烟粉虱MEAM1隐种主要内共生菌的定位和分布
2020-06-08陈吉强张毅波张桂芬
陈吉强 张毅波 张桂芬

摘要 烟粉虱MEAM 1隐种是一种重要的世界性入侵害虫,共生菌在其种群入侵和扩张中起着不可忽视的作用。本研究采用荧光原位杂交技术(FISH),明确其携带的初生共生菌(Candidatus Portiera aleyrodidarum)和次生共生菌(Candidatus Hamiltonella defensa和Rickettsia sp.)在不同发育阶段虫体中的定位和分布。结果显示,烟粉虱MEAM 1隐种卵、若虫和成虫3个阶段均携带3种共生菌,Portiera和Hamiltonella菌集中分布于含菌细胞中,其中Hamiltonella呈环形包裹于含菌细胞周边;Rickettsia既在菌胞中呈“集中”分布,也“随机”分布于虫体其他部位或器官。上述结果明确了这3种共生菌在MEAM 1烟粉虱各个发育阶段的分布特征,同时证明3种共生菌均能通过烟粉虱雌虫垂直传播,为进一步揭示内共生菌在烟粉虱入侵过程中的作用打下基础。
关键词 烟粉虱; 共生菌; 荧光原位杂交; 垂直传播
中图分类号: S 433.3 文献标识码: A DOI: 10.16688/j.zwbh.2019072
Abstract Bemisia tabaci (Gennadius) MEAM 1 is one of the most devastating invasive pests worldwide and its endosymbionts play an important role in the invasion and expansion of this pest insect. In this study, the fluorescence in situ hybridization (FISH) was used to identify the localization and distribution of the primary endosymbiont (Candidatus Portiera aleyrodidarum) and the secondary endosymbionts (Candidatus Hamiltonella defensa and Rickettsia sp.) in different developmental stages of B.tabaci MEAM 1. Three symbiotic bacteria were found in the eggs,nymphs,and adults of B.tabaci MEAM 1. Among them, Portiera and Hamiltonella were located inside the bacteriocytes and Hamiltonella around the bacteriocyte. Rickettsia was located both inside the bacteriocyte and randomly distributed in other parts or organs. These results revealed the distribution characteristics