduced serum combined and/or total bilirubin levelsvsbaseline within 8 wk of treatment initiation[30-32,36],and three of these demonstrated significant reductions within 2 wk[30-32].Since bilirubin levels are a read out of the functional capacity of the liver[6],the improvement in bilirubin levels in patients who received AdoMet treatment may therefore reflect a corresponding improvement in liver function;however,further investigations are required to validate this observation.

Table4 Summary of outcome data relating to prespecified biochemical liver parameters (randomized studies)
AdoMet is a natural compound,synthesized and metabolized mainly by the liverviamultiple pathways[24];it is likely that exogenous AdoMet is rapidly metabolized,improving overall liver metabolic homeostasis[14].AdoMet can be formulated for iv,im,or oral administration,with the three routes appearing to have comparable efficacy within 8 wk of treatment initiation,although it should be noted that most of the studies analyzed here did not directly compare different formulations.However,the trial reported by Fiorelliet al[32],which used both iv and im delivery,showed similar improvements in liver parameters with the two formulations.In several studies,initial iv administration was followed by maintenance oral administration[30,31,34,35];while a comparison of efficacy between the two formulations is difficult,it might be expected that iv administration would result in a faster onset of efficacy compared with the oral route due to increased bioavailability.Ivashkinet al[35]reported that improvements in ALP and γGT after 8 wk of treatment were greater in patients initially treated with iv AdoMetvsoral AdoMet,however the authors highlighted that patients receiving initial iv treatment had higher baseline values as the initial treatment route was based on baseline disease severity.Finally,all the studies included in this review used pharmaceutical grade AdoMet;it is uncertain whether similar findings would be observed from AdoMet formulations of different quality or from alternative manufacturing techniques.
Although this systematic review did not include an assessment of adverse events,AdoMet has a favorable tolerability profile that has been established in several clinical studies and by long-term marketing experience[45].The rapid efficacy of AdoMet,combined with its favorable tolerability profile,makes it an attractive therapeutic option for a wide range of patients.
We acknowledge that this review has several limitations.Firstly,this study retrospectively evaluated data from prospective clinical studies that were not designed to evaluate the speed of onset of the treatment effect of AdoMet.Secondly,we included non-randomized studies,which increased bias.It is noticeable that,in general,the non-randomized studies reported more substantial and rapid improvements in the measured outcomes than the randomized studies.The lack of a control arm in the non-randomized studies is a significant confounding factor;therefore,while the results of these studies support the rapid efficacy of AdoMet,more weighting must be given to the data arising from the two double-blind placebocontrolled randomized studies.Thirdly,to ensure that all eligible studies were included in the review,we did not restrict our database searches to English-language abstracts.As a result,several of the included studies were translated to English from other languages.Fourthly,we included studies of patients with a broad variety of underlying chronic liver diseases,and we recognize that the heterogeneity of the studied populations was a confounding factor that may mask the true treatment effects in specific underlying diseases.For example,it is possible that variations in the severity and duration of IHC across patients could have had an impact on clinical outcomes,and it is possible that not all patients with IHC had a deficiency in AdoMet synthesis.Fifthly,we recognize that a relatively limited number of studies were eligible for inclusion (three randomized and six non-randomized studies),and that sample sizes in some of the included studies were small.Finally,we acknowledge that a meta-analysis would have provided a more robust assessment of the early effects of AdoMet on liver enzymes,but this was not possible due to the heterogeneous nature of the available studies.
To our knowledge,this is the first systematic review investigating the efficacy of AdoMet within the early weeks of treatment in adults with IHC.Clinical data from randomized and non-randomized studies suggest that AdoMet significantly reduces plasma ALT,AST,ALP,and γGT levels,as well as clinical symptoms of cholestasis within the first 8 wk of treatment.Furthermore,AdoMet has clinical efficacy withinthe first 2 wk of treatment as some studies indicated that it reduced the levels of liver enzymes and improved clinical symptoms of cholestasis within this short timeframe.AdoMet has also been shown to have long-term efficacy in liver diseases[46],further supporting the use of AdoMet in this patient population.However,prospective,randomized,placebo-controlled clinical studies are needed to establish the speed of onset of AdoMet efficacy,and the subsequent clinical impact on patient outcomes,in the treatment of specific liver diseases.

