A型肉毒毒素影响透明质酸钠凝胶酶解反应的实验研究
2020-06-01张珍珍张向东
张珍珍 张向东

[摘要]目的:探讨A型肉毒毒素(Botulinum toxin type A,BTX-A)对透明质酸钠凝胶(Hyaluronic acid,HA)酶解反应的影响。方法:①分别取海薇和润百颜HA凝胶各5份,每份0.05ml,经2%甲苯胺蓝染色,实验组分别加入2.5U、5.0U、10.0U、20.0U BTX-A,对照组加入生理盐水1.0ml(0U BTX-A);再加入300.0U透明质酸酶,显微镜下观察HA凝胶的酶解状态并采集图片,采用Image J软件计算平均光密度值(OD)及其代谢率;②大鼠背部两侧皮下各注射润百颜HA凝胶0.25ml,左侧真皮层注射BTX-A 5.0U,右侧注射生理盐水0.1ml,分别在注射前(0d)、注射后3、7、14、30、60、90d对注射区域皮肤组织取材,检测透明质酸酶-1(HYAL-1)和透明质酸酶-2(HYAL-2)的表达。结果:①在0U、2.5U、5.0U、10.0U BTX-A作用下,随着BTX-A剂量的增加,HA凝胶的代谢率逐渐减小,润百颜分别在60min、70min、80min、100min达到酶解平衡状态,海薇分别在60min、70min、90min、100min达到酶解平衡状态;20.0U BTX-A对HA凝胶的抑制作用与10.0U BTX-A相似,两种HA凝胶均在100min达到酶解平衡状态。相同酶解条件下,在40min后,润百颜较海薇產品代谢速度增快(P<0.001);②注射后3、7d,BTX-A组大鼠皮肤组织中HYAL-1的表达较生理盐水组降低(P<0.05);注射后3、7、14d,BTX-A组HYAL-2的表达较生理盐水组降低(P<0.05)。结论:BTX-A对透明质酸酶的活性具有抑制作用,并可降低大鼠皮肤组织中HYAL-1、HYAL-2的表达。
[关键词]A型肉毒毒素;透明质酸钠凝胶;透明质酸酶;酶解作用
[中图分类号]Q946 [文献标志码]A [文章编号]1008-6455(2020)05-0110-07
Abstract: Objective To investigate the effect of botulinum toxin type A(BTX-A), on the enzymatic degradation of hyaluronic acid gel(HA). Methods ①Divided two samples of Matrifill and Biohyalux into 5 parts each with a volume of 0.05ml, and stained with 2% toluidine blue solution. The experimental group was respectively added 2.5U, 5.0U, 10.0U, 20.0U BTX-A, and the control group(0U BTX-A) was added with 1.0ml of normal saline. Then added hyaluronidase at a dose of 300.0U, observed the status of HA under the microscope and collect pictures. The average optical density value(OD), and metabolic rate were calculated by Image J software. ②Biohyalux was injected into the subcutaneous tissue of the back of rats with a volume of 0.25ml on each side. BTX-A 5.0U was injected into the dermal tissue layer on the left side, and 0.1ml of normal saline was injected on the right side.The skin tissues of the injection area were taken before the injection(0d), and 3, 7, 14, 30, 60, 90 days after the injection for detected the expression of HYAL-1, HYAL-2. Results ①Under the effects of BTX-A at 0U, 2.5U, 5.0U and 10.0U, as the dose of BTX-A increased, the metabolic rate of HA gradually decreased. The product of Biohyalux basically reached an equilibrium state of enzyme degradation at 60min, 70min, 80min and 100min. And Matrifill basically reached an equilibrium state of enzyme degradation at 60min, 70min, 90min and 100min. The inhibitory effect of 20.0U BTX-A on HA gel was similar to that of 10.0U BTX-A. Both HA gels reached the enzymatic hydrolysis equilibrium state at 100min. Under the same enzymolysis conditions, Biohyalux had a faster metabolism rate than Matrifill after 40min (P<0.001). ②On the 3 and 7 days after injection, the expression of HYAL-1 in the skin tissues of the BTX-A group was lower than those of the normal saline group (P<0.05). On the 3, 7 and 14 days, the HYAL-2 expression of the BTX-A group was lower than those of the normal saline group (P<0.05). Conclusion BTX-A has an inhibitory effect on the activity of hyaluronidase and reduces the expression of HYAL-1 and HYAL-2 in rat skin tissue.
