Aromatic compounds from Solanum nigrum L.and their antioxidant activities
2020-05-16
Key Laboratory of Computational Chemistry-Based Natural Antitumor Drug Research &Development,Liaoning Province,School of Traditional Chinese Materia Medica,Shenyang Pharmaceutical University,Shenyang 110016,China
Abstract Solanum nigrum L.,belonging to the genus Solanum of Solanaceae family,is widely distributed in temperate and tropical regions of the world.It possesses the effects of clearing away heat and detoxification.A wide spectrum of chemical constituents have been isolated from the Solanum nigrum L.,including lignans,steroids,etc.The structures of the eight compounds were determined by comprehensive spectroscopic analyses.It is noteworthy that compounds 1-4,6 and 8 have been isolated from Solanum nigrum L.for the first time.All compounds were evaluated for their antioxidant activities by ABTS and DPPH assays.Based on the results,Solanum nigrum L.could be used as a new natural antioxidant in biomedical applications.
Key words: Solanum nigrum L.;Solanaceae family;aromatic compounds;antioxidant activity
1 Introduction
Solanum nigrumL.,belonging to the genus Solanum of Solanaceae family,is also known as Ku Kui.In China,Solanum nigrumL.,a member of the Solanaceae family,is a common herbal plant growing abundantly in temperate zones[1].It originated in Southeast Asia,mainly in China andIndia,but also spread to Australia and America[2].It is found thatSolanum nigrumcontains many kinds of active substances,such as polyphenols,f lavonoids,anthocyanins,alkaloids,polysaccharides and so on,which have many kinds of activities such as antioxidation,anti-tumor,anti-diabetes and antiproliferation[3-5].
The production of reactive oxygen species(ROS),including hydrogen peroxide(H2O2),hydroxyl radical(OH),and superoxide anion(O2-),is a natural part of the oxidative metabolism process[6].However,excessive ROS could induce harmful effects on cell damage and loss of function,thus leads to many human diseases,such as cancer,diabetes mellitus,neurological disorders,and even death[7].Fresh fruits and vegetables are usually the sources of natural antioxidant compounds and provide wellness benefits to consumers[8].Epidemiological studies have suggested that certain natural ingredients with antioxidant properties might have potential in the prevention or treatment of various diseases,possibly in inhibiting or delaying oxidative reactions.This paper described the details of the isolation of the chemical constituents1-8(Fig.1).It is noteworthy that compounds1-4,6and8have been isolated fromSolanum nigrumL.for the first time.Furthermore,their antioxidant activities were also tested using DPPH and ABTS radical scavenging assays.
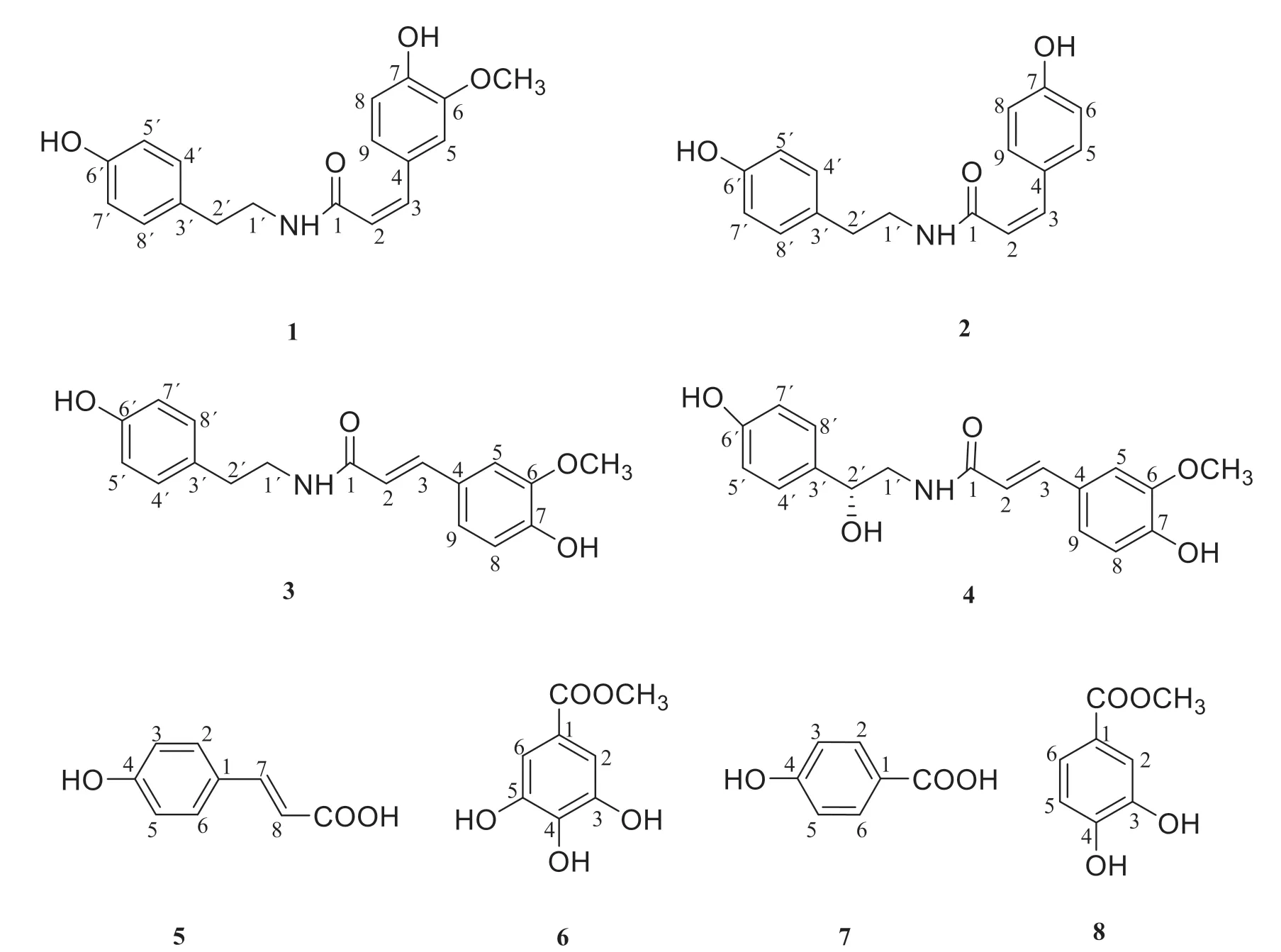
Fig.1 The structures of compounds 1-8
2 Materials and Methods
2.1 General procedures
