Altered physiology of mesenchymal stem cells in the pathogenesis of adolescent idiopathic scoliosis
2020-06-17DaiSikKoYunHakKimTaeSikGohJungSubLee
Dai Sik Ko, Yun Hak Kim, Tae Sik Goh, Jung Sub Lee
Dai Sik Ko, Division of Vascular Surgery, Department of Surgery, Gachon University Gil Medical Center, Incheoz 21565, South Korea
Yun Hak Kim, Department of Anatomy and Department of Biomedical Informatics, School of Medicine, Pusan National University, Yangsan 50612, South Korea
Tae Sik Goh, Jung Sub Lee, Department of Orthopaedic Surgery and Biomedical Research Institute, Pusan National University Hospital, Busan 49241, South Korea
Abstract
Key words: Adolescent idiopathic scoliosis; Mesenchymal stem cell; Bioinformatics;Transcriptome; Proteome
INTRODUCTION
Adolescent idiopathic scoliosis (AIS) is a complex three-dimensional deformity of the spine that is accompanied not only by coronal plane deformity but also by deformity in the sagittal and axial planes[1]. It mostly occurs in adolescent females with pubertal growth spurt[2-4]. Despite extensive research over the years, there is no established etiology for the pathogenesis of AIS[4-6]. Multiple factors such as genetics, melatonin deficiency, platelet abnormality, unique upright posture of humans, and aberrant growth of endochondral-membranous bone growth contribute to the initiation and progression of the complex three dimensional curve in AIS[4,6-13]. However, there is still a lot of controversy regarding the extent and the manner in which each of these factors contribute to the development of AIS.
Mesenchymal stem cells (MSCs) differentiate into progenitor cells of bone,cartilage, and adipose tissues[14-16]. Altered MSC proliferation, division, and differentiation could lead to abnormal physiological processes or osteogenic disease[17,18]. Functional defects and reduced cell proliferation of osteoblasts and their precursors, MSCs, cause an imbalance between bone formation and bone resorption resulting in osteoporosis, which is characterized by a reduction in bone mass[19,20]. Two major features of AIS, namely, systemic low bone mass and disproportionate endochondral-membranous bone growth, are known to be associated with abnormal differentiation of MSCs[21-26].
Since the pathogenesis of AIS is multifactorial and complex, the role of MSCs in the pathogenesis of this disease has not been reviewed yet. Herein, we will discuss the studies to date on the abnormal differentiation of MSCs in patients with AIS[27,28].Furthermore, the expression profile of genes, proteins and noncoding RNAs of MSCs,between patients with AIS and normal individuals is different[16,29-33]. This review highlights the role of MSCs in the pathogenesis of AIS, thereby contributing not only to further research but also to improve clinical outcome.
ABNORMAL DIFFERENTIATION OF HUMAN MSCS MIGHT BE INVOLVED IN THE DEVELOPMENT AND PROGRESSION OF AIS
AIS is characterized by generalized osteopenia and systemic low bone mass in both axial and peripheral skeletons and disproportionate endochondral-membranous bone growth[22,34]. The exact pathomechanism for these characteristics is mostly unknown.However, it has been predicted that osteoblasts and their progenitors, MSCs, might contribute to these features of AIS. Parket al[28]conducted an age- and sex-matched comparative study of patients with AIS and patients with lower leg fracture as control group to define the relationship between bone mineral density and differentiation potential of MSCs. Compared to the control group, the mean lumbar spinal bone mineral density was significantly low in AIS patients, while mean femoral neck bone mineral density was not altered. There was no difference in the mean doubling time and adipogenic differentiation abilities of MSCs obtained from patients with AIS and that of the control group. Both the osteogenic differentiation ability and alkaline phosphatase (ALP) activity of MSCs from patients with AIS were significantly lower than those of the control group. Correlation analysis demonstrated that there was no association between osteogenic and adipogenic differentiation abilities of MSCs and bone mineral density in either group, while there was a positive correlation between osteogenic differentiation ability of MSCs and ALP activity of patients with AIS.
