Base editing in plants:Current status and challenges
2020-06-22SutarSuhasBharatShaoyaLiJingyingLiLeiYanLanqinXia
Sutar Suhas Bharat,Shaoya Li, Jingying Li, Lei Yan, Lanqin Xia*
Institute of Crop Sciences,Chinese Academy of Agricultural Sciences,Beijing 100081,China
ABSTRACT Genome editing technologies have revolutionized the field of plant science by enabling targeted modification of plant genomes and are emerging as powerful tools for both plant gene functional analyses and crop improvement.Although homology-directed repair(HDR)is a feasible approach to achieve precise gene replacement and base substitution in some plant species, the dominance of the non-homologous end joining pathway and low efficiency of HDR in plant cells have limited its application.Base editing has emerged as an alternative tool to HDR-mediated replacement, facilitating precise editing of plant genome by converting one single base to another in a programmable manner without a doublestranded break and a donor repair template. In this review, we summarize the latest developments in base-editing technologies as well as their underlying mechanisms. We review current applications of these technologies in plant species. Finally, we address the challenges and future perspectives of this emerging technology in plants.
Contents
1. Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 385
2. Base editors:structure,mechanisms,and applications in plants . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 385
2.1. Structure and mechanisms of CBEs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 385
2.2. CBE-mediated base editing in plants. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 389
2.3. Adenine base editor-ABE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 389
2.4. ABEs mediated base editing in plants . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 390
2.5. REPAIR system. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 390
2.6. RESCUE system . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 390
3. Challenges and prospects. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 390
3.1. Off-target activity of CBEs and ABEs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 391
3.2. PAM site dependency and editing window. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 392
3.3. Random indels induced by base editors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 392
3.4. RNA base-editing technology . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 392
4. Conclusions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 392
Declaration of competing interest . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 392
Acknowledgments. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 392
References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 392
1. Introduction
Genome editing technology has experienced several stages of development: zinc finger nucleases (ZFNs), transcriptional activator-like effector nucleases (TALENs), and the newly developed Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR)-CRISPR-associated protein (Cas) (CRISPR/Cas)system.The CRISPR/Cas system,as a versatile,simple and costeffective system for genetic manipulation, has dominated the genome-editing field since its discovery [1-7], and has been widely applied in gene functional analysis and crop improvement [8-13]. Two classes of CRISPR/Cas systems have been identified [14-17], among which the class II system is widely used for DNA and RNA targeting.For type II and type V class II systems, the Cas protein induces double-strand DNA breaks(DSBs) at the targeted genomic site, after which DSBs are repaired by either non-homologous end joining (NHEJ) or homology-directed repair(HDR)[1,18-20].NHEJ often leads to a small insertion/deletion and is usually used in generating lossof-function mutants, whereas HDR is another approach to repaire DSBs in a precise manner.HDR-mediated gene replacement is now a feasible approach to achieve precise gene modification in many including some plant species[8-11,21-23]. However, despite a number of strategies have been exploited,HDR in plants still remains challenging owing to the inherently low HDR frequency as well as the limited amounts of donor repair templates (DRT) being delivered into plant cells because of the existance of cell wall[8-11,21-23].It is desirable to establish a more efficient system for precise gene modification and correction in plants.
For single-base substitution, base editing is emerging as an alternative and efficient powerful tool to HDR-mediated precise gene editing in plants[23-27].The cytidine base editor(CBE)and adenine base editor (ABE) are the two groups of base editors widely used now. For CBE, the Cas9 nickase (nCas9) or catalytically dead Cas protein (dCas9) is fused with a cytidine deaminase that catalyzes the deamination of cytosine (C) in a narrow window of the non-targeted strand. Deamination converts the original C to uracil (U), which is recognized as thymine(T)during DNA replication,ultimately resulting in a C·G to T·A single-base substitution(Fig.1A)[28].In mammalian cells,a catalytically inactive version of the Lachnospiraceae bacterium Cpf1(dCpf1)has also been reported for targeted single C·G to T·A base substitutions(Fig.1B)[29].In ABE,nCas9 or dCas9 is fused with adenosine deaminase,which catalyzes the deamination of adenine(A).Deamination converts the original A to inosine(I),which is recognized as guanine (G) during DNA replication.Thus,ABE permits A·T to G·C base substitutions(Fig.1C)[30].