The 1D NMR spectra were performed on Bruker ARX-400 MHz spectrometers with trimethylsilane(TMS)as an internal standard.The HR-ESI-MS data were taken with an Agilent G6520 Q-TOF spectrometer(Santa Clara,CA,USA).ECD spectra were recorded on a Bio-Logic MOS-450 spectrometer(Bio-Logic Science Instruments,Seyssinet-Pariset,France).Silica gel(100-200 mesh,200-300 mesh,Qingdao Marine Chemical Co.,China),Sephadex LH-20(25-100μm,Green Herbs Science and Technology Development Co.,Ltd.China)and ODS gel(60-80μm,Merck,Germany)were used for column chromatography and silica gel GF254(Qingdao Marine Chemical Co.,China)was used for TLC.Semipreparative RP-HPLC isolation was achieved with an instrument(Shimadzu,Kyoto,Japan)equipped with LC-6AD pump and SPD-20A ultraviolet-visible light absorbance detector using YMC C18column(250 mm×10 mm,5μm).In addition,2,2'-azinobis(3-ethylbenzothiazoline-6-sulfonic acid)diammonium salt(ABTS),diphenylpicrylhydrazyl(DPPH)and 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid(Trolox)as a standard were all purchased from Sigma-Aldrich(Steinheim,Germany)in order to screen the antioxidant activities of the isolated compounds.
2.2 Plant material
The herbs ofSolanum nigrumL.(Solanaceae)were collected in September 2016 from Qiaocheng District(Bozhou,Anhui,China),with the location“E115°77′,N33°88′”.The plant material was identified by Prof.Jincai Lu(School of Traditional Chinese Materia Medica,Shenyang Pharmaceutical University).A voucher specimen(No.20160704)has been deposited in the Herbarium of Shenyang Pharmaceutical University,Liaoning,China.
2.3 Extraction and isolation
The air-dried herbs ofS.nigrumL.(40 kg)were crushed and exhaustively extracted with 70% EtOH for three times(50 L,2 h for each time)under reflux.The combined extract(4600 g)was concentrated to dryness,dissolved in water,and partitioned successively with EtOAc,n-butanol(n-BuOH)in the same volume for three times.The EtOAc extract(850.6 g)was chromatographed on a silica gel column eluted with a step gradient of CH2Cl2-MeOH(from 100:1 to 1:1)to obtain three fractions(Fr.A-C).Fr.C(120.3 g)was further subjected to a HP20 column and an ODS column eluted with a step gradient of EtOH-H2O to obtain six fractions(Fr.C1-Fr.C6).Among them,fraction C1and fraction C2were further divided into ten subfractions(C1.1-C1.10)and eleven subfractions(C2.1-C2.11)respectively by silica gel with the mobile phase CH2Cl2-MeOH(from 50:1 to 2:1).Fraction C3was also chromatographed on silica gel eluted with CH2Cl2-MeOH(from 50:1 to 2:1)to obtain eleven subfractions(C3.1-C3.11).Compound6(5.30 mg),compound7(204.4 mg)and compound8(11.7 mg)were separated from subfraction C1.4by semipreparative HPLC eluted with CH3CN-H2O(25:75,v/v,2.5 mL/min).Subfractions C2.5,C2.9were purified by RP-HPLC with the mobile phase CH3CN-H2O(25:75,v/v,2.5 mL/min)to produce compound5(48.9 mg),compound1(321.7 mg),compound2(11 mg)and compound4(9.2 mg),respectively.In addition,compound3(20.8 mg)was obtained from subfraction C3.8by semipreparative HPLC eluted with CH3CN-H2O(30:70,v/v,2.5 mL/min).
