Management of adults with coarctation of aorta
2020-06-12PradyumnaAgasthiSaiHarikaPujariAndrewTsengJosephGrazianoFrancoisMarcotteDavidMajdalanyFaroukMookadamDonaldHaglerRezaArsanjani
Pradyumna Agasthi, Sai Harika Pujari, Andrew Tseng, Joseph N Graziano, Francois Marcotte,David Majdalany, Farouk Mookadam, Donald J Hagler, Reza Arsanjani
Abstract
Key words: Coarctation of aorta; Cardiac surgery; Cardiac catheterization; Balloon angioplasty; Stents
INTRODUCTION
Coarctation of the aorta (CoA) is one of the most common congenital cardiac anomalies causing a narrowing of the proximal descending[1,2]varying within a range of arch abnormalities from a discrete narrowing to a long segment of arch hypoplasia[2]. It was first described by Thiene[3]in 1760 and accounts for 4%-6% of all congenital heart defects, with an incidence is of 3-4 cases per 10000 live births[4,5].Males are commonly affected than females with a ratio between 1.27:1 and 1.74:1,respectively[1,6-8].
CLASSIFICATION AND MORPHOLOGY
The narrowing of CoA is classically situated between the left subclavian artery and the ligamentum arteriosum at the aortic isthmus. CoA can also occur in atypical locations, between the transverse aortic arch and the bifurcation of the abdominal aorta[9]. Traditionally, CoA was classified as pre-ductal, juxta-ductal, and post-ductal based on its relationship with the ductus arteriosus (Figure 1). However, the degree of narrowing of the aorta and the association with arch vessels are regarded as more clinically relevant[10]. Approximately 40%-80% of patients have associated transverse arch hypoplasia, which varies in severity and must be accurately defined prior to planned intervention[11-13]. Although the ratio of the narrowed segment's diameter to that of the distal descending aorta was formerly used to define the degree of CoA[14],arch hypoplasia is currently defined by z-scores of -2 or lower[15]. Rarely, we can observe tubular hypoplasia representing a combination of small arch diameter and increased length between segments of the arch[16]. CoA ranges in severity from complete aortic luminal atresia to a mild, precisely defined posterior shelf like lesion,and it needs to be differentiated from an interrupted aortic arch, in which there is a true discontinuity of the aortic walls (Figure 1)[17].
ASSOCIATIONS
Cardiac
Studies have shown evidence of a common molecular pathogenic mechanism for the co-existence of CoA with other left heart obstructive pathologies such as aortic stenosis and hypoplastic left heart syndrome[18-20]. Although 75% of patients with CoA have a co-existent bicuspid aortic valve (BAV)[21,22], in patients with BAV and previous repaired CoA, the probability of requiring surgery for the aortic valve or ascending aorta is lower than in isolated BAV[23]with a slower progression of ascending aorta dilation on serial echocardiography[24]. Higher prevalence of CoA in BAV patients justify the need for routine screening and arch imaging for CoA in patients with BAV[25]. Other associated cardiac anomalies include left heart obstructive lesions like cor triatriatum, parachute mitral valve and discrete subaortic stenosis, referred as Shone syndrome as well as ventricular septal defect, patent ductus arteriosus, double outlet right ventricle, transposition of great arteries, atrio-ventricular canal defects[22,26-28]and rarely, aberrant arch vessels (Figure 2).

Figure 1 Coarctation of Aorta types. A: Ductal; B: Pre-ductal; C: Post-ductal. R.S.A: Right subclavian artery; R.C.C: Right common carotid; L.C.C: Left common carotid; L.S.A: Left subclavian artery; M.P.A: Main pulmonary artery.
Non-cardiac
Chromosomal abnormalities like Turner's syndrome (23 X0) can be associated with multiple congenital extra-cardiac anomalies (short stature, wide set nipples, webbed neck and infertility), aortic aneurysm, an 18% incidence of coarctation[29]and increased risk of aortic dissection[30-32]. CoA is also associated with a number of syndromes like PHACES (posterior fossa malformations, hemangiomas, arterial anomalies, cardiac defects, eye abnormalities, sternal cleft, and supra-umbilical raphe) syndrome[33],Williams-Beuren syndrome[34], Alagille syndrome[35], and Noonan syndrome[36].Cerebral artery aneurysms and various anomalies of the pulmonary and aortic arch vessels have been associated with CoA[37].
HISTOLOGY AND PATHOGENESIS
Abnormal histology of the arterial wall adjacent and distal to CoA site often observed with medial degeneration noted in pre- and post-stenotic specimens leading to increased incidence of aortic dissection and aneurysm. Hence, CoA is considered a generalized arteriopathy[38]. The ductal tissue with in-folding of aortic media is observed in the tissue ridge, which is often noted extending from the posterior aortic wall and protruding into aortic lumen, on examination of the lesions of the coarctation[39].
The underlying pathogenesis is not completely understood. However, the most commonly accepted theories include hemodynamic, ductal hypothesis, and abnormal genetic mutations. Hemodynamic theory states that reduced anterograde intrauterine blood flow to the fetal arch leads to its underdevelopment[40,41]. Ductal hypothesis postulates the migration of ductal tissue into the wall of the fetal thoracic aorta[42-45].The NOTCH1 gene, which plays an important role in cardiovascular development,and several other genes have been implicated in the etiology of CoA[38]. Increasing evidence of genetic contribution to CoA has been documented[46,47]with siblings having a 0.5% risk of CoA at birth and a 1% risk of congenital heart disease[48]. In the development of the aorta, vascular endothelial growth factor (VEGF) plays a crucial role, acting as a chemoattractant and stimulating angioblast migration toward the midline[2]. Studies have shown that targeted VEGF disruption leads to significant impairment of aortic formation[49]with increased pre-coarctation collagen and decreased smooth muscle content when compared to the post-coarctation aorta or proximal aorta in young transplant donors[50]. Mechanical models indicate that blood flow abnormalities, defective endothelial cell migration, and excessive deposition of aortic duct tissue at the aortic isthmus can lead to coarctation[51]. Coarctation can also be acquired in inflammatory diseases of aorta, such as Takayasu arteritis[52], and also in severe atherosclerosis[53]. Environmental factors such as chemical exposures,particularly solvents have been suggested to have a possible role in development of CoA and studies noting the geographical variations in CoA also suggest the same[54].