In addition to DNA base editing, RNA base editing offers a promising tool for precise RNA modification at the RNA level.REPAIR (RNA editing for programmable A to I (G) replacement),as the first generation of RNA base-editing technology,is composed of the RNA-targeting CRISPR effector Cas13 and the catalytic domain of ADAR2, resulting in an A-to-I singlebase conversion in RNA transcripts(Fig.1D)[31].RESCUE(RNA editing for specific C-to-U exchange)comprises a catalytically dead Cas13 (dCas13) and cytidine deaminase, enabling RNA editing for a specific C-to-U exchange in RNA transcripts in mammalian cells(Fig.1E)[32].
In this review,we summarize the latest developments and breakthroughs in base editing technologies, with a focus on achievements and potential applications in plants.We further address the challenges and potential utility of these emerging technologies in the biological sciences as well as in crop improvement.
2. Base editors: structure, mechanisms, and applications in plants
Genome-wide association studies have shown that many important agronomic traits in plants are conferred by target alleles with one or several SNPs [24-27,33-35]. Introducing these favorable alleles into commercial cultivars will take breeders many years.Given that achievement of precise gene editing through CRISPR/Cas-mediated HDR in plants remains neither routine nor feasible in many laboratories,base editing is becoming an efficient technology for converting one target nucleotide into another without DSBs and DRT [28-30,36].Because of the simplicity and efficacy of the CBE and ABE base editors in precise base editing, the two systems have been widely used in many organisms including plants for both gene functional annotation and gene correction [24,37-40]. Table 1 summarizes the structures and mechanisms of base editors and their applications in plants.
2.1. Structure and mechanisms of CBEs

Fig.1-DNA and RNA base editors.(A)A CRISPR/Cas9-mediated cytosine base editing system(CBE).A sgRNA and a catalytically impaired Cas9(nCas9 or dCas9)complex binds to the target sequence in genomic DNA.The cytidine deaminase catalyzes the deamination of cytosine(C)in a narrow window of the non-targeted strand(4th-8th nt in the protospacer)and makes the base change from C to U(uracil)at a target site.U is recognized as thymine(T)during DNA replication,ultimately resulting in a C·Gto-T·A conversion.(B)CRISPR/Cpf1-mediated CBE system. In this system,catalytically inactive Cpf1(dCpf1) is fused with a cytidine deaminase,which catalyzes the deamination of cytosine in a narrow window of the non-targeted strand(8th-13th nt in the protospacer).As with the CRISPR/Cas9 mediated CBE system,C·G-to-T·A conversion is achieved in the non-targeted DNA strand.(C)CRISPR/Cas9-mediated adenine base editing system(ABE).An adenosine deaminase and catalytically impaired Cas9 fusion bind to the target site in a gRNA-programmed manner.The adenosine deaminase catalyzes an A (adenine) to I(inosine)change at the target site.During replication,the original A is replaced with G(guanine).Finally,A·T-to-G·C conversion is achieved in the DNA strand.(D) In the REPAIR system,catalytically impaired Cas13b(dCas13b)is fused to ADARDD(deaminase domain of human ADAR)fusion protein,which converts A to I in RNA transcripts.A mismatched C opposite the targeted adenosine is used to increase the deamination frequency.(E)The RESCUE system comprises a dCas13 and ADAR2DD(an evolved adenine deaminase domain with E488Q mutation),which perform RNA editing to achieve a specific C to U exchange in RNA transcripts.To reduce off-target effects,a guanine mismatch in the guide RNA is introduced.