N-cis-feruloyltyramine(1):colorless oily substance,1H NMR(400 MHz,DMSO-d6):δH8.11(1H,t,J=5.7 Hz,-NH),9.21(2H,s,6′,7-OH),7.70(1H,d,J=1.9 Hz,H-5),6.71(1H,d,J=8.2 Hz,H-8),7.09(1H,dd,J=8.2,1.9 Hz,H-9),6.99(2H,d,J=8.3 Hz,H-4′,8′),6.67(2H,d,J=8.3 Hz,H-5′,7′),5.77(1H,d,J=12.9 Hz,H-2),6.49(1H,d,J=12.9 Hz,H-3),2.62(2H,t,J=7.5 Hz,H-2′),3.27(2H,dt,J=7.5,5.7 Hz,H-1′),3.74(3H,s,6-OCH3).
N-(p-cis-coumaroyl)tyramine(2):colorless oily substance,1H NMR(400 MHz,DMSO-d6):δH8.09(1H,t,J=5.7 Hz,-NH),9.44(2H,s,6′,7-OH),7.60(2H,d,J=8.6 Hz,H-5,9),6.69(2H,d,J=8.6 Hz,H-6,8),6.99(2H,d,J=8.4 Hz,H-4′,8′),6.67(2H,d,J=8.4 Hz,H-5′,7′),5.75(1H,d,J=12.9 Hz,H-2),6.48(1H,d,J=12.9 Hz,H-3),2.61(2H,t,J=7.5 Hz,H-2′),3.27(2H,dt,J=7.5,5.7 Hz,H-1′).
N-trans-feruloyltyramine(3):colorless oily substance,1H NMR(400 MHz,DMSO-d6):δH8.00(1H,t,J=5.7 Hz,-NH),9.38(2H,s,6′,7-OH),7.11(1H,d,J=1.9 Hz,H-5),6.79(1H,d,J=8.2 Hz,H-8),6.97(1H,dd,J=8.2,1.9 Hz,H-9),7.01(2H,d,J=8.4 Hz,H-4′,8′),6.68(2H,d,J=8.4 Hz,H-5′,7′),6.43(1H,d,J=15.7 Hz,H-2),7.31(1H,d,J=15.7 Hz,H-3),3.80(3H,s,6-OCH3).
N-trans-feruloyloctopamine(4):colorless oily substance,1H NMR(400 MHz,DMSO-d6):δH7.94(1H,t,J=5.7 Hz,-NH),9.33(2H,s,6′,7-OH),7.11(1H,d,J=1.9 Hz,H-5),6.79(1H,d,J=8.1 Hz,H-8),6.98(1H,dd,J=8.1,1.9 Hz,H-9),7.14(2H,d,J=8.4 Hz,H-4′,8′),6.71(2H,d,J=8.4 Hz,H-5′,7′),6.54(1H,d,J=15.7 Hz,H-2),7.30(1H,d,J=15.7 Hz,H-3),3.18,3.38(2H,m,H-1′),3.80(3H,s,6-OCH3),5.35(1H,s,2′-OH);13C NMR(100 MHz,DMSO-d6):δc165.6(C-1),119.1(C-2),138.9(C-3),126.5(C-4),110.8(C-5),147.8(C-6),148.2(C-7),115.7(C-8),121.5(C-9),47.1(C-1′),71.2(C-2′),134.1(C-3′),127.2(C-4′),114.8(C-5′),156.4(C-6′),114.8(C-7′),127.2(C-8′),55.5(6-OCH3).
p-coumaric acid(5):colorless powder,1H NMR(400 MHz,DMSO-d6):δH7.50(2H,d,J=8.6 Hz,H-2,6),6.79(2H,d,J=8.6 Hz,H-3,5),7.46(2H,d,J=16.0 Hz,H-7),6.29(2H,d,J=15.95 Hz,H-8),10.93(1H,brs,-COOH).
methyl gallate(6):colorless powder,1H NMR(400 MHz,DMSO-d6):δH6.93(2H,s,H-2,6),3.71(3H,s,-OCH3).
p-hydroxybenzoic acid(7):colorless powder,1H NMR(400 MHz,DMSO-d6):δH8.47(2H,d,J=8.7 Hz,H-2,6),7.26(2H,d,J=8.7 Hz,H-3,5),12.62(1H,brs,-COOH).
methyl protocatechin(8):colorless powder,1H NMR(400 MHz,DMSO-d6):δH8.19(1H,d,J=2.7 Hz,H-2),7.92(1H,d,J=8.6 Hz,H-5),7.24(1H,dd,J=8.6,2.7 Hz,H-6),3.81(3H,s,-OCH3).