Figure 2 Anatomical variations of subclavian artery in coarctation of aorta. A: Right subclavian artery arising from the site of coarctation; B: Left subclavian artery arising from the site of coarctation. R.S.A: Right subclavian artery; R.C.C: Right common carotid; L.C.C: Left common carotid; L.S.A: Left subclavian artery; P.T:Pulmonary trunk.
PATHOPHYSIOLOGY
Narrowing of the aorta causes an increase in left ventricular afterload and reduced lower body perfusion, resulting in activation of the renin-angiotensin-aldosterone system and subsequent upper body hypertension. Compensatory mechanisms ensue,including left ventricular hypertrophy, pre- and post- stenotic vessel dilation and development of collateral flow in the intercostal, internal mammary and scapular vessels (Figure 3 and Figure 4). Flow disturbances in the aorta caused by the coarctation can increase the risk of endocarditis, especially when associated with congenital heart disease, such as bicuspid aortic valve[55]. The clinical presentation may vary from a critically ill neonate with heart failure to an asymptomatic hypertensive adult based on the balance between the extent of aortic narrowing and the above compensatory mechanisms[56]. Moreover, there may be an increased risk of aortic aneurysm or dissection and cerebral berry aneurysm development depending on the possible existence of underlying intrinsic aortopathy and hypertension[38].
NATURAL HISTORY
Natural history is mainly obtained from hospital post-mortem records and from case series prior to the availability of surgical correction first performed in 1948[57]. The prognosis of CoA depends on the hemodynamic severity and is generally poor without intervention. Historical data shows that patients who survived beyond infancy died at a mean age of 34 years with a 75% mortality rate by age 43 years from congestive heart failure, aortic dissection or rupture, endocarditis, endarteritis,intracranial bleed and myocardial infarction[39]. Studies suggest a less than 5% chance of developing hypertension by early adulthood in patients repaired in infancy versus 25%-33% in those operated after the age of one year[58-61].
CLINICAL PRESENTATION

Figure 3 Collateral vessels development due to luminal narrowing, allowing blood flow from high to low pressure areas. Figure shows the collaterals in intercostal, internal mammary, scapular and lumbar vessels.
A substantial number of asymptomatic subjects with aortic coarctation are not detected until adult life, underestimating the incidence at birth[5]. The age of presentation and manifestations depend on the severity of narrowing, relationship with arch vessels, and collateral vessel formation. Therefore, the clinical presentation of coarctation differs significantly in pediatric patients and adults. In the fetus and neonate coarctation may be suspected because of right ventricular enlargement associated with decreased left ventricular flow and greater flow through the ductus arteriosus. Infants may remain asymptomatic when there is associated patent ductus arteriosus. However, after ductal closure, severe CoA results in heart failure and/or shock from acute left ventricular pressure overload. Clinical presentation in children and adolescents is typically through lower extremity fatigue and exertional dyspnea.Presentation in adults is relatively rare, 10.3% of patients with CoA being diagnosed after the age of 40 years[62]. In virtually all cases, CoA presents with upper extremity hypertension and lower blood pressure in the lower extremities with delayed femoral pulses. Pulse and blood pressure reading patterns will vary with the origin of the subclavian arteries relative to CoA site. The classic bilateral upper extremity hypertension occurs when the left subclavian artery is proximal to the coarctation.Occasionally, left subclavian originates distal to the coarctation, resulting in unequal arm blood pressure readings with diminished left brachial pulse and pressure. Rarely,when both the right and left subclavian originate distal to the coarctation as in anomalous origin of the subclavian artery from the descending aorta, pulses and pressures in all four extremities are equally decreased. Despite the blood pressure variability in the extremities, regional blood flow is usually maintained by autoregulatory mechanisms causing vasoconstriction in hypertensive areas and vasodilation in hypotensive areas[63]. Several theories support the etiology of hypertension: Imbalance within the autonomic nervous system[64], impaired vascular function[65], and hyperactivation of the renin-angiotensin system[66,67], which are the most widely accepted. According to the renin-angiotensin system theory, lower body hypoperfusion results in activation of the renin-angiotensin-aldosterone system and consequent upper body hypertension. Symptoms and complications secondary to hypertension include headaches, epistaxis, exercise intolerance, angina, shortness of breath due to left ventricular dysfunction, heart failure and ruptured cerebral artery aneurysms[37,68]. Other potential mechanisms for hypertension in CoA include endothelial dysfunction, reduced arterial compliance, and blunted baroreceptor function[69].
Patients can also present with colder and fatigued lower extremities, claudication,and abdominal angina. Occasionally, an initial presentation of CoA may include dissection and/or aneurysm/pseudoanuerysm of the aorta and branch vessels,including the spinal and intercostal arteries[70,71]. Complications such as premature coronary atherosclerosis, cerebrovascular events, left ventricular systolic dysfunction,and endocarditis can occur. Timely recognition of CoA and risk factor modification and treatment improves survival but life expectancy remains lower compared to normal individuals Analysis of 80 adolescents who underwent surgical repair of CoA in childhood did not find intracranial aneurysms, implying the possibility of aneurysms developing with age and preferentially in the presence of hypertension[72].Late hypertension may be associated with residual or recurrent obstruction[2]. Hence,ambulatory blood pressure monitoring use can help in early diagnosis of late hypertension[73]. Blood pressure is usually 10%-20% higher in the lower extremities due to wave amplification. An upper to lower extremity pressure gradient of 10 mmHg should raise suspicion of coarctation, and a gradient of 35 mmHg or greater is considered highly specific for CoA[74]. Occasionally, claudication can be noted due to the ischemia of the lower limb. Cardiac auscultation may demonstrate a harsh systolic murmur in the left sternal border with radiation to the inter-scapular region in the back. In the suprasternal notch, an associated thrill may also be palpable, and occasionally, a left ventricular lift can be observed of there is left ventricular pressure or volume overload. The development of arterial collaterals is suggested by continuous murmur, especially in patients with long-standing unrepaired coarctation and may diminish the pressure gradient.