Key words: botulinum toxin type A(BTX-A); hyaluronic acid(HA); hyaluronidase(HYAL); enzymatic hydrolysis
近年来,随着医学美容技术的成熟,多种药物联合注射广泛应用于面部微整形领域。其中,A型肉毒毒素(Botulinum toxin type A,BTX-A)和透明质酸(Hyaluronic acid,HA)作为最常用的注射药物,两者联合使用因其协同作用备受临床医师及就医者的青睐,但鲜有相关文献指出其协同作用的发生机制。另外,临床上因注射HA导致组织缺血坏死、视力受损甚至失明等严重并发症也时有报道[1-4],需及时注射透明质酸酶进行溶解,目前尚无实验证实两者联合注射对透明质酸酶活性的影响。因此,本实验初步探究BTX-A对透明质酸酶的影响,探讨两者联合使用的协同作用机制并提供实验依据。
1 材料和方法
1.1 主要实验材料:注射用交联透明质酸钠凝胶(海薇)(上海其胜生物制剂有限公司,0.5ml);注射用修饰透明质酸钠凝胶(润百颜)(山东济南华熙福瑞达生物医药有限公司,0.5ml);注射用玻璃酸酶(上海第一生化药业公司,1 500U/支);注射用A型肉毒毒素(衡力,兰州生物制品研究所有限责任公司,100U/瓶);甲苯胺蓝粉剂(武汉赛维尔生物科技有限公司,5g);透明质酸酶-1抗体(北京博奥森生物技术有限公司,50μl)、透明质酸酶-2抗体(武汉三鹰生物技术有限公司,100μl)。注:玻璃酸酶,即透明质酸酶,全文统称为透明质酸酶。
1.2 动物模型:SPF级wistar大鼠28只(雌雄各半),购买于中国农业科学院兰州兽医研究所[许可证号:SCXK-(甘)2015-001],体重(200±20)g。实验经过兰州大学第二医院动物伦理委员会批准,实验在兰州大学第二医院实验动物中心进行[实验动物使用许可证号:SYXK-(甘)2018-0003]。
1.3 實验方法