2.4 Antioxidant assay
2.4.1 DPPH radical scavenging activity assay
Since DPPH is an intensely colored radical,the DPPH-based photometric measurement is widely used for antioxidant screening as it is a simple and rapid method.DPPH radical scavenging activity was measured using a modified established method[9].In DPPH assay,the absorbance decreases as a result of a color change from purple to yellow as the radical is scavenged by antioxidants through a donation of hydrogen to form the stable DPPH-H molecule[10].This article describes free radicalscavenging activities of extracts fromSolanumnigrumL.through the DPPH assay.A series of tested samples and DPPH were added to 96-well plates,absolute ethyl alcohol was used instead of samples as the blank control,trolox was used as a positive reference.The mixture plate was shaken vigorously and then immediately incubated in the dark for 30 min.The absorbance of the reaction solution was determined using a Bio-Tek microplate reader(Bio-Tek,Winooski,VT)at an absorbance of 517 nm,all compounds were analyzed at five different final concentrations ranging from 10 to 200μg/mL.The DPPH radical-scavenging activity in percentage of sample was calculated as follows:
DPPH scavenging rate=[1-(AS-ASB)/(AC-ACB)]×100%
Where AS,ASB,ACand ACBare the absorbancies of the sample,the blank sample,the control and the blank control,respectively.
2.4.2 ABTS radical scavenging activity assay
ABTS is a nitrogen-centered free radical and is used to evaluate the antioxidant activity of compounds1-8.The ABTS assay was based on the procedure described in a previous report with some modifications[11].The ABTS radical was produced by reacting ABTS with a solution of potassium persulfate at room temperature for 12 h-16 h.The absorbance of the ABTS radical produced was adjusted to 0.70±0.02 at 734 nm before use.The ABTS solution(150μL)was mixed with the samples(100μL)at different concentrations and the absorbance was measured after 20 min at 734 nm using a microplate reader(Epoch,Bio-Tek).Absolute ethyl alcohol as negative control and sample without ABTS as blank,trolox was used as positive control.The inhibition percentage of the radical scavenging activity was calculated using the equation:
ABTS·+scavenging rate=[1-(AS-ASB)/(AC-ACB)]×100%
Where AS,ASB,ACand ACBare the absorbancies of the sample,the blank sample,the control and the blank control,respectively.
3 Results and discussion
3.1 Structure elucidation
The1H NMR spectrum of compound1showed the presence of one nitrogen proton signal atδH8.11(1H,t,J=5.7 Hz),one hydroxyl proton signal atδH9.21(2H,s),one 1,3,4-trisubstituted benzene ring signal atδH7.70(1H,d,J=1.9 Hz,H-5);6.71(1H,d,J=8.2 Hz,H-8),7.09(1H,dd,J=8.2,1.9 Hz,H-9),onep-substituted benzene ring signal atδH6.99(2H,d,J=8.3 Hz,H-4′,8′),6.67(2H,d,J=8.3 Hz,H-5′,7′).5.77(1H,d,J=12.9 Hz,H-2),6.49(1H,d,J=12.9 Hz,H-3)are hydrogen signals of a group ofcis-double bonds,2.62(2H,t,J=7.5 Hz,H-2′),3.27(2H,dt,J=7.5,5.7 Hz,H-1′)are a group of coupling methylene signals,one methoxy signal atδH3.74(3H,s).Based on these results,by comparison between experimental spectroscopic data and reported literature value[12],the structure of1was determined asN-cis-feruloyltyramine.
The molecular formula of compound2was determined as C17H17NO3.Analysis of 1D NMR data of2revealed that it possesses a nitrogen proton signal atδH8.09(1H,t,J=5.7 Hz),a hydroxyl proton signal atδH9.44(2H,s),twop-substituted benzene rings signal atδH7.60(2H,d,J=8.6 Hz,H-5,9),6.69(2H,d,J=8.6 Hz,H-6,8);6.99(2H,d,J=8.4 Hz,H-4′,8′),6.67(2H,d,J=8.4 Hz,H-5′,7′).5.75(1H,d,J=12.9 Hz,H-2),6.48(1H,d,J=12.9 Hz,H-3)are hydrogen signals of a group ofcis-double bonds,2.61(2H,t,J=7.5 Hz,H-2′),3.27(2H,dt,J=7.5,5.7 Hz,H-1′)are a group of coupling methylene signals.Based on these results,by comparison between experimental spectroscopic data and reported literature value[13],the structure of2was determined asN-(p-ciscoumaroyl)tyramine.