Figure 4 Magnetic resonance imaging demonstrating extensive collaterals in patient with unrepaired coarctation of aorta.
DIAGNOSIS
Initial workup includes blood pressure measurements in all four extremities. Clinical diagnosis is usually based upon the characteristic findings on physical examination such as delayed or diminished femoral pulses, systolic hypertension in the upper extremities, and low or undetectable blood pressure in lower extremities.Confirmation is usually done by imaging techniques; echocardiography being the most widely used initially but three-dimensional techniques like contrast enhanced computed tomography (CT) or cardiovascular magnetic resonance imaging (MRI) are the most cost-effective.
Electrocardiogram
Patients with CoA may have a normal Electrocardiogram (EKG) or show the evidence of left ventricular hypertrophy and dilatation from long-standing left ventricular pressure overload. Ischemic changes may also be found infrequently[38].
Chest radiography
Chest radiograph may show a normal cardiac contour or can be mildly enlarged. A characteristic finding of “Figure of 3” beneath the aortic notch suggests the narrowing of the descending aorta at the level of coarctation and dilatation pre and post coarctation (Figure 5). Bilateral inferior rib notching may also be seen in the third to eighth ribs suggesting the presence of dilated intercostal collateral arteries[68].
Echocardiography
Transthoracic echocardiography (TTE) is the imaging modality most often used in the assessment of cardiac disease but has limitations in evaluating extra cardiac structures and collateral circulation. TTE can help confirm suspected CoA, assessing pressure gradient severity and providing diagnosis of other associated cardiac and valvular abnormalities, most commonly, left-sided obstructive lesions (subvalvular and supravalvular aortic stenosis parachute mitral valve and cor-triatriatum) but especially ruling out the presence of a bicuspid aortic valve.

Figure 5 Chest X-Ray demonstrating rib notching and Figure 3 sign.
The presence of any associated aortopathy can be evaluated by following the serial measurements of aortic root and ascending dimensions. Long axis (candy cane) view demonstrates a focal area of narrowing of the proximal descending thoracic aorta distal to the origin of subclavian artery and color flow Doppler demonstrates associated flow turbulence (Figure 6). The severity of CoA can be estimated using continuous-wave Doppler, by calculating the pressure gradient across the narrowed area, although appropriate correction for velocity proximal to the site of coarctation is important to avoid overestimation of the true gradient[1]. The mean systolic gradient often provides an excellent estimate of coarctation gradient. The severity of CoA can also be estimated by calculating the ratio of the maximal velocity across the coarctation in the suprasternal view to the peak velocity in the abdominal aorta in the subcostal view[75]. If transverse arch hypoplasia is present, the proximal velocity increases as well; therefore, the systolic pressure gradient should be calculated with the Bernoulli equation[76]. Diastolic flow persistence with velocities over 1 m/s is a characteristic finding in CoA (Figure 7). Sometimes, in the presence of collateral blood flow, pressure gradients across the coarctation, may be less severe than expected[77].Severe obstruction, eccentric gradient, or long, tortuous vessels may also affect the Doppler gradient by potentially under or overestimating the degree of severity and echo derived gradient.
Subcostal views are used to assess the distal thoracic and upper abdominal aorta. In healthy individuals without obstruction, pulse wave Doppler in the abdominal aorta shows a rapid systolic upstroke, short deceleration time, followed by a brief early diastolic flow reversal and little anterograde flow throughout diastole. The presence of coarctation causes a delay (> 50 ms from the EKG R wave) and reduction in systolic upstroke velocity (< 55 cm/s) with anterograde diastolic flow persistence and loss of early diastolic flow reversal[2,78](Figure 7). Prolonged diastolic flow and reduced peak systolic velocity are sensitive indicators of aortic obstruction. Transesophageal echocardiography (Figure 8) can provide accurate imaging of the descending aorta,but has limited acoustic windows in the arch because trachea-bronchial shadowing and is not widely used in the diagnosis of CoA.
Cardiac magnetic resonance imaging
Cardiac magnetic resonance imaging (CMR) demonstrates superior visualization of the thoracic aorta when compared to echocardiography and can clearly define the location and severity of CoA, collaterals, branching patterns, and CoA pressure gradients[75,79,80]. Gadolinium-enhanced magnetic resonance angiography, provides excellent flow-independent resolution of vascular structures and is helpful in providing anatomic information in adults prior to surgical or catheter-based intervention, and is also recommended for follow-up imaging[81]. CMR is generally preferred over cardiovascular CT for serial imaging because it lacks ionizing radiation[82]. However, CMR is more susceptible to metallic artifacts than CT, leading to difficulties in the assessment of vessel lumen patency, identifying re-coarctation,aneurysm, or fracture stented patients[68].
Phase-contrast flow imaging is useful to quantify the flow volumes and velocity[83]and to determine the degree of collateral flow. Collateral flow joining the descending aorta is identified by the increase in flow by 30% or more from proximal to distal aorta[84]. Four-dimensional flow MRI is an emergent tool in evaluating the hemodynamic significance of collateral blood flow[85]. CMR should be routinely applied in adult patients who previously had surgical or interventional treatment of coarctation in childhood.