1.3.1 体外酶解实验:取海薇及润百颜样本0.05ml置于直径3.5cm的培养皿中,0.025ml的2%甲苯胺蓝染色10min后,加入2.0ml蒸馏水稀释,放置于4℃冰箱中,12h后取出,25℃室温下恢复1h,分别加入2.5U(2.5U/ml)、5.0U(5.0U/ml)、10.0U(10.0U/ml)、20.0U(20.0U/ml) BTX-A及生理盐水1.0ml(0U BTX-A),再加入300.0U透明质酸酶[5],混匀,立即在显微镜(40×)下观察HA凝胶的酶解状况。
1.3.2 BTX-A对大鼠皮肤组织中HYAL-1、HYAL-2的影响:大鼠经10%水合氯醛(0.03ml/kg)腹腔注射麻醉,注射部位选择在背部两侧距离脊柱1.0cm处,剃除注射部位毛发,以注射点为中心设置边长约1.5cm正方形区域,记号笔标记,用75%医用酒精消毒后,于大鼠两侧注射点皮下注射HA各0.25ml;5min后于左侧背部真皮层注射BTX-A 5.0U(5.0U/0.1ml),右侧注射生理盐水0.1ml;采用30G注射针头朝上斜行进针、回抽无血液后进行注射(见图1)。
于注射前(0d)、注射后3、7、14、30、60、90d对注射区域皮肤组织进行取材。大鼠麻醉后,沿标记线用眼科剪剪开皮肤全层,完整剔除剩余HA凝胶,分离皮下组织,将皮肤组织固定于4%多聚甲醛溶液中,行免疫组化染色检测HYAL-1、HYAL-2的表达。
1.3.3 Image J软件进行半定量分析:使用ImageJ 2016软件计算HA凝胶及HYLA-1、HYAL-2的平均光密度值(OD)。并用Origin 2018软件绘制相关统计图。
1.4 统计学分析:使用SPSS 22.0软件进行统计学分析,针对计量资料先进行正态性检验,符合正态分布的数据采用(x?±s)表示,采用单因素方差分析测试多组间统计学显著性,t检验比较组间有无差异。P<0.05表示差异有统计学意义。
2 结果
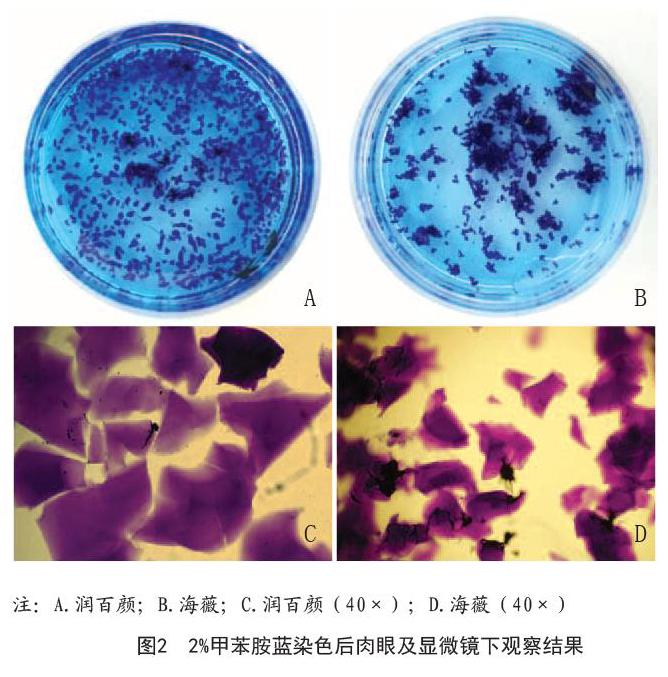