Table5 Summary of outcome data relating to prespecified biochemical liver parameters (non-randomized studies)
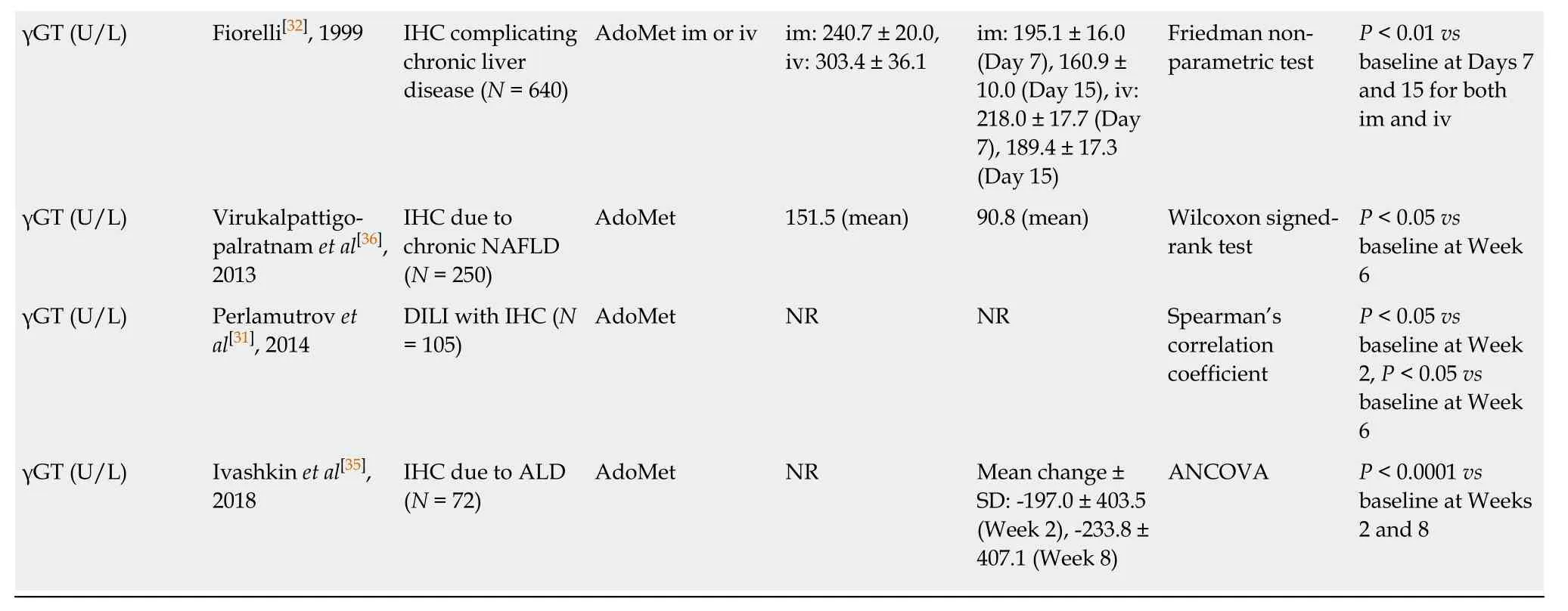
1Published studies translated to English from original language.AdoMet:S-adenosylmethionine;ALD:Alcoholic liver disease;ALP:Alkaline phosphatase;ALT:Alanine aminotransferase;ANOVA:Analysis of variance;ANCOVA:Analysis of covariance;AST:Aspartate aminotransferase;CI:Confidence interval;CT:Chemotherapy;DILI:Drug-induced liver injury;γGT:Gamma-glutamyl transferase;IHC:Intrahepatic cholestasis;im:Intramuscular;iv:Intravenous;NAFLD:Non-alcoholic fatty liver disease;NR:Not reported;SD:Standard deviation;SE:Standard error.
In summary,this systematic review provides new insight into the efficacy of AdoMet in treating IHC,and demonstrates that most studies that investigated the efficacy of AdoMet within 2 wk of treatment initiation,showed improvements in some biochemical liver parameters and symptoms of cholestasis (such as fatigue and symptoms of depression) within this short timeframe;further improvements were observed in some studies after 4 and 8 wk of treatment.