Figure 6 Echocardiographic findings in coarctation of aorta. A: Two-dimensional suprasternal view on transthoracic echocardiogram demonstrating narrowing in the aortic lumen at the isthmus (arrow); B: Color Doppler imaging demonstrating turbulent flow at the site of the coarctation (arrow).
Computed tomographic angiography
Computed tomographic angiography (CTA) also provides invaluable information in the diagnosis and management of patients with CoA. CTA has better spatial resolution, shorter acquisition times, causes less claustrophobia than CMR and allows for the presence of metallic implants as well as previous stent implantation without a signal noise artifact[68]. Similar to CMR, it can be performed to visualize the CoA segment, any aneurysms distal to CoA, hypoplasia of aortic arch, re-coarctation post repair, to follow serial aortic dimensions, and to demonstrate associated vascular anomalies (Figure 9 and Figure 10). However, it requires ionizing radiation and contrast, and cannot provide hemodynamic information such as peak pressure gradient and the degree of collateral circulation[38]. However, dose saving algorithms reduce the radiation exposure.
Cardiac catheterization and angiography
Cardiac catheterization and angiography (CA) i s the gold standard in assessing the pressure gradients across the CoA and provides high-resolution images of the aorta[38].Coarctation is classically defined as a catheterization-measured peak systolic gradient> 20 mmHg. Hemodynamic assessment should include coarctation gradient assessment, right and left heart catheterization including assessment of left ventricular end diastolic pressure. Aortic angiography is obtained to determine the site, severity of obstruction and associated vascular abnormalities[86].
TREATMENT
Indications for intervention (surgical or transcatheter intervention) in coarctation of aorta are: (1) Peak to peak coarctation gradient ≥ 20 mmHg: This gradient can be measured as a difference between systolic blood pressures from the upper and lower extremities, from catheterization data in which peak pressure distal to the coarctation is subtracted from peak pressure proximal to the coarctation, or with echocardiography. However, the resting gradient may be lower in significant left ventricular systolic dysfunction due to the low forward stroke volume[81]; (2)Radiographic evidence of significant collateral flow; (3) Coarctation-attributed systemic hypertension; (4) Coarctation-attributed heart failure; and (5) Exercise limitations from limited lower extremity blood flow (i.e., claudication).
Surgical management
Various surgical techniques have been utilized for CoA repair.