of the three endosymbionts in different developmental stages of B.tabaci MEAM 1, and provided evidence that all the three endosymbionts are vertically transmitted through the female of B.tabaci, and thus lay a foundation for further understanding the roles of endosymbionts in the invasion of B.tabaci.
Key words Bemisia tabaci; endosymbionts; fluorescence in situ hybridization; vertically transmitted
烟粉虱Bemisia tabaci (Gennadius)属半翅目Hemiptera,粉虱科Aleyrodidae,是一種典型高度多食性的取食植物韧皮部汁液的世界性入侵害虫。烟粉虱通过直接取食为害植物每年可导致数亿美元的经济损失,还可传播100多种植物病毒及分泌蜜露污染植物[1-4]。烟粉虱被认为是一个独特的,由超过35种形态上难以区分的隐种组成的复合种[5-9]。烟粉虱隐种以前被称为生物型,其中在我国分布最广泛的入侵种是B型和Q型,而B型也被称为“中东-小亚细亚1”隐种(MEAM 1)或者Bemisia argentifolii,Q型也被称为“地中海”隐种(MED)[10]。在过去的30年里,这两个隐种在世界范围造成了巨大的农业损失[11-12]。
烟粉虱MEAM 1隐种在20世纪90年代中期传入我国,并且很快取代了中国本地种,而MED隐种于2003年第一次在我国云南昆明的观赏性植物一品红上被检测到[13]。之后,MED隐种在许多省市和区域逐渐取代了MEAM 1隐种。从2008年起,在我国大部分地区的烟粉虱种群变成了以MED隐种为主[14-15];而烟粉虱MEAM 1隐种主要分布于我国长江以南和西南沿海地区,包括广东、福建、浙江等省份,另外新疆、甘肃和辽宁等地区也零星分布[4]。MEAM 1隐种具有较高的入侵能力和竞争力,MED隐种的入侵性较差,但其具有较强的抗药性[16-18]。有研究表明双重感染Rickettsia-Arsenophonus菌或Wolbachia-Arsenophonus菌的MED烟粉虱对啶虫脒、噻虫嗪、吡虫啉和螺甲螨酯的抗药性较高[19]。这可能是MED隐种取代MEAM 1隐种成为我国主要入侵烟粉虱的原因之一。近年来,也有研究者认为,共生菌在烟粉虱种群扩散和入侵方面发挥了重要作用,因此,可把内共生菌作为对象来研究其对烟粉虱不同隐种的生物学特性、入侵能力及隐种间取代的影响[20-21]。