The pathoetiology of AIS has been reported to be associated with a dysfunctional melatonin pathway including melatonin receptor 1B gene polymorphism and melatonin deficiency[35-37]. MSCs of patients with AIS showed lower expression of melatonin receptor 2 (MT2) than those of controls, while the expression of melatonin receptor 1 had no significant difference between the two groups. Melatonin did not alter the differentiation ability in patients with AIS. However, melatonin promoted osteogenic and chondrogenic differentiation by increasing ALP activity and glycosaminoglycan synthesis and upregulating the expression of genes including ALP, osteopontin, osteocalcin, and collagen type II in normal controls. Decreased osteogenic differentiation ability, relatively lower expression of MT2, and lack of response of MSCs to melatonin might contribute to generalized low bone mass and abnormal dissociation of endochondral-membranous bone growth resulting in the development and progression of AIS.
TRANSCRIPTOMIC CHARACTERIZATION OF MSCS IN AIS PATIENTS
Zhuanget al[32]identified 1027 novel differentially expressed genes (DEGs) in MSCs of patients with AIS compared to normal controls. Of these 1027 DEGs, 551 genes were upregulated includingSMAD3andHOXC69and 476 genes were downregulated includingMAP2K1andHSPA6. Gene ontology (GO) analysis confirmed that the upregulated DEGs belonged mainly to categories such as small GTPase-mediated signal transduction, DNA-dependent transcription, and cytokinesis. The downregulated DEGs included genes involved in small-molecule metabolic process,cell adhesion,etc. Pathway analysis demonstrated that several biological pathways were significantly upregulated and downregulated including mitogen-activated protein kinase (MAPK) signaling pathway, peroxisome proliferator-activated receptor signaling pathway, calcium signaling pathway, and Notch signaling pathway. All these identified pathways were known to have an important role in controlling the osteogenic and adipogenic differentiation of MSCs. Finally, 24 genes including mitogen-activated protein kinase kinase 1 (MAP2K1), SMAD family member 3(SMAD3), homeobox C6 (HOXC6), heat shock 70 kDa protein 6 (HSPA6), and general transcription factor IIi (GTF2I), were identified as the most significant genes, which might play essential roles in the pathogenesis of AIS, through network analysis of DEGs from the above-mentioned significant pathways.
Among the 1027 DEGs,SPRY4was the most significantly downregulated gene involved in MAPK signaling, and has been reported to be crucial for both osteogenic differentiation and melatonin response. A previous study has reported thatSPRY4expression was significantly decreased in MSCs of patients with AIS[32]. Knockdown ofSPRY4in MSCs of healthy controls using siRNAs showed impairment in osteogenic differentiation of MSCs. Moreover, ectopic expression ofSPRY4in MSCs of healthy controls by lenti-SPRY4infection showed enhancement in osteogenic differentiation of MSCs. Melatonin treatment increasedSPRY4expression in MSCs, and upregulation ofSPRY4gene in these cells facilitated the differentiation of osteoblasts from MSCs. Collectively, impaired expression ofSPRY4in MSCs might contribute to aberrant bone metabolism and skeletal growth in AIS by desensitizing melatonin response.
Research regarding the role of MSCs in patients with AIS has been largely focused on osteogenic or chondrogenic differentiation[27,28,38,39]. Recently, abnormal adipogenesis in AIS has been considered in addition to leptin might contribute to the pathogenesis of AIS[31].In vitroadipogenic differentiation of MSCs from AIS patients and normal controls followed by microarray analysis detected 300 DEGs. Seven genes includingSpot14(THRSP, thyroid hormone responsive protein) showed the most marked differential expression between MSCs of patients with AIS and normal controls.Spot14mRNA and protein were highly expressed during adipogenic differentiation of MSCs in AIS patients. Immunohistochemistry indicated that the ratio of positively stainedSpot14cells in adipose tissue samples from AIS patients was higher than that of normal controls. Although the study was limited toSpot14, which plays an important role in the regulation of adipose tissue differentiation, adipogenic differentiation was found to be different in AIS patients and normal individuals.Based on this study, the exact role of adipogenic differentiation in AIS needs to be further studied.