Figure 7 Doppler echocardiographic findings in coarctation of aorta. A: Continuous wave Doppler across the coarctation segment in suprastenrtal view demonstrating significant increase in flow velocity with a peak velocity of 4 m/s, comparable to a peak gradient of 64 mmHg based on simplified Bernoulli's equation;B: Abnormal Doppler pattern in abdominal aorta in a patient with severe coarctation demonstrating blunted velocity with delayed systolic upstroke and continuous diatonic run-off.
Resection with end-to-end anastomosis:Crafoord[87]and Gross[88]described the first successful surgical interventionviaa left lateral thoracotomy in 1945. The intervention involves resection of the coarcted segment followed by direct suture anastomosis of the transected ends (Figure 11). The incidence of re-coarctation was comparatively high following the initial repair, with the incidence rates of 41% to 51%[89-92]and highest in neonates.
Prosthetic patch aortoplasty:Vosschulte first described the usage of a prosthetic patch to augment the aorta (Figure 12)[93]. Dacron grafts showed to have a lower rate of re-coarctation[94], but had a high incidence of aortic aneurysm formation (20%-40%)and are no longer performed[95]despite patch material evolution from Dacron to polytetrafluoroethylene (PTFE), which, reduced aneurysm formation to 7%, but increased the rate of re-coarctation to 25%[96].
Subclavian flap aortoplasty:Waldhausenet al[97]first described the technique in 1966.A flap is generated from the subclavian artery, which is ligated close to the left vertebral artery origin by an incision extending down onto aortic isthmus and across the coarcted segment. Re-coarctation rates are close to 3% in older children, but up to 23% in neonates[98-100]. Sacrificing the subclavian artery might cause claudication in the left arm in the long term, although it usually does not result in left arm ischemia[99].
Extended end-to-end anastomosis:Amatoet al[101]first described the technique in 1977 and is currently frequently applied. The aortic arch is clamped proximally,including the subclavian artery and distally below the coarcted segment (Figure 13).An end-end anastomosis of the aortic arch, which has been opened on its inferior aspect and the descending aorta, is performed after the division of ductus arteriosus.This technique showed lower re-coarctation rates of 4%-13% and low perioperative mortality[102].
Interposition graft:The resection and graft interposition were first described by Gross in 1951[88,103,104]and is the preferred approach in adults. A tube graft of either aortic homograft or Dacron is sewn into the aorta, after the cross-clamping of the aorta and resection of the coarcted segment. It is useful in patients with long-segment CoA; however, it requires longer cross-clamp time and does not grow with the patient thus being unsuitable for pediatric patients[2]. A surgical follow-up study described a high prevalence of dilatation of interposition grafts to 150% of the original diameter,with most dilatation occurring in the first year after the procedure[105]. Dilatation was most pronounced in knotted (Gelseal and Gelsoft; Vaskutek Ltd., Inchinnan,Renfrewshire, Scotland) grafts compared with woven (Gelweave; Vascutek Ltd.,Renfrewshire, United Kingdom) grafts[105]. Hence, routine serial arch imaging is recommended for patients after surgical repair. Another study reported no perioperative mortality or re-coarctation events during a mean follow-up of 10 years ± 7.6 years[106].
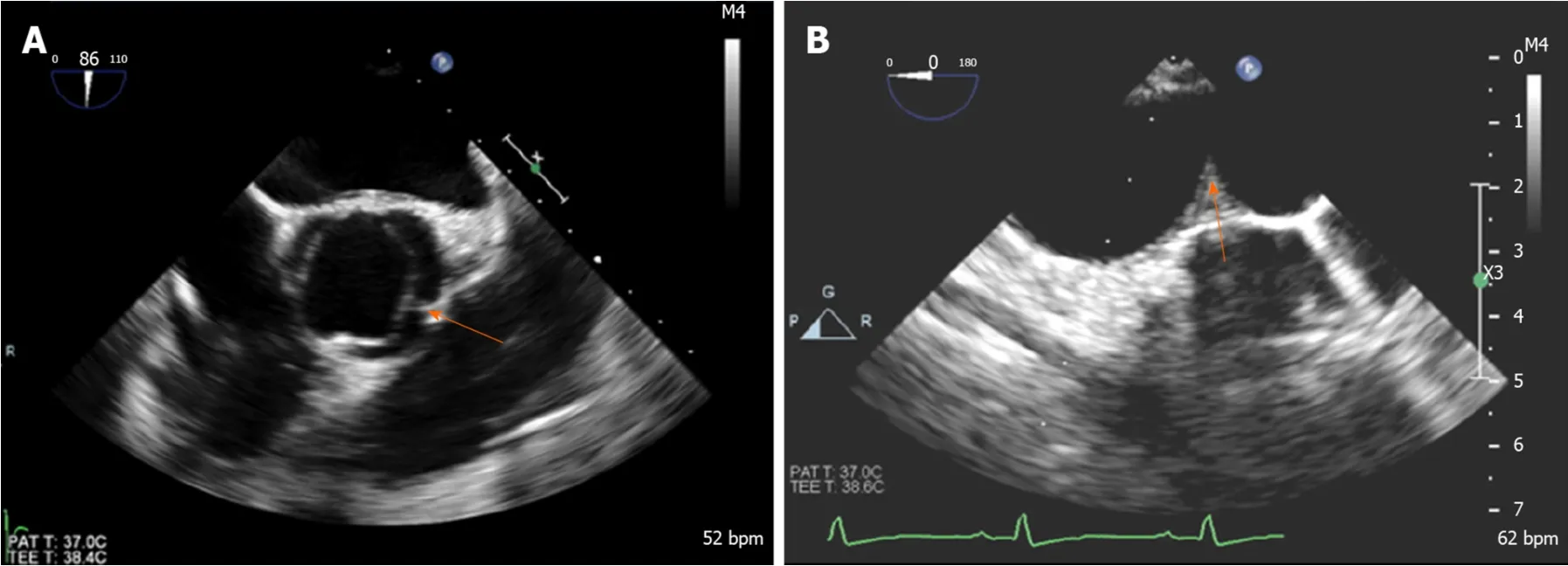
Figure 8 Transesophageal echocardiogram in a patient with coarctation demonstrating. A: Bicuspid aortic valve with raphe between left and right coronary cusps (arrow); B: Coarctation in distal aortic arch (arrow).
Extra-anatomical correction: This procedure is possible when concomitant cardiac procedures such as coronary artery bypass grafting or aortic valve replacement need to be addressed[107]. It is performed in adults through median sternotomy with cardiopulmonary bypass support and provides additional blood flow to the distal aorta leaving the stenosed aorta in situ[108]. A prosthetic conduit is anastomosed proximally to the ascending aorta or distally to the subclavian artery[109], bypassing the coarcted segment. A study of 80 patients who underwent ascending-to-descending aortic bypass (Figure 14) followed for 7 years ± 6 years showed improvement in blood pressure, no early deaths and no paraplegia or stroke. Late deaths occurred in 6%,while 4% required re-intervention (for peri-prosthetic regurgitation and mitral valve replacement)[110]. Some have noted anastomotic stenoses and pseudoanuerysm formation.
When considering surgery, the prognostic benefit and age-related risks for procedural complications need to be discussed. A study assessing re-coarctation 20 years ± 7 years after surgical repair in infancy showed moderate-to-severe recoarctation in 34% of patients highlighting that re-coarctation is common in follow-up patients after surgical repair of CoA[111]. However, surgery might still be the preferred option in the setting of significant arch hypoplasia or long coarctation segments[112].
Catheter based therapies/ endovascular management
Transcatheter procedures play an important and evolving role in the repair of native CoA and the treatment of re-coarctation or aneurysm formation after the initial repair.
Balloon angioplasty:Transcatheter balloon angioplasty was first described by Singeret al[113]in 1982, and became widely used over the following two decades. A balloon catheter is advanced up to the obstructed segment and inflated to induce intimal tear and limited medial tear in the arterial wall. There remains a potential risk for later aneurysm formation with this approach, especially when treating a native CoA[38].Short-term results were satisfactory in infants and children, but long-term outcomes less favorable, with re-coarctation rates of up to 80%[114-117].
A follow-up study of 99 patients who underwent balloon angioplasty for recoarctation showed that re-intervention was required in 28 patients[118]. Comparison of balloon angioplasty with surgery in 80 patients aged 12 years ± 10 years, showed increased aortic complications during follow-up, with 26% requiring re-intervention for re-coarctation and 21% for aneurysm formation[119]. Hence, this approach may be reasonable to stabilize critically ill infants as a bridge to surgery and an alternative for mild coarctation in adolescents. A recent meta-analysis showed that balloon dilation is less likely to achieve treatment success as measured by the proportion of patients achieving a blood pressure gradient ≤ 20 mmHg when compared to stenting. Odds ratio for re-coarctation in patients undergoing balloon dilation versus stenting was 7.01 and 3.340 for aortic wall injuries[120].
Stent implantation:Stent implantation has become the mainstay of interventional treatment for CoA, with a reduction in clinically significant residual gradient and aortic wall abnormalities when compared with balloon angioplasty alone[121]. It improves luminal diameter, results in minimal residual peak pressure gradient and sustained hemodynamic benefit. Additionally, it prevents vascular recoil resulting in re-coarctation and can tack intimal flaps to the aortic wall allowing the wall to heal,reducing the risks of dissection and aneurysm[38]. Balloon-expandable intravascular stents have emerged as the preferred treatment option for patients with native CoA and re-coarctation (Figure 15)[122].