烟粉虱体内的共生菌分为初生内共生菌和次生内共生菌。初生内共生菌为“Candidatus Portiera aleyrodidarum”,能够为其提供维持生命所需的必需氨基酸和类胡萝卜素来弥补取食韧皮部汁液所缺乏的营养[22-24]。此外,不同烟粉虱隐种通常还含有不同的次生内共生菌,因此,烟粉虱复合种中可能含有一种或多种次生内共生菌,目前已在烟粉虱复合种中发现了7属/种次生内共生菌,分别命名为Candidatus Hamiltonella defensa、Candidatus Wolbachia spp.、Arsenophonus spp.、Candidatus Cardinium hertigii、Candidatus Fritschea bemisiae、Rickettsia sp.和Candidatus Hemipteriphilus asiaticus[25-29]。目前对烟粉虱次生内共生菌的研究主要集中在其传播方式、分布形式以及功能上[20,25,30-34]。烟粉虱次生内共生菌的功能主要表现在提高烟粉虱的适合度(包括产卵量、存活率和性比偏雌性)[32-33]、高温耐受性和提高番茄黄化曲叶病毒(TYLCV)的传播速率[34]以及增加烟粉虱的抗药性[35]等方面。烟粉虱次生内共生菌是一个复杂的群体,其可随物种、寄主植物和地理分布的不同而变化[32]。例如,以色列的MEAM 1隐种携带有Hamiltonella和Rickettsia菌,而MED隐种则携带Rickettsia、Wolbachia和Arsenophonus菌,但Cardinium和Fritschea菌在这两个隐种中均未检测到,并且鼠尾草上的MED隐种中Rickettsia和Arsenophonus菌的感染率显著高于其他寄主植物上的MED隐种[36]。在我国,除了Fritschea菌外,Wolbachia、Rickettsia、Hamiltonella、Cardinium和Arsenophonus菌在MEAM 1和MED隐种中均能检测到,其中Hamiltonella菌在两个种群中的感染率均最高,Arsenophonus却相反,并且Wolbachia、Rickettsia和Hamiltonella的感染率在MEAM 1中显著高于MED隐种,而在MEAM 1中,Cardinium的感染率却显著低于MED隐种[21]。
目前,针对我国烟粉虱MEAM1隐种体内主要共生菌的定位和分布还不是很清楚。本研究选取我国境内的烟粉虱MEAM 1隐种为对象,采用荧光原位杂交技术(fluorescence in situ hybridization,简称FISH技术),明确烟粉虱MEAM1隐种体内3种主要内共生菌Portiera、Hamiltonella和Rickettsia的具体定位和分布,研究结果将为进一步揭示内共生菌对烟粉虱生活史和入侵能力的影响打下基础。
1 材料与方法
1.1 供试昆虫
试验使用的原始种群于2017年采自中国甘肃酒泉。参照罗晨等[37]的方法,采用分子方法对种群进行鉴定,确定为MEAM 1隐种,同时建立室内种群。
1.2 供试植物
寄主植物为棉花Gossypium spp.,品种为‘创优棉168。将种子播种至塑料花盆中,每盆3粒,并置于大型养虫笼中,保证寄主植物的清洁。培养条件:28℃±2℃,相对湿度:70%~80%。待棉花长出4~6片叶时供试。
1.3 试验方法
1.3.1 探针的制备
本试验所使用的不同共生菌的探针序列如下:初生内共生菌Portiera:5′-Cy3-TGTCAGTGTCAGCCCAGAAG-3′,次生内共生菌Rickettsia:5′-Cy5-TCCACGTCGCCGTCTTGC-3′,Hamiltonella:5′- Cy5-CCAGATTCCCAGACTTTACTCA-3′[38],探针由生工生物工程(上海)股份有限公司合成。