The first-generation cytosine base editor (CBE1) was engineered by fusing cytidine deaminase,a rat apolipoprotein B mRNA-editing enzyme (rAPOBEC1), to the amino terminus of dCas9 using a 16-amino-acid linker [28]. In a narrow window of the non-targeted strand (4th-8th nt in the distal region of the protospacer), CBE1 converts C to U. U is then recognized by cell replication machinery as T, resulting in a C·G to T·A transition[28](Fig.1A).However,the conversion of C·G to T·A is usually reversed by base-excision repair,and U in the C·G to U·A pair is generally removed by uracil DNA Nglycosylase (UNG) [41]. To improve base editing efficiency, a second-generation cytosine base editor(CBE2)was developed by incorporating a uracil DNA glycosylase inhibitor (UGI) into BE1 to prevent the activity of UNG[42],resulting in a threefold increase in editing efficiency [28]. The third-generation base editor, CBE3, was generated by replacing the dCas9 in CBE2 with Cas9 nickase (nCas9) containing the D10A mutation(Fig. 2A). The use of nCas9 (D10A) increased the base editing activity of CBE3. The fourth-generation cytosine base editor(CBE4)was generated by fusing rAPOBEC1 to the N terminus of nCas9 (D10A) using a 32 amino acid linker and two copies of UGI appended to the C terminus using a nine-amino acid linker. Further optimization of CBE was performed to reduce indel formation during base editing, improve editingefficiency,and narrow the editing window.For example,YEEBE3, developed by creation of a mutation in rAPOBEC1(Fig.2B),had a narrow editing window (5th-6th nt) compared to CBE3[43].Lamprey cytidine deaminase(pmCDA1),which is similar to rAPOBEC1 in structure and function,was combined with activation-induced cytidine deaminase (AID), nCas9 (D10A), and UGI to generate an activation-induced cytidine deaminase (AID) base editor that showed high on-target activity and low frequency of indel mutations [30] (Fig. 2C).Gam, a DNA-binding protein from bacteriophage Mu, can form a complex with ends of DSBs and protect them from degradation, thus reducing indel formation during base editing [44]. Thus, when the Mu Gam protein was fused separately with BE3,SaBE3,BE4,and SaBE4,the resulting BE3-Gam, SaBE3-Gam, BE4-Gam and SaBE4-Gam base editors reduced random indel frequency [45]. The SaBE4-Gam base editor enabled C·G to T·A conversion with reduced indels and increased the frequency of targeted single-base conversion in comparison with SaBE4 base editors [45] (Fig. 2D). Subsequently, other DNA base-editing technologies such as targeted AID-mediated mutagenesis system (TAM), which was developed by fusion of a mutant AID (AIDx) to dCas9 in combination with UGI (Fig. 2E), showed narrowed the C·G to T·A substitution in the position from −12 nt to −16 nt in the protospacer region in mammalian cells[46].

Table 1-Base editing in various plant species using various base editors.

Table 1 (continued)

Fig.2-Structural representations of some developed cytosine base editors(CBEs).(A)CBE3 base editor.It is composed of a Cas9 nickase(nCas9)(D10A),a cytidine deaminase rAPOBEC1(orange)and a uracil DNA glycosylase inhibitor(UGI)(blue).(B)YEE-BE3 base editor.It is composed of nCas9,YEE-rAPOBEC1(a mutation in rAPOBEC).(C)Target-AID base editor.In this system,cytidine deaminase(pmCDA1),is combined with nCas9 and UGI.(D)SaBE4-Gam base editor.SaBE4-Gam is composed of a nSaCas9(D10A),two UGI molecules,and a cytidine deaminase connected to a Gam protein(green).(E)Targeted AID-mediated mutagenesis (TAM)base editor.TAM is engineered by fusing activation-induced cytidine deaminase(AIDX)with dCas9.
CBEs based on SpCas9 are limited by their G/C-rich protospacer-adjacent motif (PAM) sequences. CRISPR/Cpf1,as a new class 2,type V CRISPR/Cas system,has been used as a new alternative tool for genome editing in various organisms [10,11,47-50]. Cpf1 recognizes the thymidine-rich TTTN protospacer-adjacent motif (PAM) complementary with the widely used SpCas9 system (NGG PAM), enabling the editing of AT-rich regions such as 5′ and 3′ UTRs and promoter regions[51].A Cpf1-based CBE,LbCpf1-BE0,was developed by fusing the rat cytosine deaminase rAPOBEC1 to dCpf1 together with UGI (Fig. 1B). The Cpf1 base editor recognizes a T-rich PAM sequence and catalyzes C·G to T·A conversion in a narrow window (8th-13th nt of the protospacer) in human cells with reduced levels of random indels, non-C·G to T·A substitutions,and off-target effects[34].
To further expand the base editing toolkit,engineered Cas9 variants with altered PAM sequences and improved cleavage specificity have been developed [52,53]. Two xCas9 variants,xCas9 3.6 and xCas9 3.7, recognize a broad range of PAM sequences including NG, GAA, and GAT, and reduce the generation of off-target mutation in the human genome [52].Also, SpCas9-NG induced the C·G to T·A conversion at target sites with NG PAMs in human cells [54]. These Cas9 variants have the potential to expand the scope of DNA targeting and improve base-editing efficiency in plants.