2.1 BTX-A对体外HA凝胶酶解反应的影响:通过肉眼及显微镜(40×)下观察,两种产品形态学差异较大,在水溶液中润百颜较海薇分布分散,其颗粒尺寸也较海薇大(润百颜:130.0~1 400.0?m,海薇:100.0~800.0?m),见图2。
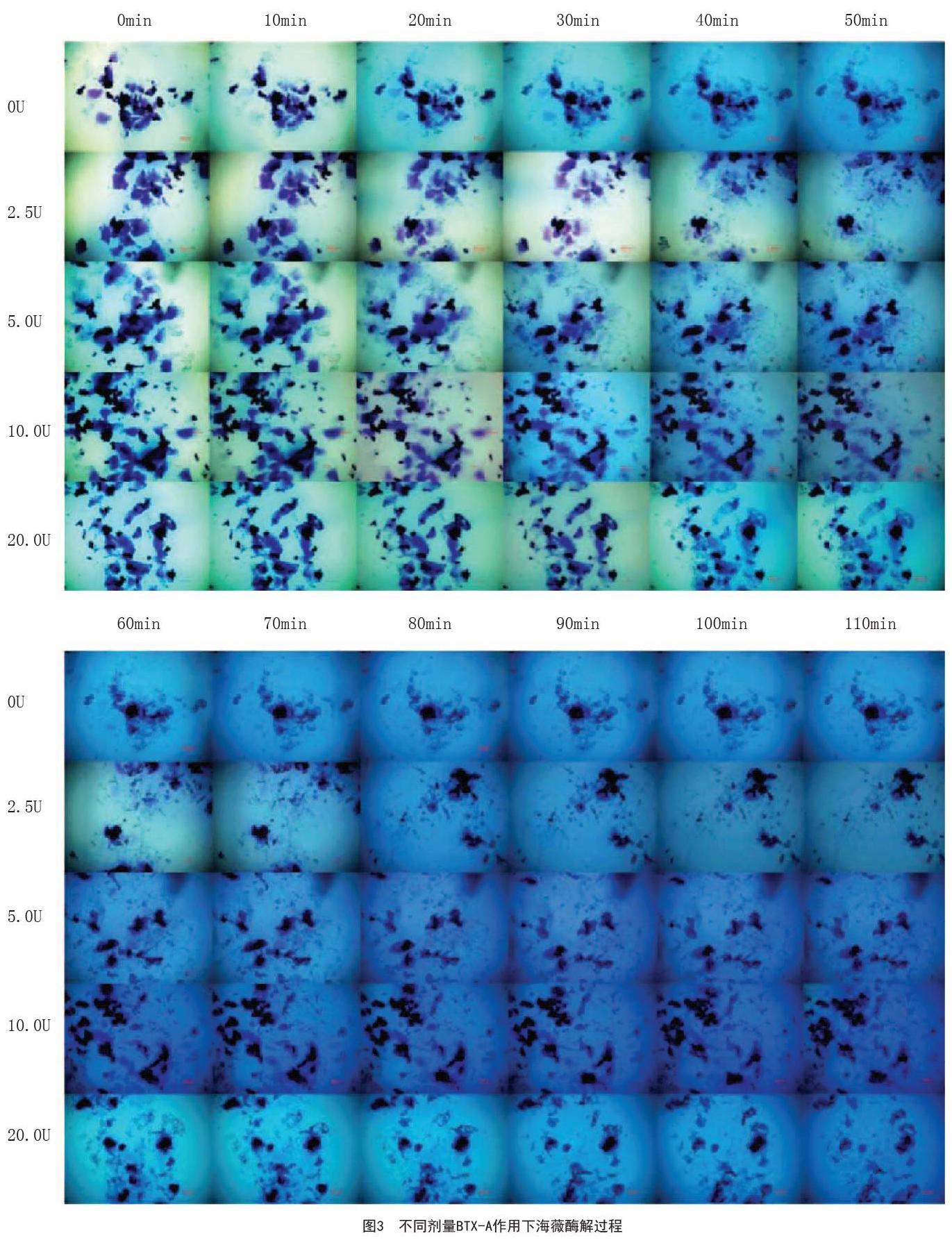
在0U、2.5U、5.0U、10.0U BTX-A作用下,随着BTX-A剂量的增加,HA凝胶的代谢率逐渐减小,润百颜分别在60min、70min、80min、100min达到酶解平衡状态,而海薇分别在60min、70min、90min、100min达到酶解平衡状态。BTX-A在0~10.0U,其抑制作用呈剂量依赖性,随着BTX-A剂量的增加,代谢率逐渐减小。另外,20.0U BTX-A对HA凝胶的抑制作用与10.0U BTX-A相似,两种HA凝胶均在100min达到酶解平衡状态。海薇:BTX-A剂量为10.0U及20.0U,在10min时HA凝胶的代谢速率较生理盐水组明显减慢,差异有统计学意义(P<0.01);2.5U、5.0U组与生理盐水组比较,分别在40min、20min时代谢速率明显减慢(P<0.05),见图3~4。润百颜:BTX-A剂量分别为5.0U、10.0U、20.0U时,在10min时HA凝胶的代谢速率较生理盐水组均明显减慢(P<0.05);30min时,2.5U组也较生理盐水组的代谢速率减慢(P<0.01),见图5~6。
相同酶解条件下,不同HA凝胶酶解速率具有明显差异。20min后,在10.0U、20.0U BTX-A作用下,润百颜凝胶较海薇代谢明显增快(P<0.01);在0U、5.0U BTX-A作用下,在30min后润百颜较海薇凝胶代谢增快(P<0.05);40min后,2.5U BTX-A作用下,润百颜代谢与海薇比较显著增快(P<0.001)。见图7。
[4]Shi H,Liang LL,Cui ZH.Ophthalmic artery occlusion after cosmetic facial filler injections[J].JAMA Ophthalmol,2018,136(6):e180764.