1.3.2 各阶段虫态的收集
在显微镜下用解剖针分别挑取卵、不同龄期若虫100头,用吸虫管取成虫若干头,置于1.5 mL的离心管中,3个重复。卵从卵柄部位进行挑取,其他龄期若虫从虫体一侧进行挑取。
1.3.3 原位雜交
首先将收集的样本分别置于适量Carnoy固定液中过夜处理,然后弃掉固定液;用50%乙醇漂洗3次;加入200 μL Triton X-100(细胞渗透液),金属浴35℃,30 min,然后弃掉细胞渗透液;加入400 μL蛋白酶K,金属浴56℃,30 min,然后弃掉蛋白酶K;加入适量的Carnoy固定液固定过夜,弃掉后加入200 μL含有6% H2O2的乙醇溶液4℃过夜处理;用杂交缓冲液洗涤3次,加入100 μL杂交缓冲液和50 μL 10 pmol/mL荧光探针,金属浴60℃,避光处理24 h;杂交缓冲液洗涤2次。
采用LSM T-PMT Carl Zeiss蔡司激光共聚焦显微880观察拍照,Carl Zeiss蔡司照片处理软件ZEN 2012对照片进行处理。
2 结果与分析
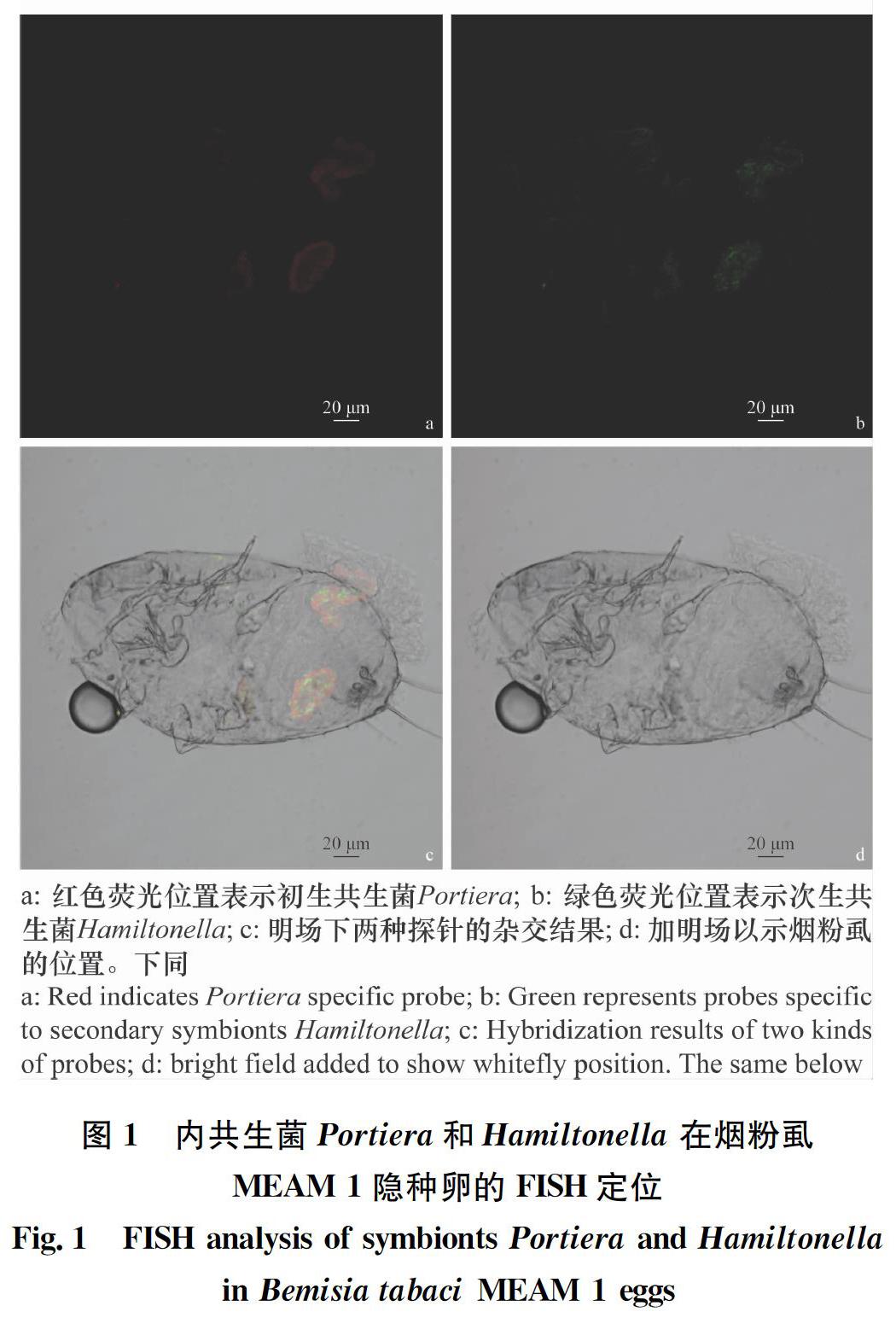
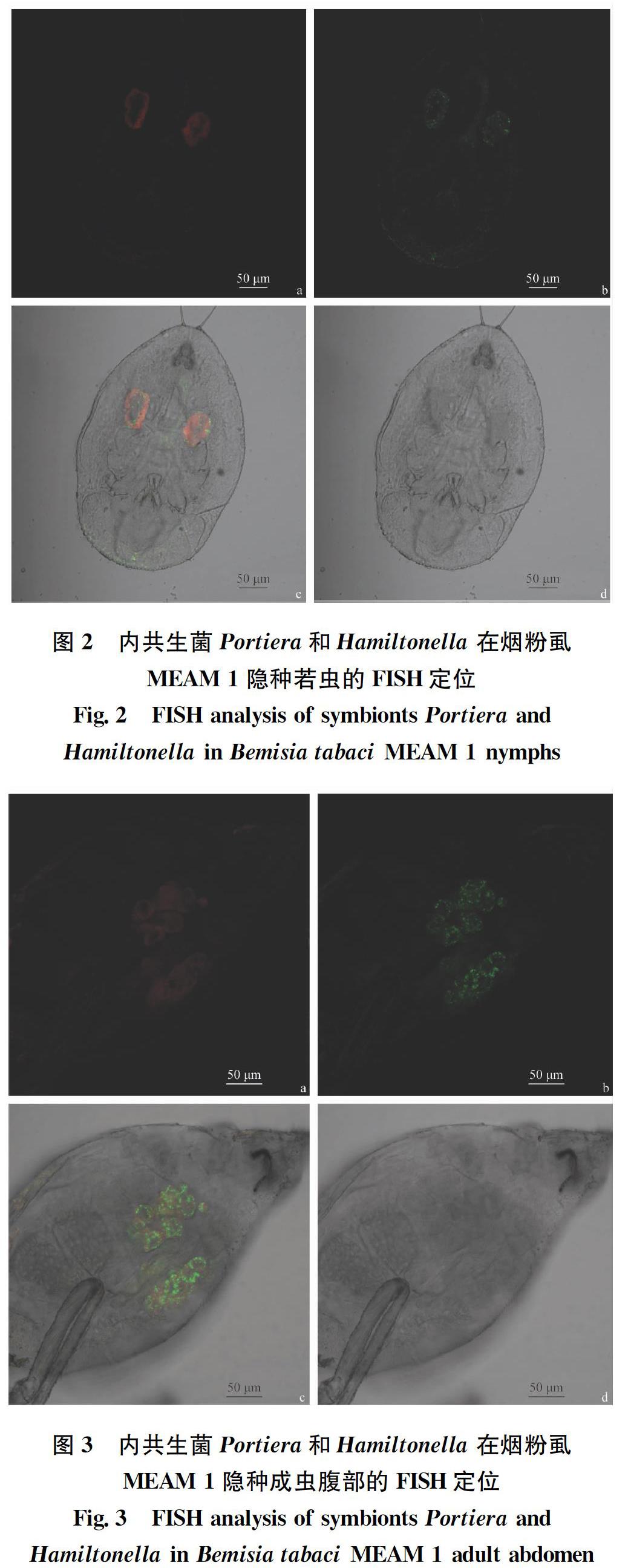
2.1 烟粉虱MEAM 1隐种中Portiera和Hamiltonella的定位 经FISH检测Portiera和Hamiltonella在烟粉虱MEAM 1隐种卵(图1)、若虫(图2)和成虫(图3)3个阶段均有分布,且两种共生菌均“集中”分布于含菌细胞内,其中Portiera聚集分布于含菌细胞的中心部位,Hamiltonella呈环形包裹于含菌细胞周边。
2.2 烟粉虱MEAM1隐种携带Portiera和Rickettsia的定位 经FISH检测发现Portiera和Rickettsia在烟粉虱MEAM 1隐种各虫态均有分布,初生内共生菌Portiera“集中”分布于含菌细胞内,次生内共生菌Rickettsia分布在含菌细胞内,也“随机”分布于虫体其他部位或器官的两种分布类型(图4~6)。
3 討论
烟粉虱的初生内共生菌Portiera集中分布于含菌细胞中,能够为烟粉虱补充植物韧皮部汁液中缺失的营养物质,比如类胡萝卜素等。Gottlieb等[38]证实以色列的MEAM 1隐种和MED隐种的初生内共生菌均集中分布于含菌细胞中。Singh等[39]发现印度的Asia Ⅰ和Asia Ⅱ两个隐种均携带有初生内共生菌且均集中分布于含菌细胞中。我们的试验结果与前人的研究结果是一致的。
次生内共生菌Hamiltonella也广泛分布于不同的烟粉虱隐种和不同地理种群中。在我国,除了几种烟粉虱本地隐种外,入侵烟粉虱MEAM 1和MED两种隐种均携带有此种内共生菌[40]。本研究结果发现入侵我国的烟粉虱MEAM 1隐种携带的次生共生菌Hamiltonella分布于含菌细胞中但呈环形分布,包裹在初生共生菌的周边。Su等[41]发现我国烟粉虱MED种群携带有次生内共生菌Hamiltonella,且通过原位杂交技术证实该菌的分布特征跟初生共生菌Portiera类似,主要分布于含菌细胞中。Gottlieb等[38]和Singh等[39]均在不同烟粉虱隐种和不同地理种群中发现类似的结果。