Figure 9 Contrast-enhanced computed tomography demonstrating coarctation of aorta. A: 3D reconstruction image; B: Sagittal cross-sectional view.
Stent placement is considered a reasonable treatment option in patients weighing more than 30 kg, as children weighing less than 30 kg may need repeated interventions for stent expansion in the face of growth with the potential injury risk to the femoral arteries during vascular access for stent delivery[123,124]. Studies support the safety and efficacy of stent placement for both native and recurrent coarctation[125-127].Stent placement was successful in 99% in the Coarctation of the Aorta Stent Trial(COAST) I trial in which 105 children and adults underwent stent implantation for the treatment of native CoA and re-coarctation[127]. Nine patients required re-intervention for aneurysms during the first two years, and 10 patients required re-intervention after two years. Another study of 578 CoA patients who underwent stent placement showed a 98% implantation success rate, which was defined as a reduction in the pressure gradient to < 20 mmHg or a ratio of post-stent coarctation to descending aorta of > 0.8[125,128].
Stents can be classified based on the design as closed-cell, open-cell, and hybrid design, or as bare metal or covered. Cheatham-platinum (CP) stent, Palmaz series,Genesis XD and Intra Stent are some of the stents commonly used. Rarely, formula or Vallejo stents are used in small children as a bridge to anticipated surgery. In closedcell design, all internal inflection points of the structural components are connected,whereas they are not in open-cell design. Open-cells have an advantage of longitudinal flexibility of the cells due to the absence of connections[38]. Stents can be made of different metals such as stainless steel, platinum-iridium alloy, and chromium-cobalt alloy. Covered stents usually have a polytetrafluoroethylene sleeve that can exclude aneurysms from the circulation and may prevent endoleaks within the sleeved section of the stent. Deployment of a second covered stent may be of utility in the acute management of aortic rupture at the time of stenting[129].Premounted covered stents are potentially very valuable for rapid deployment in the event of significant aortic wall injury. Some studies have suggested routine use of covered stents in face of more severe degree of coarctation and in older patients.
Previous studies suggested that bare metal stents might have a higher risk of aneurysm development[95,130,131]but a more recent study compared did not observe this difference[132]. Serial re-dilation of purposely under-deployed, covered stents is a strategy considered in growing children and when there is a concern of aortic rupture by deployment to the size of the normal aorta[133,134]. Table 1 summarizes the major studies that used covered stents in the treatment of CoA.
Complications
Re-coarctation:Factors predisposing to re-coarctation include: (1) Patient age < 30 days[135]; (2) Isthmic hypoplasia[136]; and (3) Coarctation segment diameter of 3.5 mm or less and 6 mm or less prior to angioplasty and post-intervention respectively[135].Persistent post-procedural hypertension, post-procedure peak pressure gradient of ≥20 mmHg, or collaterals usually warrant re-intervention[137-139].