2.2. CBE-mediated base editing in plants
In recent years, CBEs have been used to edit various plant traits(Table 1)[24,25,33,34,38,55-57].The CBE system was first established in plants by two laboratories simultaneously[24,25]. The feasibility and efficiency of CBE3 were tested in rice at three chosen targets: one target (P2) in OsPDS, which encodes a phytoene desaturase,and two targets(S3 and S5)in OsSBEIIb,which encodes starch branching enzyme IIb.Precise point mutations were successfully introduced into the three target sites, and the efficiencies of obtaining the desired mutations at the S5,S3,and P2 targets were 19.2%,10.5%,and 1.0%, respectively. Disruption of an intron-exon boundary resulted in high-amylose rice [24]. In rice, stable NRT1.1B and SLR1 base-edited plants were obtained with the CBE3 base editor,with editing efficiencies of respectively 2.7%and 13.3%[25].Successfully edited NRT1.1B in rice improved nitrogen use efficiency [58]. A marker-free and herbicide-resistant tomato that contained multiple point mutations was generated using CBEs by editing the acetolactate synthase (ALS) gene [33].Targeted C·G to T·A transitions in OsCDC48, OsSPL14, TaLOX2,ZmCENH3 genes were achieved at efficiencies up to 43.5% in rice, wheat and corn by using a nCas9-cytidine deaminase fusion [34]. The feasibility and editing efficiency of CBE was tested in Arabidopsis. A maize-codon optimized cytidine deaminase-Cas9n-UGI (CBE3) was transformed into Arabidopsis via floral dip to alter the target codon CCT for Pro197of the acetolactate synthase (ALS) gene. A 1.7% C·G to T·A mutation efficiency was obtained in the T1generation,and plants in the T2generation containing these point mutations showed herbicide resistance [38]. In rice, rBE5,developed by attaching a codon-optimized gene fragment of the human AID gene(hAID*D)to the 5′terminal end of Cas9n-NLS using a polypeptide linker, successfully edited Pi-d2 and OsFLS2 at efficiencies of respectively 30.8% and 57.0%. Plants with edited Pi-d2 showed blast resistance[55].A3A-PBE,a base editor composed of human APOBEC3A, nCas9, and UGI,enabled the efficient conversion of C·G to T·A within a wider 17-nt deamination window in regions with high GC contexts in wheat,rice,and maize than previous base editors[56].The C·G to T·A substitution in the codon of Pro190(CCG) of ALS successfully conferred herbicide resistance on watermelon,and the base-edited point mutations were passed to the next generation with high efficiency[35].In allotetraploid cotton,a BE3 system was generated to achieve point mutation in GhCLA and GhPEBP genes [57]. The ALS gene has also been successfully edited in tomato and potato plants using CBE3[26].CBEs with engineered SpCas9 and SaCas9 variants enabled C·G to T·A conversion at frequencies up to 80% in rice [59]. To evaluate the C·G to T·A base-editing activity of xCas9-3.7 in plants,SpCas9 was substituted with xCas9-3.7 in CBE3,giving rise to xBE3. xBE3 has the ability to edit sites with NGN, GAA and GAT PAMs. However, CBE and ABE containing the xCas9 variant failed to edit most target sites in plants [54,60,61]. In rice protoplasts and stable lines,Cas9-NG variants,Cas9-NGv1 and Cas9-NG,showed reduced activity at canonical NGG PAM sites, but showed detectable mutation frequency at noncanonical NG PAM sites [54,61,62]. Collectively, the versatile base editing tools that have been developed will certainly expand the target scope in rice and other crop plants.