本研究发现,烟粉虱MEAM 1隐种携带的Rickettsia不仅存在于含菌细胞中,还随机分布于其他不同的器官和部位,证明Rickettsia在MEAM 1隐种中的分布包含“集中”分布和“随机”分布两种不同类型。类似地,Gottlieb等[25]对MEAM1 烟粉虱中不同发育阶段的Rickettsia进行了定位观察,发现在卵期、若虫期以及成虫期均有分布。蛹期烟粉虱的Rickettsia主要存在肠道中,成虫期主要分布于腹部和肠道附近,集中分布于中肠中,推测其可能在食物消化过程中发挥作用;此外,Rickettsia还随机分布于寄主全身[25]。然而,Brumin等[31]利用电子荧光显微镜来研究Rickettsia入侵的特性,发现除含菌细胞外,烟粉虱的消化器官、唾液腺、生殖器官均被感染;同时观察到类似Rickettsia的信号附着在菌胞外表面并可能被菌胞吞噬,该现象与Munderloh和Kurtti[42]所报道的Rickettsia能通过引起吞噬泡的形成而频繁地在寄主细胞和组织之间移动的结论一致。总之,昆虫体内Rickettsia的分布研究为未知的细菌-昆虫间特殊相互作用和可能的传播途径提供了线索。
共生菌在寄主中的传播方式主要有水平传播和垂直传播两种。Caspi-Fluger等[30]研究发现Rickettsia菌可以通过寄主转移到植物韧皮部细胞中,植物作为Rickettsia菌水平传播的储存器介导了昆虫与共生菌之间的水平传播。而烟粉虱体内携带的内共生菌的垂直传播与含菌细胞有密切联系。Himler等[20]研究发现Rickettsia菌在烟粉虱体内通过母代垂直传播率达到99.17%,在同一寄主植物上,感染Rickettsia菌的雄虫与未感染的雌虫之间没有水平传播。Brumin等[31]研究发现Rickettsia菌没有分布在含菌细胞内,而是通过早期侵入卵母细胞并存在于卵泡细胞和细胞质中,待卵子成熟后,它大部分被排除在外,然而,一些细菌细胞留在卵子中,确保它们转移到后代。Rickettsia菌的垂直传播主要由卵母细胞介导而不是含菌细胞。初生内共生菌Portiera和次生内共生菌Hamiltonella存在于含菌细胞内[38-39],并通过烟粉虱雌虫由含菌细胞垂直传递给子代。本研究结果证明3种共生菌均能通过烟粉虱雌虫垂直传播给子代,但是垂直传播的过程和机制有待一进步研究。
利用荧光原位杂交技术对烟粉MEAM1隐种携带的初生内共生菌Portiera和次生内共生菌Hamiltonella和Rickettsia定位研究表明,在烟粉虱各个发育阶段,3种内共生菌均有分布,其中Portiera和Hamiltonella集中分布于含菌细胞中,而Rickettsia既分布于含菌细胞中,也均匀分布于虫体其他部位。这些结果一方面证明了3种共生菌均能通过烟粉虱雌虫完成垂直传播,同时也明确了这些共生菌在入侵烟粉虱MEAM 1隐种各个发育阶段的分布特征,研究结果为进一步揭示内共生菌参与的烟粉虱入侵机制打下基础。
参考文献
[1] BROWN J K, FROHLICH D R, ROSELL R C. The sweet potato or silverleaf whiteflies: Biotypes of Bemisia tabaci or a species complex?[J]. Annual Review of Entomology, 1995, 40(1): 511-534.