PROTEOMIC CHARACTERIZATION OF MSCs IN AIS PATIENTS
Differential proteome analysis revealed 25 significantly distinct proteins in MSCs of AIS patients compared to that of normal controls[16]. Among the 25 distinct proteins,five were associated with bone growth and metabolism. These included pyruvate kinase M2 (PKM2), annexin A2, β-actin, γ-actin, and heat shock 27 kDa protein(HSP27). Downregulation of β-actin and γ-actin in MSCs of AIS patients indicated impaired osteogenic differentiation potential, which was consistent with previous studies. Downregulation of proteins such as β-actin, γ-actin, HSP27, WD repeatcontaining protein 1, and moesin which were localized in the cytoskeleton might contribute to the initiation and progression of AIS. Downregulation of β-actin, γ-actin,HSP27, and annexin A2 and upregulation of PKM2 indicated decreased osteogenic differentiation capacity and increased proliferation ability of MSCs in AIS, and the former might contribute to low bone mass in AIS.
NON-CODING RNA OF MSCs IN AIS PATIENTS
Non-coding RNA (ncRNA), is a functional RNA molecule, which is transcribed from DNA but not translated into protein, includes microRNA (miRNA), long non-coding RNA (lncRNA),etc. and plays a role in regulating gene expression[40]. Recently, studies regarding the roles ofncRNAs in the pathogenesis of AIS have been reported[29,33,41].
Zhuanget al[33]identified 1483 differentially expressedlncRNAs by microarray analysis of bone marrow MSCs from AIS patients and normal controls.LncAIS (gene symbol: ENST00000453347) was defined to be most significantly downregulated among the 10lncRNAs from MSCs of patients with AIS. Silencing oflncAIS in normal MSCsin vitroleads to inhibition of osteogenic differentiation and ectopic bone formationin vivo. Inversely, overexpression oflncAIS in normal MSCs enhances osteogenic differentiation and bone formation. In normal MSCs,lncAIS interacts with NF90 to stabilizeHOXD8mRNA. StableHODX8promotesRUNX2expression,resulting in osteogenic differentiation whereas downregulation oflncAIS impairs NF90 recruitment and destabilizesHOXD8mRNA and MSCs, resulting in decreased osteogenic differentiation in AIS patients.
Huiet al[29]identified novel 54 differentially expressedmiRNAs in bone marrow mesenchymal cells of AIS patients through microarray analysis. Of the 54 differentially expressedmiRNAs, 42miRNAs includingmiR-17-5p andmiR-106a had increased expression andmiRNAs such asmiR-199a-3p,miR-29c-3p had decreased expression compared to that of non-AIS controls. Comprehensive bioinformatics studies including functional analysis by GO analysis, KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway analysis, and network analysis were performed.UpregulatedmiRNAs were associated with transmembrane transport, small-molecule metabolic process, and immune response, while downregulatedmiRNAs were involved in DNA-dependent transcription, cytokinesis, and small GTPase-mediated signal transduction. Pathway analysis showed that calcium signaling, Notch signaling, and ubiquitin-mediated proteolysis pathways were significantly upregulated, while MAPK signaling and phosphatidylinositol-3-kinase-Akt signaling pathways were significantly downregulated. Network analysis of differentially expressedmiRNAs, GO categories, and significant biological pathways revealed seven most significantmiRNAs (miR-17-5p,miR-106a-5p,miR-106-5p,miR-16-5p,miR-93a-5p,miR-15a-5p, andmiR-181b-5p). Previously, Zhuanget al[33]identified 1027 DEGs in bone marrow mesenchymal cells of AIS patients. Corresponding with the differentially expressedmiRNAs, 204 target genes such asMAP2K1,SMAD3,GTF21,andDUSPwere associated with the predictedmiRNAs. The study uncovered several novel biological pathways that were hitherto not found to be associated with osteogenic differentiation of MSCs.