Table6 Summary of outcome data relating to prespecified symptoms and consequences of cholestasis
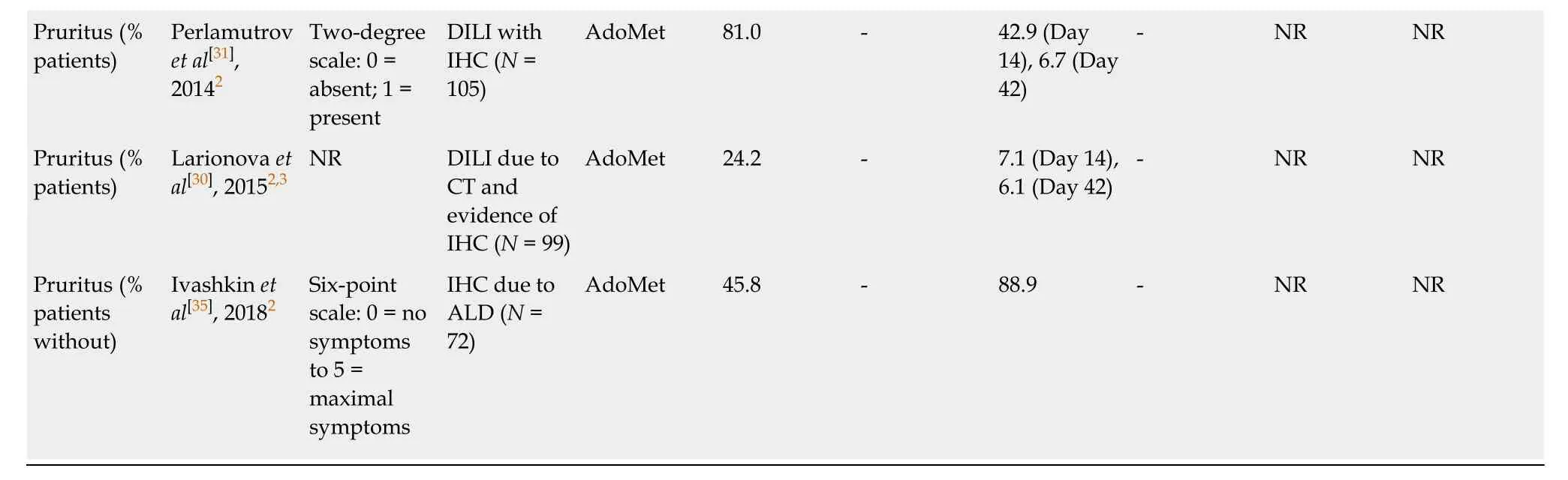
1Randomized studies;2Observational studies;3Published studies translated to English from original language;4Typically involving irritability,weakness,fatigue,and unstable mood.AdoMet:S-adenosylmethionine;ALD:Alcoholic liver disease;CT:Chemotherapy;DILI:Drug-induced liver injury;IHC:Intrahepatic cholestasis;NAFLD:Non-alcoholic fatty liver disease;NR:Not reported;SE:Standard error.
ARTICLE HIGHLIGHTS
Research background
Intrahepatic cholestasis (IHC) is a key feature of several chronic liver diseases and is associated with clinical signs and symptoms such as pruritus,jaundice,and fatigue,which may subsequently be associated with depression,autonomic dysfunction,and sleep disturbances.Sadenosylmethionine (AdoMet) is a metabolically pleiotropic molecule that is used to treat IHC.
Research motivation
The efficacy of AdoMet has been demonstrated by s everal clinical studies and a previous systematic review and meta-analysis;however,the efficacy of AdoMet in the early weeks of treatment has not been systematically investigated.
Research objectives
The primary objective of this systematic review was to evaluate the efficacy of AdoMet in improving biochemical liver parameters [alanine aminotransferase (ALT),aspartate aminotransferase (AST),alkaline phosphatase (ALP),and gamma-glutamyl transferase (γGT)] in adult patients with IHC within 8 wk of initiating treatment.The secondary objective was to assess the efficacy of AdoMet in improving the clinical symptoms of cholestasis within this timeframe.
Research methods
Published clinical trials reporting the efficacy of AdoMet (intravenous,intramuscular,or oral forms) within 8 wk of treatment initiation in adult patients with IHC were considered for inclusion in the review.Mean serum levels of ALT,AST,ALP,and γGT following AdoMet treatmentvsplacebo,comparator,or baseline were summarized.Changes in patient-reported clinical symptoms of cholestasis (such as fatigue and depression) were also reviewed.
Research results
In total,three randomized and six non-randomized (observational) studies of patients with IHC were included in the systematic review.Of the three randomized studies,two were double-blind and placebo-controlled,and one was comparator-controlled with unclear blinding and a relatively high risk of bias.Both of the double-blind placebo-controlled randomized studies and all of the non-randomized studies (4/4) that investigated changes in ALT,AST,ALP,and/or γGT within 2 wk of AdoMet treatment initiation reported significant reductions in at least two of these parameters.Reductions in patient-reported fatigue and depression were also reported within 2 wk in some studies.
Research conclusions
Clinical data from the randomized and non-randomized studies included in this systematic review suggest that AdoMet shows clinical efficacy within the first 2 wk of treatment,as some studies reported reductions in liver enzymes and improvements in clinical symptoms of cholestasis within this short timeframe.
Research perspectives
Sustained treatment efficacy is crucial in patients with IHC,but the early onset of efficacy may also be a key consideration to facilitate rapid improvements in liver function,leading to a prompt reduction in the distressing symptoms of cholestasis.In terms of future research,further targeted clinical studies are desired to determine the speed of onset of the clinical impact of AdoMet in patients with specific liver diseases.
ACKNOWLEDGEMENTS
The authors thank Dr Shelly Lu for her critical review of the manuscript.Editorial support was provided by Josh Lilly of Alpharmaxim Healthcare Communications and funded by Abbott.