Compound3was found to have the molecular formula C18H19NO4,identical to that of structure1.Comparison of the1H NMR data of3and1indicated that these two compounds have similar proton signals.The1H NMR data of compound3showed the existence of one nitrogen proton signal atδH8.00(1H,t,J=5.7 Hz)and one hydroxyl proton signals atδH9.38(2H,s)in the downfield region.One 1,3,4-trisubstituted benzene ring signal atδH7.11(1H,d,J=1.9 Hz,H-5),6.79(1H,d,J=8.2 Hz,H-8),6.97(1H,dd,J=8.2,1.9 Hz,H-9),onep-substituted benzene ring signal atδH7.01(2H,d,J=8.4 Hz,H-4′,8′),6.68(2H,d,J=8.4 Hz,H-5′,7′).6.43(1H,d,J=15.7 Hz),7.31(1H,d,J=15.7 Hz)are hydrogen signals of a group oftrans-double bonds,one methoxy signal atδH3.80(3H,s).Based on these results,by comparison between experimental spectroscopic data and reported literature value[14],the structure of3was determined asN-transferuloyltyramine.
The1H NMR data of compounds1-4were highly similar,with only slight changes of some signals,compound4was identified the molecular formula C18H19NO5on the basis of1H and13C NMR data,based on the molecular formula,the unsaturation number of4was deduced as 10.Combined analysis of1H NMR data showed the existence of one nitrogen proton signal atδH7.94(1H,t,J=5.7 Hz)and a hydroxyl proton signal atδH9.33(2H,s)in the downfield region.One 1,3,4-trisubstituted benzene ring signal atδH7.11(1H,d,J=1.9 Hz,H-5),6.79(1H,d,J=8.1 Hz,H-8),6.98(1H,dd,J=8.1,1.9 Hz,H-9),onep-substituted benzene ring signal atδH7.14(2H,d,J=8.4 Hz,H-4′,8′),6.71(2H,d,J=8.4 Hz,H-5′,7′).6.54(1H,d,J=15.7 Hz),7.30(1H,d,J=15.7 Hz)are hydrogen signals of a group oftrans-double bonds,one methoxy signal atδH3.80(3H,s).The13C NMR data of compound4showed 14 carbon signals,including one carbonyl carbon(δC165.6),two phenyl carbons(δC126.5,110.8,147.8,148.2,115.7,121.5,134.1,127.2×2,114.8×2,156.4),one methoxy carbon(δC55.5).The absolute configurations of compound4was determined by comparison of the experimental and calculated ECD.The measured ECD spectra of4matched well with the calculated ECD spectra for 2′R(Fig.2).Therefore,the absolute configurations of4was determined as 2′R.Based on these results,by comparison between experimental spectroscopic data and reported literature value[15],the structure of4was determined asN-transferuloyloctopamine.
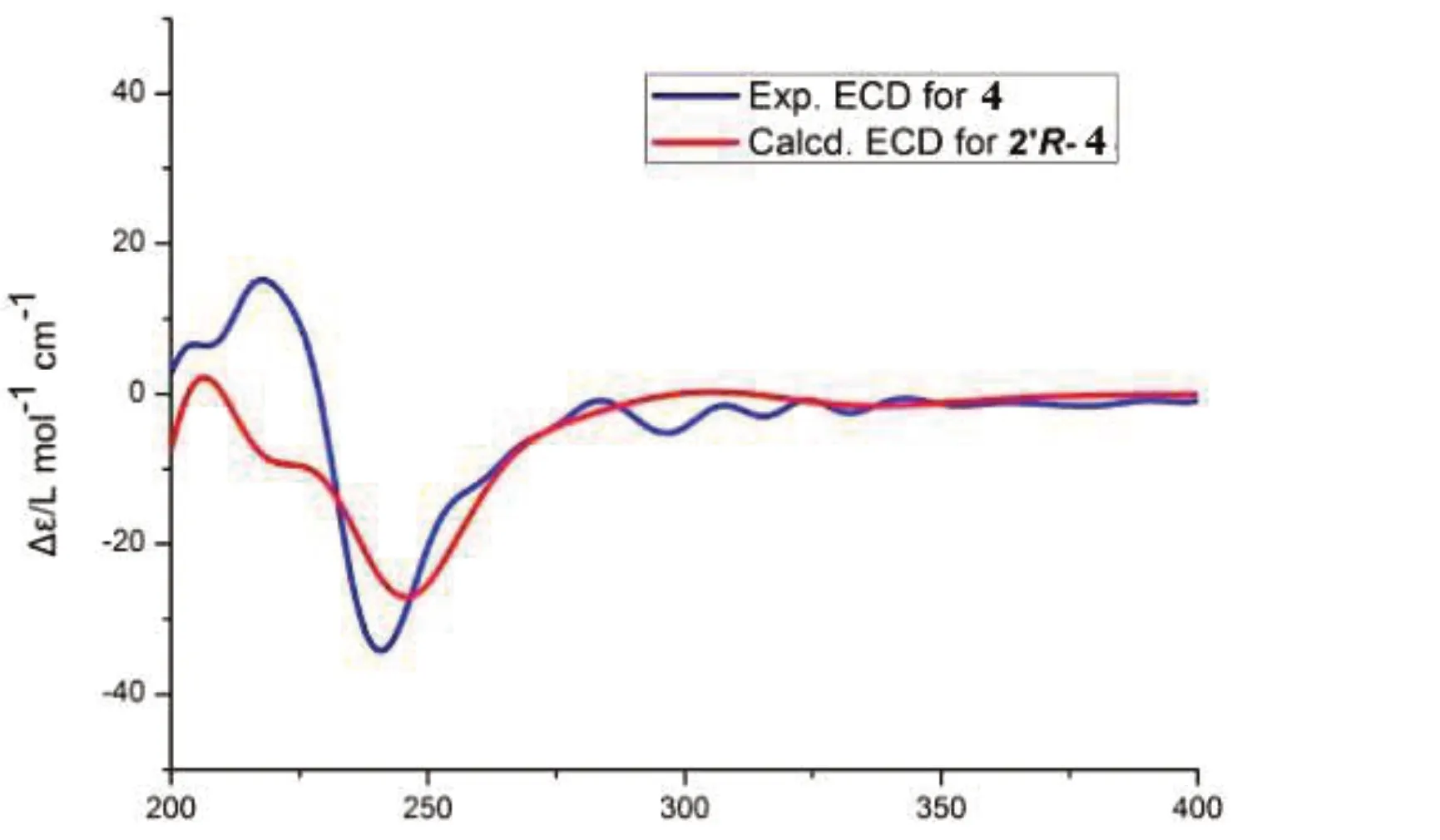
Fig.2 The ECD spectra of 4
It is worth emphasizing that compound5is different from the above compounds,it is a monobenzene ring compound.The1H NMR spectrum of compound5showed the presence of onep-substituted benzene ring signal atδH7.50(2H,d,J=8.6 Hz,H-2,6),6.79(2H,d,J=8.6 Hz,H-3,5).7.46(2H,d,J=16.0 Hz,H-7),6.29(2H,d,J=15.95 Hz,H-8)are hydrogen signals of a group oftrans-double bonds,and connected to the benzene ring.10.93(1H,brs)is one carboxyl proton signal.After carefully examining these data,by comparison between experimental spectroscopic data and reported literature value[16],the structure of5was determined asp-coumaric acid.