2.3. Adenine base editor-ABE
An adenine base editor(ABE)has been developed to edit A·T to G·C [63]. The first generation ABE1.2 was generated by combining the N-terminus of the nCas9 with the TadATadA* heterodimer using the 16-amino-acid linker XTEN,with the C terminal of nCas9 fused with a nuclear localization signal (NLS) (Fig. 2-F). Unlike CBEs, ABEs mediate the conversion of A·T to G·C in genomic DNA (Fig. 1C) [63]. ABE contains a hypothetical deoxyadenosine deaminase and an nCas9(D10A).ABE binds to a target DNA sequence in a guide RNA-programmed manner, exposing a small bubble of single-stranded DNA, and the hypothetical deoxyadenosine deaminase domain catalyzes an A to I transition within this bubble.Following DNA repair or replication,I is recognized as G,and finally the original A·T base pair is replaced with a G·C base pair at the target site [27,64,65]. To improve editing efficiency,further optimization of ABE was performed, including using various TadA mutations and fusing the TadA (2.1)* domain to the C-terminus of nCas9(D10A),varying linker lengths between TadA (2.1)* and nCas9 (D10A), or using an inactivated Nterminal TadA* subunit. Extensive directed evolution and protein engineering resulted in seventh-generation ABEs (e.g.,ABE7.10),which converted target A·T to G·C efficiently(~50%)in human cells with very high product purity(typically ≥99.9%)and very low occurrence of indels(typically ≤0.1%) [66]. SpCas9-NG also generated targeted mutations at various NG PAM sites with high efficiency in human cells[54],providing the oppitunity to expand the editing scope of ABEs. Recently, a simplified base editor ABE-P1S containing ecTadA*7.10-nSpCas9(D10A)showed much higher editing efficiency in rice than the widely used ecTadA-ecTadA*7.10-nSpCas9 (D10A) fusion. The ecTadA*7.10-nCas9 fusion can also be used to improve the editing efficiency of other ABEs containing SaCas9 or the SaKKH-Cas9 variant[67].Exploiting more efficient ABEs will facilitate the application of adenine base editing in crop improvement.
2.4. ABEs mediated base editing in plants
Adenosine base editors efficiently mediate A·T to G·C conversion in plants (Table 1) [27,40,59,68]. ABEs, together with previous CBEs, enable the introduction of all four transitions(C·G to T·A or A·T to G·C)at target loci in the genome in a userdefined way,greatly expanding the capability of base editing.
A fluorescence-tracking adenine base editor (rBE14) using the nCas9 (D10A)-guided TadA:TadA7.10 heterodimer has been developed. It efficiently and cleanly introduced a A·T to G·C conversion in OsMPK6, OsSERK2, and OsWRKY45 at respective frequencies of 16.7%, 32.1%, and 62.3% in rice [39].A new plant ABE based on an evolved tRNA adenosine deaminase fused to the nCas9 enabled A·T to G·C conversion at frequencies up to 7.5% in protoplasts and 59.1% in regenerated rice and wheat plants. An OsACC-T1 with C2186R substitution endowed rice plants with herbicide tolerance [27]. A plant ABE-P1 (adenine base editor, plant version) was developed by fusing recombinant ecTadA*7.10 protein to the N terminus of the nCas9 (D10A) which had a strong VirD2 nuclear localization signal at the C terminus.The editing effect of ABE-P1 was evaluated in rice at the OsSPL14 and OsSLR1 gene loci, and the editing efficiencies were 26.0%and 12.5%, respectively. ABE-P1 also enabled multiplex base editing with high efficiency [68]. Four plant-compatible ABE binary vectors (pcABEs) were created by fusing differently modified ecTadAs (E. coli TadAs) with nCas9 (D10A) [40].Sequencing showed that pcABE7.10 had A·T to G·C editing activity with respective efficiencies of up to 4.1% and 8.8% in Arabidopsis and Brassica napus (rapeseed). To expand the targetable sites of ABE in the rice genome, new adenosine base editors were developed using engineered SpCas9 variants.The editing efficiencies of two target genes,OsSPL14 and OsSPL17, were respectively 25% and 45%. The base editing efficiencies of ABE-NG at the OsSPL14 (CGG), LF1(AGC), and OsIAA13 target sites were 2.6%, 2.9%, and 11.9%, respectively.These results showed that ABEs containing SpCas9-NG worked efficiently in rice with broadened PAM compatibility,expanding the applications of ABE in plants [54]. Moreover, it has been shown that adenine and cytosine base editing could be simultaneously performed in rice[59].