PERSPECTIVES ON THE ROLE OF MSCS IN THE CLINICAL PROGRESSION OF AIS
As discussed above, studies have been conducted to determine the exact role of MSCs in the pathogenesis of different diseases. Attempts have been made to treat various diseases using MSCs[42]. Previously, MSCs were solely used in the field of cell replacement such as autologous iliac bone graft in bone-related surgery[43,44].Currently, MSCs are being used to treat diseases, by regulating the surrounding cytokine milieu,viasecretion of paracrine factors[45]. Indeed, MSCs have been useful for the treatment of inflammatory diseases such as rheumatoid arthritis and psoriasis,as well as for left ventricular failure following myocardial infarction and aging frailty[46-49]. Furthermore, research has demonstrated the positive effects of MSCscontaining scaffold grafting in angular deformity after growth plate injury[50].Recently, Brzoskaet al[51]evaluated the contribution of muscle component in the initiation of AIS in various ways and examined the possibility of applying regenerative medicine using stem cells in AIS compared to the results of other diseases. Therapeutic strategy of precisely injecting myogenic precursor cells, which are reinforced through genetic modulation of MSCs, into the target muscle using imaging modality will soon begin. Collectively, after more accurately understanding the pathomechanism of AIS, treatment using MSCs will also be initiated. In the near future, it is expected that treatments to prevent AIS would be developed and widely used, possibly by regulating growth rate in the x, y, and z axes by injecting MSCs into growth plates or muscle, or by functional modulation of MSCs through ncRNAs.
CONCLUSION