[2] OLIVEIRA M R V, HENNEBERRY T J, ANDERSON P. History, current status, and collaborative research projects for Bemisia tabaci [J]. Crop Protection, 2001, 20(9): 709-723.
[3] JONES D R. Plant viruses transmitted by whiteflies [J]. European Journal of Plant Pathology, 2003, 109(3): 195-219.
[4] WAN Fanghao, YANG Nianwan. Invasion and management of agricultural alien insects in China [J]. Annual Review of Entomology, 2016, 61(1): 77-98.
[5] BOYKIN L M, SHATTERS R G J R, ROSELL R C, et al. Global relationships of Bemisia tabaci (Hemiptera: Aleyrodidae) revealed using Bayesian analysis of mitochondrial COI DNA sequences[J]. Molecular Phylogenetics and Evolution, 2007, 44(3): 1306-1319.
[6] DE BARRO P J, LIU Shusheng, BOYKIN L M, et al. Bemisia tabaci: a statement of species status [J]. Annual Review of Entomology, 2010, 56(1): 1-19.
[7] DINSDALE A, COOK L, RIGINOS C, et al. Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries [J]. Annals of the Entomological Society of America, 2010, 103(2): 196-208.
[8] LIU Shusheng, COLVIN J, DE BARRO P J. Species concepts as applied to the whitefly Bemisia tabaci systematics: How many species are there?[J]. Journal of Integrative Agriculture, 2012, 11(2): 176-186.
[9] FIRDAUS S, VOSMAN B, HIDAYATI N, et al. The Bemisia tabaci species complex: additions from different parts of the world [J]. Insect Science, 2013, 20(6): 723-733.