[5]王慕瑶,蒋伊晨,吴可伦,等.六种注射用交联透明质酸钠的形态学观察及体外酶降解实验[J].中国美容医学,2017,26(4):52-56.
[6]Yamazaki K,Fukuda K,Matsukawa M,et al.Reactive oxygen species depolymerize hyaluronan: involvement of the hydroxyl radical[J].Pathophysiology,2003,9(4):215-220.
[7]Ricciardiello F,Oliva F,Mesolella M,et al.Effect of silver vitellinate, hyaluronic acid and sodium benzoate nasal spray after septoplasty[J].J Biol Regul Homeost Agents,2019,33(1):303-308.
[8]Csoka AB,Frost GI,Stern R.The six hyaluronidase-like genes in the human and mouse genomes[J].Matrix Biol,2001,20(8):499-508.
[9]Kontis TC.Update on hyaluronic acid fillers[J].Current Otorhinolaryngol Reports,2015,3(1):21-27.
[10]Molina B,David M,Jain R,et al.Patient satisfaction and efficacy of full-facial rejuvenation using a combination of botulinum toxin type A and hyaluronic acid filler[J].Dermatol Surg,2015,41 Suppl 1:S325-332.
[11]Beer KR,Julius H,Dunn M,et al.Remodeling of periorbital, temporal, glabellar, and crow,s feet areas with hyaluronic acid and botulinum toxin[J].J Cosmet Dermatol,2014,13(2):143-150.
[12]Kü?üker I,Aksakal IA,Polat AV,et al.The effect of chemodenervation by botulinum neurotoxin on the degradation of hyaluronic acid fillers: an experimental study[J].Plast Reconstr Surg,2016,137(1):109-113.
[13]程辰,謝芸,金锐,等.抗毒素治疗八例A型肉毒毒素中毒疗效的初步观察[J].中华整形外科杂志,2019,35(3):282-284.
[14]盛杰,张福琴,杨萍.A型肉毒毒素用于医学美容的进展与安全用药策略[J].中国美容医学,2014,23(13):1124-1127.
[15]Landau M.Hyaluronidase caveats in treating filler complications[J].Dermatol Surg,2015,41(Suppl 1):S347-S353.
[16]Alam M,Hughart R,Geisler A,et al.Effectiveness of low doses of hyaluronidase to remove hyaluronic acid filler nodules[J].JAMA Dermatol,2018,154(7):765-772.
[17]Hwang CJ.Periorbital injectables: understanding and avoiding complications[J].J Cutan Aesthet Surg,2016,9(2):73-79.
[18]Wang M,Li W,Zhang Y,et al.Comparison of intra-arterial and subcutaneous testicular hyaluronidase injection treatments and the vascular complications of hyaluronic acid filler[J].Dermatol Surg,2017,43(2):246-254.
[19]DeLorenzi C.Commentary on: efficacy of retrobulbar hyaluronidase injection for vision loss resulting from hyaluronic acid filler embolization[J].Aesthet Surg J,2017,38(1):23-27.
[20]Schelke LW,Decates TS,Velthuis PJ,et al.Ultrasound to improve the safety of hyaluronic acid filler treatments[J].J Cosmet Dermatol,2018,17(6):1019-1024.
[21]倪啸晓,谢秋幼,曾燕苗,等.早期高压氧治疗透明质酸钠凝胶注射隆鼻术并发症的疗效观察[J].中国美容医学,2017,26(2):47-50.
[22]陈光宇.贝丽姿?-注射用交联透明质酸钠凝胶专家共识(2018)[J].中国美容医学,2019,28(6):73-77.
[收稿日期]2020-02-12
本文引用格式:张珍珍,张向东,赵琳,等.A型肉毒毒素影响透明质酸钠凝胶酶解反应的实验研究[J].中国美容医学,2020,29(5):110-116,152.