定义1 [3,4]设H是 Hilbert空间,G:H→2H 是极大单调映射,对常数 ρ>0,定义映射 JG:H→H 为:JG(u)=(1+ρG)-1(u),u∈H 称为 G 的预解算子,其中 I是H上的恒等映射。
2.5. REPAIR system
Both CBE and ABE base editors were developed for targeted single-base substitutions at the DNA level. To enable gene correction at the RNA level, a RNA base-editing technology using the type VI CRISPR-associated RNA-guided Cas13 was established [69,70]. In Cas13 enzymes, two higher-eukaryote and prokaryote nucleotide-binding (HEPN) endoRNase domains are able to mediate precise RNA cleavage [69,70]. Four Cas13 proteins have been identified to date: Cas13a (previously known as C2c2), Cas13b, Cas13c, and Cas13d [71-73]. In the mammalian cell, a Cas13b ortholog from Prevotella sp. P5-125 (PspCas13b), was more efficient and specific than the other three Cas 13 proteins.ADAR1 and ADAR2,the orthologs of ADAR, contain two domains: a double-stranded RNAbinding domain and a catalytic deamination domain. The latter domain can deaminate target A without any protein cofactors in vitro.The E488Q mutation in ADAR2 improved its deamination activity [74]. The first generation of the REPAIR system was engineered by fusing the E488Q-mutated ADAR2 deaminase domain (ADARDD) to catalytically inactive PspCas13b (dCas13) (Fig. 1D). REPAIR has no strict sequence constraints and is capable of editing full-length transcripts containing disease-relevant mutations in a mammalian cell.Next, by introduction of a variation in RNA editing construct PspAs13b-ADARDD,REPAIRv2 was generated with >919-fold higher specificity [31]. However, the REPAIR system has not yet been used in plants for RNA editing.
2.6. RESCUE system
Several RNA-editing technologies, including REPAIR, allow Ato-I conversions[32].Development of an efficient RNA editing system for precise C-to-U conversion will enable precise correction of disease-relevant transcripts in human cells.The programmable RNA Editing for Specific C to U Exchange(RESCUE), generated by fusing cytidine deaminase to catalytically inactive PspCas13b (dCas13b) [31], is capable of precise C-to-U conversion in RNA (Fig. 1E). Thus, RESCUE, together with REPAIR, doubles the number of mutations targetable by RNA editing. Also, RESCUE allows modulation of more posttranslational modifications, such as phosphorylation, glycosylation,methylation,and disease-relevant transcript correction in human cells. Like REPAIR, RESCUE also has not yet been used in plants for RNA editing.
3. Challenges and prospects
The CRISPR/Cas base editing technology enables precise and efficient single-base conversion at targeted genomic sites,and has been widely used in various organisms including plants[25-27,33-35].However,many barriers limit the widespread use of base editors, such as high off-target activity, limited PAM sites, and wide editing window (Fig. 3). Accordingly, many attempts have been made to minimize the limitations and improve the specificity of base editors in the last two years.In years to come, more research remains to be performed for modifying the existing CBE and ABE tools,applying the REPAIR and RESCUE systems in plants, and developing novel base editors with the capacity of directing transversion mutations.The CBE system can also generate base substitutions simultaneously at multiple loci in rice[75].Multiplex base editing of the CBE system enables the creation of edited crop plants with various desired SNPs at multiple gene loci for crop improvement.The RNP method[76]has also shown increased accuracy in base editing despite its low editing efficiency,making DNAfree base editing feasible and providing a potential path for bypassing regulatory obstacles in commercializing crops with improved agronomic traits(Fig.3).
3.1. Off-target activity of CBEs and ABEs
Two recent studies [77,78] have shown genome-wide offtarget activity of CBE but not ABE base editors in rice and mouse cells.It was proposed[77]that the occurrence of C·G to T·A conversion at whole genome level was caused by the increased expression level of deaminase and UGI.However,it was shown [79] that ABEs could cause widespread off-target effects at the RNA level in mammalian cells. ABEs not only induced off-target single-nucleotide conversions at the DNA or RNA level in the presence or absence of sgRNAs, but displayed unexpected cytosine deamination activity in human cells, raising another biosafety concern [80]. A recent human cell study showed that CBEs and ABEs that display RNA off-target editing activity can also self-edit their own transcripts, leading to heterogeneity that might result in the generation of new epitopes or other gain- or loss-of-function effects [81]. These findings compromise the advantages of base editing technology and its future applications. The limitations of CBEs could be resolved by 1) engineering of cytosine deaminase to reduce sgRNA-independent deamination activity, 2) regulating the expression level of cytosine deaminase-UGI protein complex, 3) developing an more precise CBE by using different cytosine deaminases and Cas nuclease fusions [82], and 4) engineering DNA base editors that have reduced unwanted RNA-editing activity [81]. Offtarget effects at the DNA level and transcriptome-wide offtarget RNA editing induced by CBE and ABE base editors have become a major concern for gene therapy in animal cells[83].However, these effects might not pose a barrier in plants if only the desired base substitutions were obtained and offtarget effects did not affect major agronomic traits.