Through careful analysis of the1H NMR data,and by comparison between experimental spectroscopic data and reported literature value[17],the structure of6was determined as methyl gallate.The specific data are as follows:the1H NMR data displayed one hydrogen signal on the benzene ring atδH6.93(2H,s),and the chemical environment of the two hydrogens is the same,3.71(3H,s)is one methoxy signal.
Structure elucidation of7manifested that it shared the same structure with the previously reportedp-hydroxybenzoic acid[18].The1H NMR data showed the existence of onep-substituted benzene ring signal atδH8.47(2H,d,J=8.7 Hz,H-2,6),7.26(2H,d,J=8.7 Hz,H-3,5),one carboxyl proton signal atδH12.62(1H,brs).Therefore the structure of7was determined asp-hydroxybenzoic acid.
The1H NMR spectrum of compound8showed the existence of one 1,3,4-trisubstituted benzene ring signal atδH8.19(1H,d,J=2.7 Hz),7.92(1H,d,J=8.6 Hz),7.24(1H,dd,J=8.6,2.7 Hz),one methoxy signal atδH3.81(3H,s).Based on these results,by comparison between experimental spectroscopic data and reported literature value[19],the structure of8was determined as methyl protocatechin.
3.2 Antioxidant activity
The imbalance between the formation of ROS and antioxidant defense leads to oxidative stress,resulting in potential cellular damage[20].A natural plant contains various antioxidant constituents that act differently and are used in traditional medicine[21].The goal of our study was to evaluate the free radical scavenging potency of extracts fromSolanum nigrumL.,considering the different mechanisms of the various antioxidant assays,all compounds were tested for their antioxidant activities by DPPH and ABTS radical scavenging assays.The antioxidant results of all compounds(1-8)are shown in Table 1.DPPH,ABTS radicalscavenging activity of the compound fractions were shown as the percentage increase with the increased concentrations(Table 2),compounds exhibited weak free radical scavenging activity in the DPPH assay and higher antioxidant activity in the ABTS assay at relatively high concentration[22].With this kind of investigations it would be easier the establishment of natural extracts supposed to functionalize formulations to treat and prevent the human damages occurring due to free radicals and also to replace synthetic antioxidants in industry.Compounds1-7were found to exhibit more significant antioxidant activity than trolox(the positive control)following comparison of the results in the ABTS assay.Compounds1,3-4,and6exhibited potent antioxidant activities compared with the standard,while the other compounds only showed weak antioxidant activities evaluated by DPPH assay.