AIS is a unique disease that occurs only in people walking upright. With the development of various surgical treatment techniques and the development of clinical guidelines, patient satisfaction and treatment outcomes are getting better. However,as discussed above, no clear pathogenesis is known yet, and research is being conducted in various fields. Herein, we reviewed and summarized the basic and bioinformatics-based researches regarding the expression levels of mesenchymal stem cell-derived genes, proteins, and noncoding RNAs and their role in the pathogenesis of adolescent idiopathic scoliosis (Figure 1, Table 1). This review shows that the low bone mass in the body, which is one of the major features of AIS, is closely related to decreased osteogenic differentiation of MSCs and the aberrant response of these cells to melatonin (Figure 2, Table 1). Although studies on MSCs that have been reviewed so far are related to AIS and MSCs, they are not completely definitive and can be considered as a basis for further research. Based on this, multidisciplinary research and clinical trials utilizing MSCs for AIS treatment would be attempted for advancing clinical treatment.
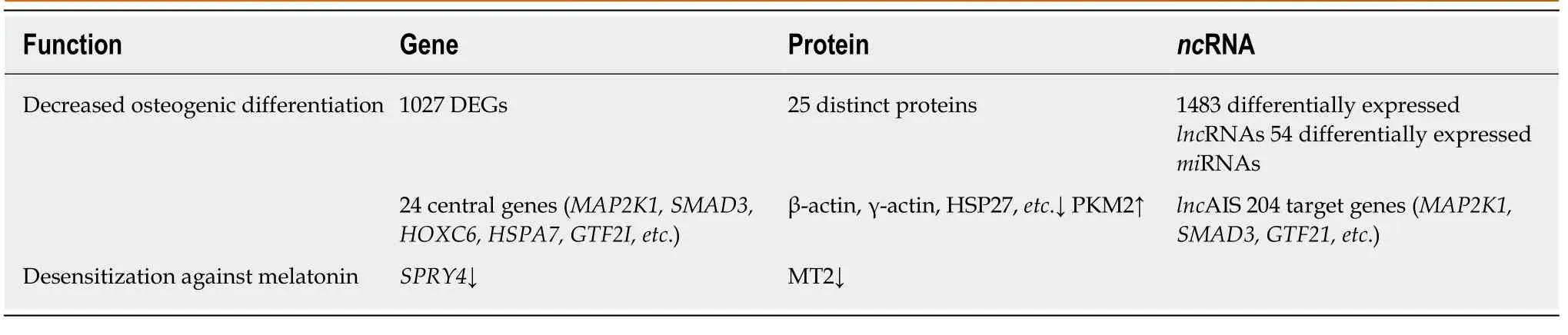
Table 1 Summary table showing differentially expressed genes, proteins, and non-coding RNAs of adolescent idiopathic scoliosis patient-derived mesenchymal stem cells compared to normal controls
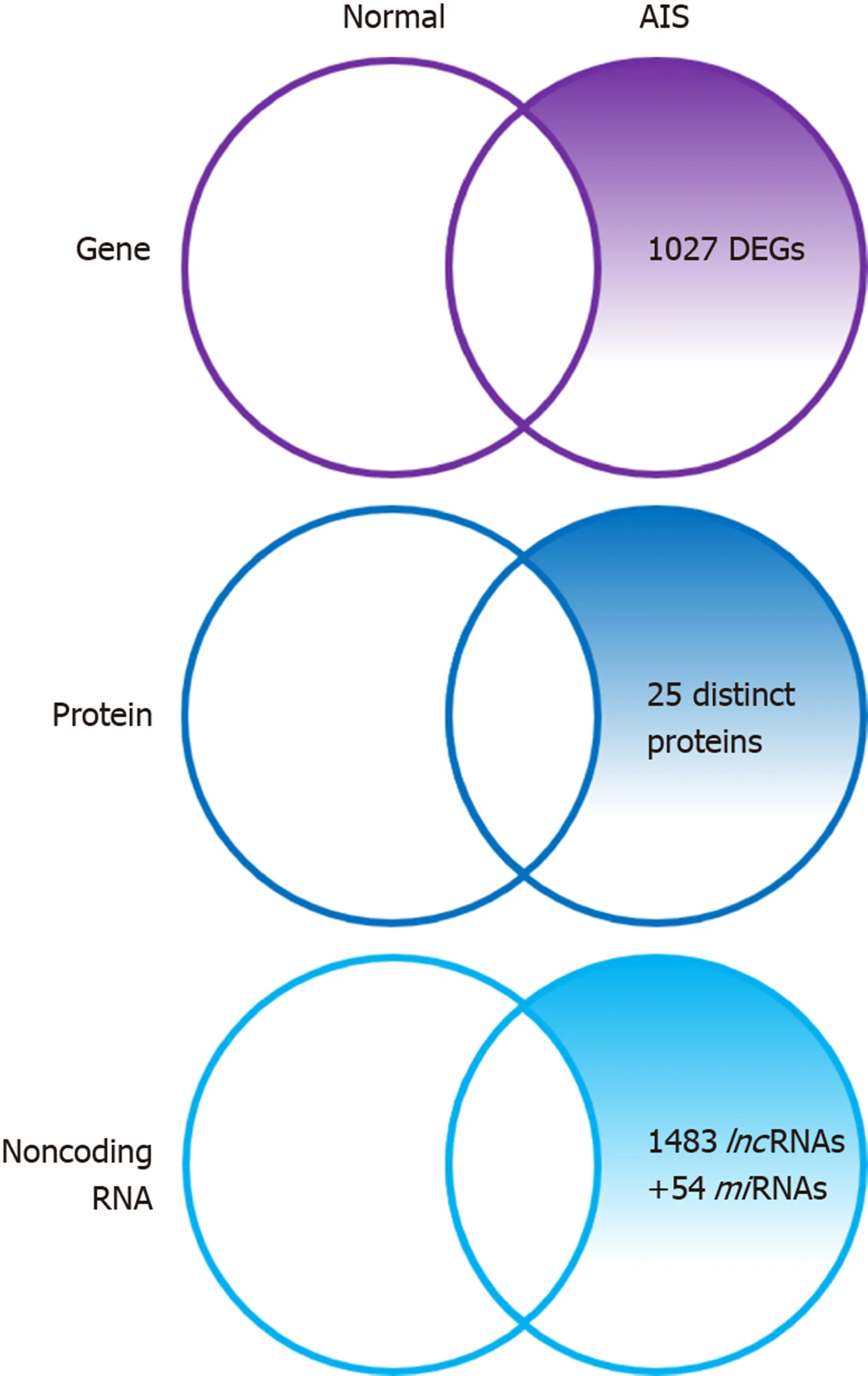
Figure 1 Summary diagram illustrating the differentially expressed genes, proteins, and non-coding RNAs of adolescent idiopathic scoliosis patientderived mesenchymal stem cells compared to normal controls. DEGs: Differentially expressed genes; AIS: Adolescent idiopathic scoliosis; lncRNAs: Long noncoding; miRNAs: microRNA.