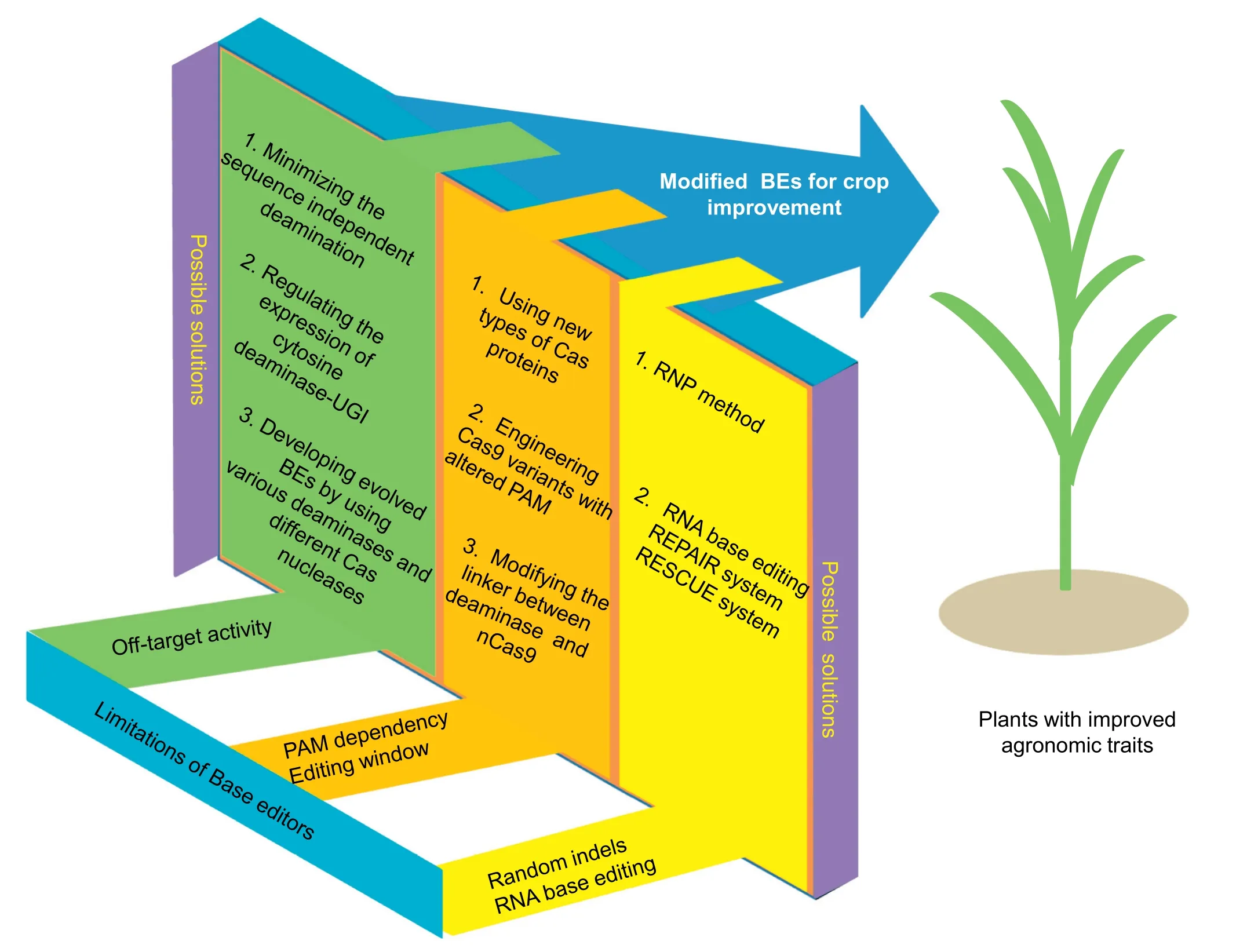
Fig.3- Challenges and future prospects of base editing.