Figure 10 Computed tomography 3D reconstruction images of aortic arch. A: Pre-intervention; B: Postintervention.
Aneurysm:Aneurysm development is dependent on abnormal aortic tissue at the site of CoA. The pathological aorta consists of a medial layer with fragmented elastic fibers, fewer smooth muscles, and a higher amount of ground substance, unlike a pseudoanuerysm which, forms between the tunica media and tunica adventitia[140].Aneurysm can arise proximal or distal to the coarctation and occasionally from the origin of branch vessels such as the left subclavian artery (Figure 16). Incomplete removal of the pathological tissue during surgical repair[141,142]or balloon angioplasty also favors the development of an aneurysm. The distal aortic arch aneurysm formation is highest after subclavian flap and lowest with end-to-end anastomosis[143].Balloon angioplasty is reported to have a higher incidence of aneurysm formation compared to surgery by Shaddyet al[144], while other studies reported a lower incidence at catheterization after balloon angioplasty[145,146]. Stents were shown to have a lower likelihood of aneurysm formation (as low as in 5% of patients) due to reducing trauma to the vessel wall through the dispersion of force[147-149].
Late systemic hypertension:Hypertension is frequently found and a great concern post CoA repair. Age at repair is perhaps the most important predictor[59,150,151].O'Sullivanet al[187]showed a prevalence of hypertension of 30% in patients repaired at a mean age of 0.2 years, at a mean follow-up of 12 years after repair. An abnormal structure and function of the pre-coarctation arterial conduits is the pathological mechanism thought to be involved. Arterial wall compliance is diminished, and rigidity is increased due to more collagen and less smooth muscle mass[50,152-155]. Other factors contributing to hypertension in post-coarctectomy patients are reduced baroreceptor sensitivity and residual aortic arch gradients[156-158]. Hence, patients with persistent hypertension after the repair may need long-term anti-hypertensive treatment.
Need for re-intervention
Surgical treatment for re-intervention:Surgical repair is mandatory when there is a persistent endoleak or prosthetic infection after stent graft placement. The mortality rate in surgical repair for re-intervention is higher than the native CoA repair and ranging from 1%-3%, to 5%-10% in patients with significant comorbidities, although guidelines recommend an endovascular approach initially for re-coarctation[159].
Perioperative complications are often aggravated by dense adhesions including revision for bleeding (2%), pulmonary-associated complications such as pneumothorax and pleural effusion (8%), and trauma of the thoracic duct or the esophagus (0%-4%)[160,161]. Other complications are lower body ischemia and spinal cord trauma with subsequent paralysis.
Endovascular treatment for re-intervention:Guidelines recommend an interventional approach as the initial treatment strategy for re-coarctation[138,139,162,163].Balloon angioplasty is the preferred treatment option for recurrent coarctation after previous surgical repair, although it has a high rate of re-coarctation after native CoA repair[159]. The scar tissue has a lower tendency for vascular remodeling and aortic wall injury occurring in only 1%-2% of cases[118,164]. Studies have shown good outcomes with balloon angioplasty of re-coarctation[164-171], with a high procedural success rate and lower complication rate when compared to surgical repair[145,172]. The procedural success rate ranges between 80%-100%[118,125,126,173], and a simple re-dilation often leads to satisfying results when re-coarctation occurs[174].

Figure 11 Surgical repair of coarctation of aorta. A: End to end anastomosis- Resection of the coarctation segment followed by direct suture anastomosis of the transected ends with associated patent ductus arteriosus; B: End-end anastomosis of coarctation without associated patent ductus arteriosus; C: End-end anastomosis of coarctation with associated aneurysm. L.C.C: Left common carotid; L.S.A: Left subclavian artery; Lig. a: Ligamentum arteriosum.
First described in 1991, stent graft placement is an alternative approach to balloon angioplasty[175]and has been used in treating re-coarctation. The procedural success rates ranges between 94%-97%[149,176]. Figure 17 demonstrates exclusion of aneurysm of proximal descending thoracic aorta by deploying a covered stent across the aneurysm.
OUTCOMES AND FOLLOW-UP
A number of studies have described the outcomes of interventions in CoA patients.The Congenital Cardiovascular Interventional Study Consortium trial and other studies[125,177-180]showed a successful outcome following stent implantation in children and adults, with a 98% success rate of reducing peak pressure gradient to less than 20 mmHg. The COAST[127]trial, which assessed the safety and efficacy of CP stents in children and adults with native or re-coarctation showed no deaths, serious adverse events or need for surgical interventional during the two-year follow-up.Complication rates were low, including aneurysms (5.7%) and stent fracture (11%).Many other studies support a low rate of complications and mortality[177,181]. The COAST II trial studied 158 patients with native and re-coarctation, showing a success rate of 92% with covered CP stent[127]. However, there was no significant difference between bare metal and covered stents in a randomized trial of 120 patients[132]. A study by Forbeset al[182]showed a lower rate of acute complications after stent implantation compared to balloon angioplasty or surgery, similarly to another study by Carret al[183].