[10] TAY W T, EVANS G A, BOYKIN L M, et al. Will the real Bemisia tabaci please stand up?[J/OL]. PLoS ONE, 2012, 7(11): e50550.
[11] DALTON R. Whitefly infestations: the christmas invasion[J]. Nature, 2006, 443(7114): 898-900.
[12] ZHANG Changrong, SHAN Hongwei, XIAO Na, et al. Differential temporal changes of primary and secondary bacterial symbionts and whitefly host fitness following antibiotic treatments [J/OL]. Scientific Reports, 2015, 5: 15898.
[13] CHU Dong, ZHANG Youjun, BROWN J K, et al. The introduction of the exotic Q biotype of Bemisia tabaci from the Mediterranean region into China on ornamental crops [J]. Florida Entomologist, 2006, 89(2): 168-174.
[14] CHU Dong, WAN Fanghao, ZHANG Youjun, et al. Change in the biotype composition of Bemisia tabaci in Shandong province of China from 2005 to 2008 [J]. Environmental Entomology, 2010, 39(3): 1028-1036.
[15] PAN Huipeng, CHU Dong, GE Daqing, et al. Further spread of and domination by Bemisia tabaci biotype Q on field crops in China [J]. Journal of Economic Entomology, 2011, 104(3): 978-985.
[16] PASCUAL S, CALLEJAS C. Intra- and interspecific competition between biotypes B and Q of Bemisia tabaci (Hemiptera: Aleyrodidae) from Spain[J]. Bulletin of Entomological Research, 2004, 94(4): 369-375.
[17] HOROWITZ A R, GORMAN K, ROSS G, et al. Inheritance of pyriproxyfen resistance in the whitefly, Bemisia tabaci (Q Biotype) [J]. Archives of Insect Biochemistry and Physiology, 2003, 54(4): 177-186.
[18] DENNEHY T J, DEGAIN B A, HARPOLD V S, et al. Extraordinary resistance to insecticides reveals exotic Q biotype of Bemisia tabaci in the New World [J]. Journal of Economic Entomology, 2010, 103(6): 2174-2186.
[19] GHANIM M, KONTSEDALOV S. Susceptibility to insecticides in the Q biotype of Bemisia tabaci is correlated with bacterial symbiont densities [J]. Pest Management Science, 2009, 65(9): 939-942.
[20] HIMLER A G, ADACHI-HAGIMORI T, BERGEN J E, et al. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias [J]. Science, 2011, 332(6026): 254-256.
[21] CHU D, GAO C S, DE BARRO P, et al. Further insights into the strange role of bacterial endosymbionts in whitefly, Bemisia tabaci: comparison of secondary symbionts from biotypes B and Q in China [J]. Bulletin of Entomological Research, 2011, 101(4): 477-486.
[22] SLOAN D B, MORAN N A.Endosymbiotic bacteria as a source of carotenoids in whiteflies [J]. Biology Letters, 2012, 8(6): 986-989.
[23] SANTOS-GARCIA D, FARNIER P A, BEITIA F J, et al. Complete genome sequence of “Candidatus Portiera aleyrodidarum” BT-QVLC, an obligate symbiont that supplies amino acids and carotenoids to Bemisia tabaci [J]. Journal of Bacteriology, 2012, 194(23): 6654-6655.
[24] RAO Qiong, ROLLAT-FARNIER P A, ZHU Dantong, et al. Genome reduction and potential metabolic complementation of the dual endosymbionts in the whitefly Bemisia tabaci [J]. BMC Genomics, 2015, 16(1): 226-239.
[25] GOTTLIEB Y, GHANIM M, CHIEL E, et al. Identification and localization of a Rickettsia sp. in Bemisia tabaci (Homoptera: Aleyrodidae) [J]. Applied and Environmental Microbiology, 2006, 72(5): 3646-3652.
[26] NIRGIANAKI A, BANKS G K, FROHLICH D R, et al. Wolbachia infections of the whitefly Bemisia tabaci [J]. Current Microbiology, 2003, 47(2): 93-101.