3.2. PAM site dependency and editing window
The ABE and CBE base editors can mediate efficient base editing within a target window in DNA. However, the wider applications of DNA base editors are limited by the editing window and a PAM at the target locus. The first generation of base editors was engineered based on SpCas9, which recognizes only the NGG PAM site[28].To further expand the technology,new types of Cas protein and engineered Cas9 variants with altered PAM sequences have been introduced into base editors [52,53]. For example, LbCpf1-BE0, a Cpf1-based CBE, recognizes a T-rich PAM sequence and catalyzes C·G to T·A conversion in a narrow window (8th-13th nt of the protospacer) [24]. The use of modified SpCas9 and SaCas9 to create an evolved adenine and cytosine base editors considerably broadened the range of targets[59].C·G to T·A and A·T to G·C conversions at target sites with NG PAMs has been achieved by xCas9 and SpCas9-NG base editors in plants[54,61,62].
The wide editing window is also a limitation of DNA base editors.To overcome this limitation,high-precision base editors with narrowed editing windows were generated by modifying the linker sequences and removing nonessential sequences (Fig. 3).Engineered YEE-BE3 base editors that contain mutated cytidine deaminase domains have narrowed the editing window from ~5 to as few as 1-2 nucleotides, thus enabling discrimination of neighboring C nucleotides,which would otherwise be edited with similar efficiency in HEK293T cells[43].The improved base editor increases the specificity of base editing and is expected to be used for precise single-base substitutions in plants.
3.3. Random indels induced by base editors
Base editors enable single-base substitutions without creating a DSB in the genome.However,albeit at low frequency,random indels have been detected along with precise base-substitution events.It has been speculated[84]that the production of these indels was triggered by nCas9 (D10A) cleavage of singlestranded DNA. To avoid such cleavage, a Cas9 mutant dCas9-HF2 (D10A/N497A/R661A/H840A/) with higher fidelity and no nuclease activity was engineered to generate the base editor rAPOBEC1-XTEN-dCas9-HF2-UGI (HF2-BE2) [85]. Unfortunately,this strategy failed to improve the specificity of the CBE system as expected. It may be noted that if the targeted base changes are not accompanied by random indels simultanously in the same DNA strand, plants with desired base conversions could be recovered following segregation.
3.4. RNA base-editing technology
The REPAIR and RESCUE systems have no strict sequence constraints and are capable of editing RNA transcripts containing disease-relevant mutations in mammalian cells.These new systems will greatly expand the use of CRISPR technology in RNA editing in plants.RNA base editing may be particularly important for plant biological research,such as in functional analysis of genes involved in a complex metabolic pathway. However, it is difficult to distinguish phenotypic variation caused by base changes in RNA transcripts from the position effect of a base editor transgene, if such effect is present. The use of RNA base editing in crop improvement awaits future exploitation.
4. Conclusions
Base editing, as a hot topic in biological research, has become efficient means of precisely converting one base to another.Diverse base editing systems have been successfully established to modify genes in plants for both biological functional analysis and crop improvement.Several Cas9 variants and mutations of cytidine deaminase and adenine deaminase have been generated and incorporated into CBE and ABE base editors to expand their editing scope as well as to improve their specificity and editing efficiency in plants.With the development of novel base editing technology and further improvement of specificity,base editing will have unparalleled potential in both plant biological science and crop improvement.
Declaration of competing interest
The authors declare there is no conflicts of interest regarding the publication of this paper.
Acknowledgments
Some studies cited in this review were partly funded by the Transgenesis Initiative Project supported by the Ministry of Agriculture and Rural Affairs of China (2019ZX08010001,2019ZX08010003) and the Central Non-Profit Fundamental Research Funding supported by the Institute of Crop Sciences,Chinese Academy of Agricultural Sciences(S2018QY05).
猜你喜欢
杂志排行
The Crop Journal的其它文章
- Brief Guide for Authors
- Crop genome editing: A way to breeding by design
- Less and shrunken pollen 1 (LSP1) encodes a member of the ABC transporter family required for pollen wall development in rice (Oryza sativa L.)
- OsABA8ox2, an ABA catabolic gene, suppresses root elongation of rice seedlings and contributes to drought response
- Mutagenesis reveals that the rice OsMPT3 gene is an important osmotic regulatory factor
- Targeted mutagenesis of amino acid transporter genes for rice quality improvement using the CRISPR/Cas9 system