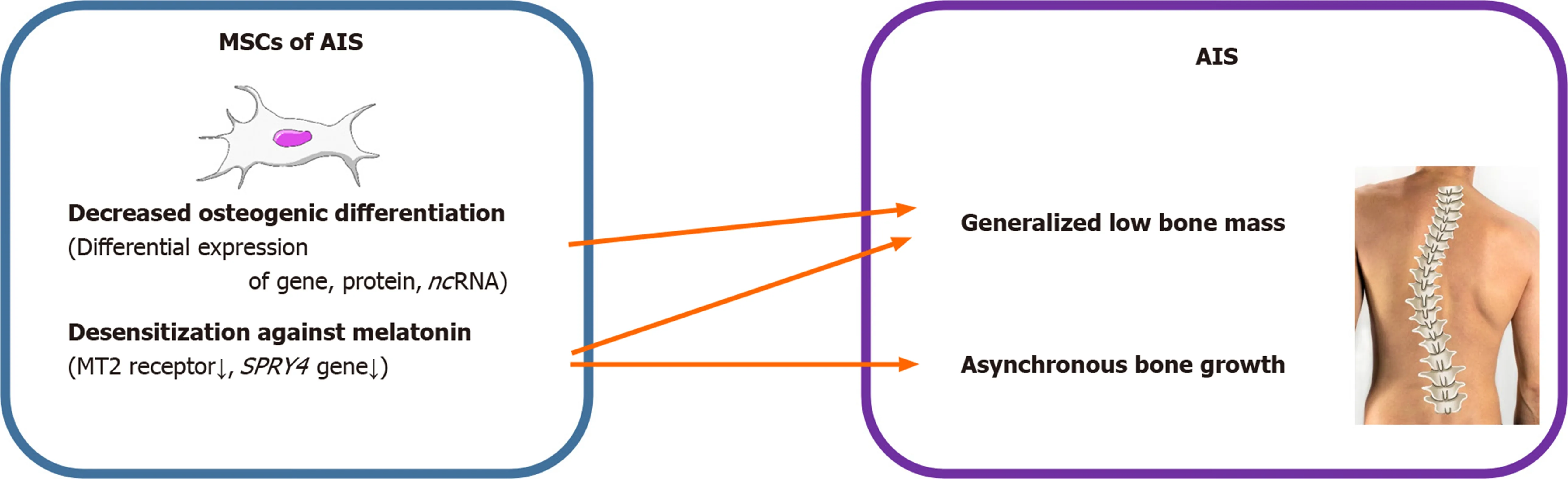
Figure 2 Summary diagram illustrating the different effects of mesenchymal stem cells resulting in adolescent idiopathic scoliosis. MSCs: Mesenchymal stem cells; AIS: Adolescent idiopathic scoliosis.
ACKNOWLEDGEMENTS
We would like to express gratitude for MD Yoon Jae Cho for his working on figures of this manuscript.
杂志排行
World Journal of Clinical Cases的其它文章
- Tumor circulome in the liquid biopsies for digestive tract cancer diagnosis and prognosis
- Isoflavones and inflammatory bowel disease
- Cytapheresis for pyoderma gangrenosum associated with inflammatory bowel disease: A review of current status
- Association between liver targeted antiviral therapy in colorectal cancer and survival benefits: An appraisal
- Peroral endoscopic myotomy for management of gastrointestinal motility disorder
- Clinical prediction of complicated appendicitis: A case-control study utilizing logistic regression