[27] BING Xiaoli, YANG Jiao, ZCHORI-FEIN E, et al. Characterization of a newly discovered symbiont in the whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) [J]. Applied and Environmental Microbiology, 2013, 79(2): 569-575.
[28] EVERETT K D E, THAO M L, HORN M, et al. Novel chlamydiae in whiteflies and scale insects: endosymbionts ‘Candidatus Fritschea bemisiae strain Falk and ‘Candidatus Fritschea eriococci strain Elm [J]. International Journal of Systematic and Evolutionary Microbiology, 2005, 55(4): 1581-1587.
[29] ZCHORI-FEIN E, BROWN J K. Diversity of prokaryotes associated with Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) [J]. Annals of the Entomological Society of America, 2002, 95(6): 711-718.
[30] CASPI-FLUGER A, INBAR M, MOZES-DAUBE N, et al. Horizontal transmission of the insect symbiont Rickettsia is plant-mediated [J]. Proceedings of the Royal Society B: Biological Sciences, 2012, 279(1734): 1791-1796.
[31] BRUMIN M, LEVY M, GHANIM M. Transovarial transmission of Rickettsia spp. and organ-specific infection of the whitefly Bemisia tabaci [J]. Applied and Environmental Microbiology, 2012, 78(16): 5565-5574.
[32] CASS B N, HIMLER A G, BONDY E C, et al. Conditional fitness benefits of the Rickettsia bacterial symbiont in an insect pest [J]. Oecologia, 2015, 180(1): 169-179.
[33] CASS B N, YALLOUZ R, BONDY E C, et al. Dynamics of the endosymbiont Rickettsia in an insect pest [J]. Microbial Ecology, 2015, 70(1): 287-297.
[34] KLIOT A, CILIA M, CZOSNEK H, et al. Implication of the bacterial endosymbiont Rickettsia spp. in interactions of the whitefly Bemisia tabaci with tomato yellow leaf curl virus [J]. Journal of Virology, 2014, 88(10): 5652-5660.
[35] KONTSEDALOV S, ZCHORI-FEIN E, CHIEL E, et al. The presence of Rickettsia is associated with increased susceptibility of Bemisia tabaci (Homoptera: Aleyrodidae) to insecticides[J]. Pest Management Science, 2008, 64(8): 789-792.
[36] CHIEL E, GOTTLIEB Y, ZCHORI-FEIN E, et al. Biotype-dependent secondary symbiont communities in sympatric populations of Bemisia tabaci [J]. Bulletin of Entomological Research, 2007, 97(4): 407-413.
[37] 羅晨, 姚远, 王戎疆, 等. 利用 mtDNA COI 基因序列鉴定我国烟粉虱的生物型[J]. 昆虫学报, 2002, 45(6): 759-763.
[38] GOTTLIEB Y, GHANIM M, GUEGUEN G, et al. Inherited intracellular ecosystem: symbiotic bacteria share bacteriocytes in whiteflies [J]. The FASEB Journal, 2008, 22: 2591-2599.
[39] SINGH R H, SINGH A, POPLI S, et al. Infection of bacterial endosymbionts in insects: a comparative study of two techniques viz PCR and FISH for detection and localization of symbionts in whitefly, Bemisia tabaci [J/OL]. PLoS ONE, 2015, 10(8):e0136159.
[40] BING Xiaoli, RUAN Yongming, RAO Qiong, et al. Diversity of secondary endosymbionts among different putative species of the whitefly Bemisia tabaci [J]. Insect Science, 2013, 20(2): 194-206.
[41] SU Qi, OLIVER K M, PAN H P, et al. Facultative symbiont Hamiltonella confers benefits to Bemisia tabaci, an invasive agricultural pest worldwide[J]. Environmental Entomology, 2013, 42: 1265-1271.
[42] MUNDERLOH U G, KURTTI T J. Cellular and molecular interrelationships between ticks and prokaryotic tick-borne pathogens[J]. Annual Review of Entomology, 1995, 40: 221-243.
(责任编辑: 田 喆)