Figure 12 Prosthetic patch aortoplasty. A: Coarctation before intervention; B: Aorta is incised longitudinally through the coarctation to increase vessel diameter; C:A prosthetic patch used for augmentation of aorta.
Patients with CoA have a reduced life expectancy and increased risk of morbidity after intervention, despite good long-term survival rates. Follow-up protocols after intervention vary by institution but most commonly, routine visits at 3, 6, and 12 mo in the first year are followed by annual visits, obtaining blood pressure in all limbs and EKG at each visit with consideration for echocardiograms for concerns about restenosis or ventricular function. CTA or CMR can be done at 6-12 mo after the intervention to detect late complications such as aneurysm and repeated at five years or less depending on the findings. Perhaps the most important and prevalent longterm complication is chronic hypertension, occurring in 35%-68% of patients[58,184-185].Furthermore, exercise-induced hypertension occurs in over one-third of normotensive patients at rest[186]. Follow-up should include ambulatory 24 h blood pressure measurement and exercise testing, as exercise-induced hypertension can predict future systemic hypertension[58]. Usually, normotensive patients at rest and with exercise lead normal lives without restriction, excluding extensive static sports.Patients with chronic hypertension, residual obstruction, or other complications should avoid strenuous sports and heavy exercises[162].
PREGNANCY AND COARCTATION OF AORTA
Patients with CoA should be evaluated before pregnancy for appropriate counseling and undergo arch imaging to assess aneurysm formation that might result in complications related to the increased cardiac output during pregnancy. Possible recoarctation should be managed before conception to avoid uncontrolled upper body hypertension and possible reduced placental perfusion. Case studies have reported aortic rupture and dissection occurring with pregnancy after CoA repair but remain rare. However, there is a greater risk of developing hypertension during pregnancy[187]. A study reported acceptable outcomes of pregnancy in patients after CoA repair with no significant cardiovascular complications during pregnancy and delivery[188]. Another study showed that serious complications were uncommon in women with a hemodynamically significant gradient (≥ 20 mmHg) after repair[189].However, women with unrepaired and repaired CoA are at increased risk of arterial hypertension, re-coarctation, aortic dissection or rupture, and rupture of a cerebral aneurysm during pregnancy and delivery.
CONCLUSION
Coarctation of the aorta is a relatively common congenital cardiac defect that often has few symptoms and therefore can be difficult to diagnose. The hallmark physical exam finding is upper extremity hypertension, and for this reason the diagnosis of coarctation of the aorta should be considered in any patient with systemic hypertension with assessment of a lower extremity blood pressure. The presence of a significant arm to leg gradient is highly suggestive of the diagnosis. Early diagnosis and treatment are important as long-term data consistently demonstrate that patients with CoA continue to have a reduced life expectancy and increased risk of cardiovascular complications. Surgical repair has traditionally been the mainstay of therapy for correction but advances in endovascular technology with covered stents and stent grafts now permit nonsurgical approaches for the management of children and adults with native CoA and complications. Persistent hypertension and vascular dysfunction can lead to an increased risk of coronary disease, which remains the greatest cause of long-term mortality. Thus, blood pressure control and periodic reassessment with TTE and CMR for evaluation of the heart and aorta should be performed regularly as cardiovascular complications may occur decades after the intervention.

Figure 13 Extended resection and end-end anastomosis. PDA: Patent ductus Arteriosus; L.C.C: Left common carotid artery; L.S.A: Left subclavian artery.

Table 1 Covered stents in the treatment of coarctation of aorta

Figure 14 Ascending-to-descending aortic bypass. ASC: Ascending; IVC: Inferior venacava; DESC: Descending.

Figure 15 Transcatheter repair of Coarctation of Aorta. A, B: Angiogram of aortic arch demonstrating coarctation of aorta in antero-posterior and lateral views; C,D: Deployment of covered stent in antero-posterior and lateral views; E, F: Post-intervention aortic arch angiogram demonstrating resolution of coarctation in anteroposterior and lateral views.

Figure 16 Aneurysm at the origin of left subclavian corrected by deploying a covered stent across the origin of subclavian artery and concomitant surgical anastomosis of left subclavian to left common carotid artery.

Figure 17 Exclusion of aneurysm of proximal descending thoracic aorta by deploying a covered stent across the aneurysm and concomitant surgical anastomosis of left subclavian to left common carotid artery.
杂志排行
World Journal of Cardiology的其它文章
- New guidelines for the diagnosis and management of pulmonary embolism: Key changes
- Nicotine-induced adrenal beta-arrestin1 upregulation mediates tobacco-related hyperaldosteronism leading to cardiac dysfunction
- Access to smart devices and utilization of online health resources among older cardiac rehabilitation participants
- Preoperative nuclear stress testing in the very old patient population
- Incidental discovery of right ventricular lipoma in a young female aImaging investigations and diagnosissociated with ventricular hyperexcitability: An imaging multimodality approach