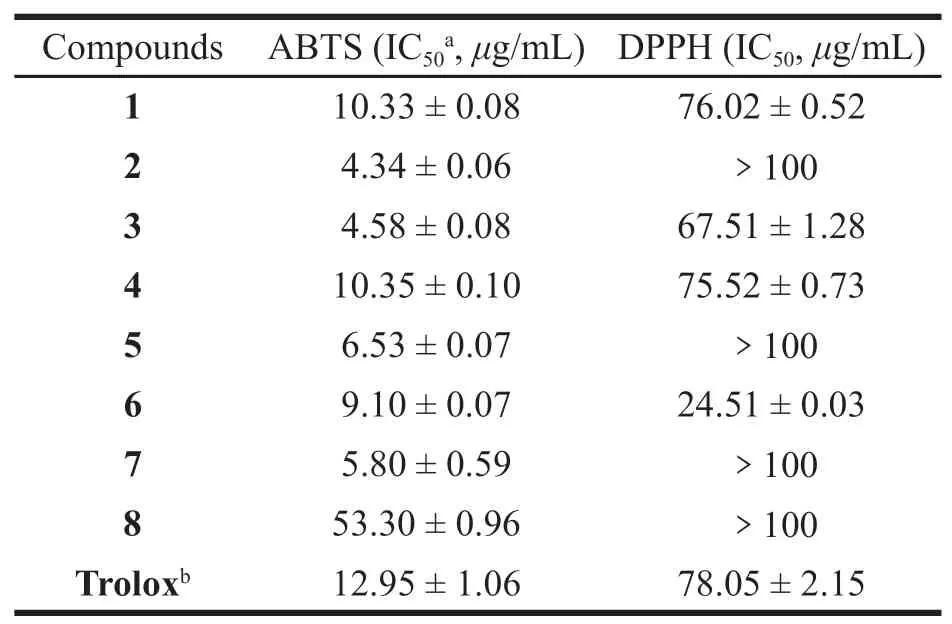
Table 1 Antioxidant activity of compounds 1-8
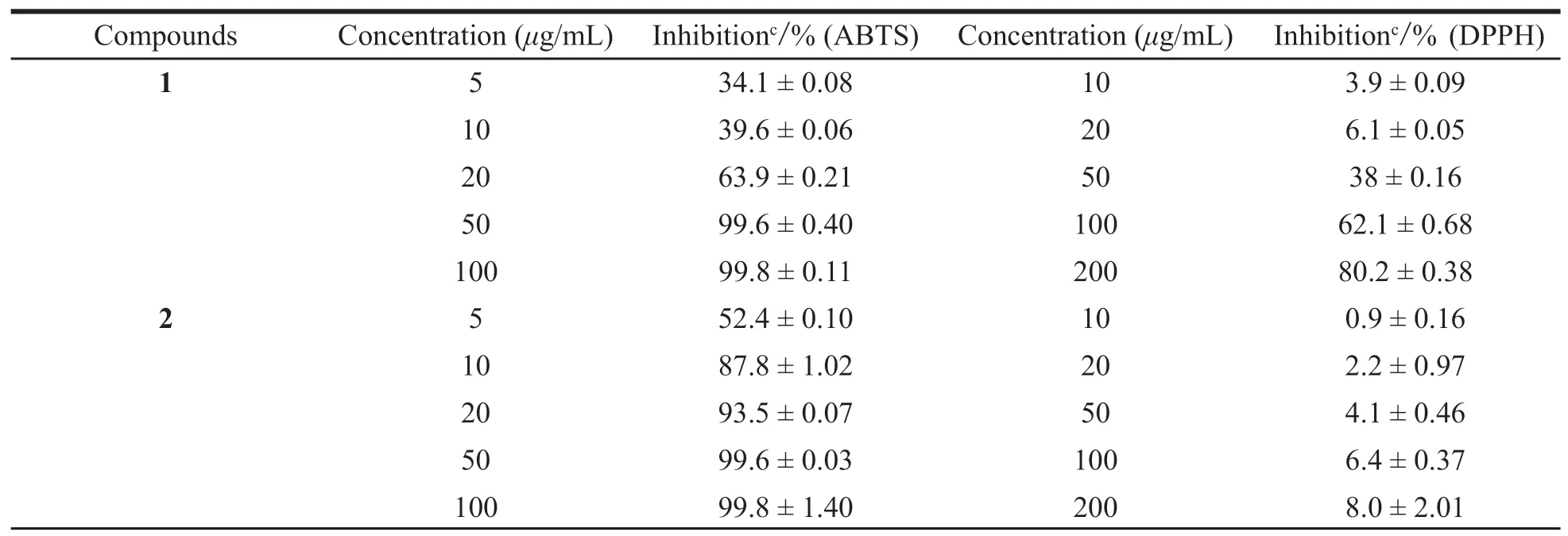
Table 2 Percentage inhibition of compounds by ABTS assay and DPPH assay
(to be continued)
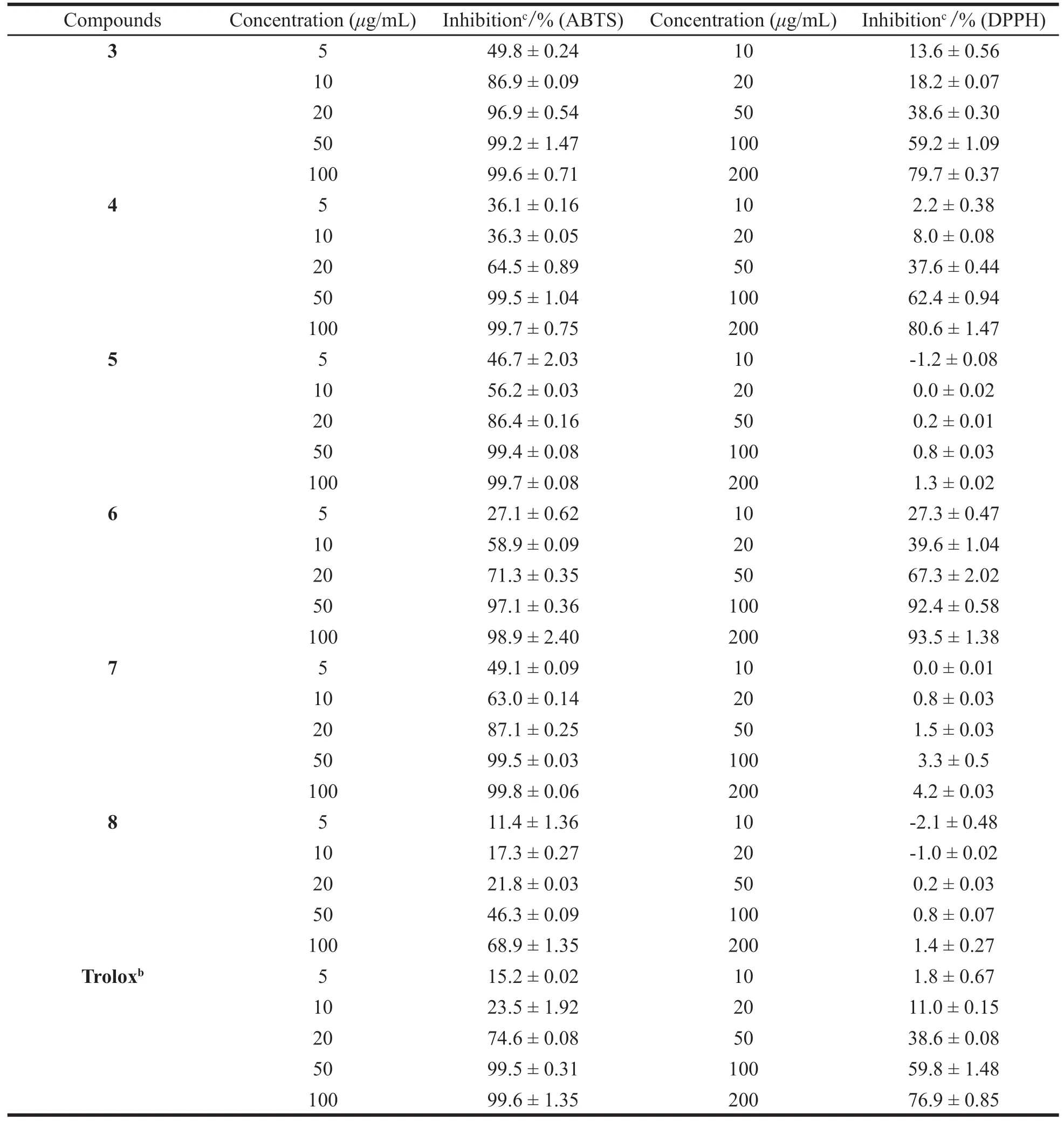
Continued table 2
4 Conclusion
In summary,eight compounds were isolated fromSolanum nigrumL..Their antioxidant activities were investigated by two different assays,and most compounds showed excellent antioxidant activities.These assays indicated that this plant extract might help prevent the progress of various oxidative stresses.Solanum nigrumL.could be used as a new natural antioxidant in biomedical applications.
Acknowledgements
We thank Lili Lou,Chao Huang and Wen Li of Shenyang Pharmaceutical University for their help in NMR measurements,and we greatly thank Prof.Ying Peng(Shenyang Pharmaceutical University)for measuring MS.
