Radiation recall dermatitis with dabrafenib and trametinib: A case report
2020-05-14
Mesut Yilmaz, Medical Oncology Department, Bakirköy Dr. Sadi Konuk Training and Research Hospital, Istanbul 34144, Turkey
Ugur Celik, Dermatology Department, Medipol Kosuyolu Hospital, Istanbul 34134, Turkey
Seyhan Hascicek, Pathology Department, Sisli Hamidiye Etfal Training and Research Hospital,Istanbul 34134, Turkey
Abstract
BACKGROUND
Radiation recall dermatitis has been defined as the “recalling” by skin of previous radiation exposure in response to the administration of certain response-inducing drugs. Although the phenomenon is relatively well known in the medical world,an exact cause has not been documented.
CASE SUMMARY
Here, we report the rare occurrence of radiation recall dermatitis after palliative radiotherapy for bone metastases in a metastatic melanoma patient treated with a combination of dabrafenib and trametinib.
CONCLUSION
We present a case of radiation recall dermatitis after completion of palliative radiotherapy while being treated with a combination of dabrafenib and trametinib. This is a very rare toxic event, and there is insufficient data to describe prevention strategies. Increased awareness and reporting of cases will help to better explain the association between targeted therapy and the radiation recall phenomenon.
Key words: Radiation recall dermatitis; Melanoma; Radiotherapy; Dabrafenib;Trametinib; Case report
INTRODUCTION
Radiation recall dermatitis is an acute inflammatory reaction confined to previously irradiated skin, which occurs after the administration of certain drugs, mainly cytotoxic chemotherapeutics such as anthracyclines (doxorubicin), taxanes(paclitaxel), and antimetabolites (gemcitabine, capecitabine), but other triggers include several antibiotics, antituberculosis drugs, and simvastatin[1,2]. The reaction can present days or even years after radiotherapy[1,2].
Dabrafenib is a potent, ATP-competitive inhibitor of RAF kinases, including BRAF,which is highly active in melanoma, both as monotherapy and in combination with MEK inhibitors. Dabrafenib plus trametinib is a standard treatment option for advanced BRAF V600 mutated melanoma[3]. We report a case of radiation recall dermatitis occurring 30 d after completion of palliative radiotherapy while the patient was treated with a combination of dabrafenib and trametinib.
CASE PRESENTATION
Chief complaints
Discolored and inflamed skin.
History of present illness
One month after completion of radiotherapy, a 76-year-old man was admitted to the oncology clinic due to discolored and inflamed skin limited to the region that was previously irradiated while still taking BRAF and MEK inhibitor combination therapy(Figure 1). Two weeks later, a similar lesion was seen in the left lower extremity(Figure 2).
History of past illness
The patient who was previously healthy was admitted to the hospital with an ulcerated lesion located in the right parietal region of the scalp in July 2015. After excision of the lesion, a pT4bN0M0 nodular melanoma was detected. He was started on adjuvant interferon therapy but this was discontinued due to grade 3 hepatotoxicity and fatigue. Bone and subcutaneous metastases were detected in August 2017 and BRAF V600E mutation was positive. Dabrafenib and trametinib were prescribed, and subcutaneous metastases regressed with this treatment, while bone metastases were stable. In January 2019, he complained of right lower leg pain and palliative radiotherapy, 20 gray in 5 fractions, was applied to bone metastasis in the right tibia region.
Personal and family history
He was treated for stage four melanoma with dabrafenib plus trametinib. He did not receive any other medications except dabrafenib and trametinib.
Physical examination upon admission
One month after completion of radiotherapy, the patient detected discolored and inflamed skin limited to the region previously irradiated, while he was still receiving BRAF and MEK inhibitor combination therapy (Figure 1). He did not take any other medications except dabrafenib and trametinib.
Laboratory examinations
Laboratory tests such as normal C-reactive protein levels and complete blood count without leucocytosis ruled out infection.
Imaging examinations
A dermal biopsy was performed and orthokeratotic epidermis with lymphocytic infiltration was observed in the pathologic specimen, with no sign of malignancy(Figure 3).

Figure 1 One month after completion of radiotherapy, discolored and inflamed skin limited to the region previously irradiated was observed while being treated with BRAF and MEK inhibitor combination therapy. A:Front view of right leg-discolored and inflamed skin limited to the region previously irradiated; B: Back view of right leg-discolored and inflamed skin limited to the region previously irradiated; C: Front view of right leg; D: Back view of right leg.
FINAL DIAGNOSIS
Radiation recall dermatitis.
TREATMENT
He was treated with topical steroids, nonsteroidal anti-inflammatory agents, and antihistamines. Two weeks after this intervention all skin lesions had disappeared.
OUTCOME AND FOLLOW-UP
Two weeks after topical therapy all skin lesions had disappeared. He is still receiving BRAF-MEK inhibitor combination therapy and continues to benefit from this treatment.
DISCUSSION
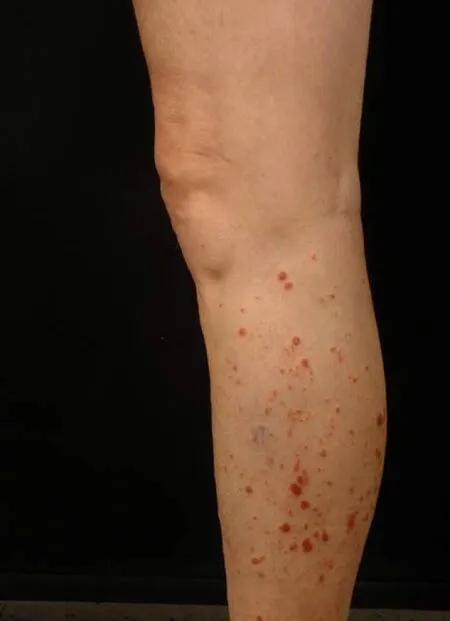
Figure 2 Similar lesions on the opposite leg.
Radiation recall phenomenon was firstly described by D’Angioet al[4]in 1959, but the pathophysiology of radiation recall dermatitis is still not well understood. Clinical diagnosis is based on patient history, symptoms, physical exam findings, and the exclusion of other causes of dermatitis. Vascular damage, epithelial stem cell depletion, epithelial stem cell sensitization, and an idiosyncratic drug hypersensitivity reaction have been proposed as potential mechanisms of this phenomenon[1,5,6]. It is mostly seen after the administration of standard cytotoxic chemotherapy agents, and the incidence of radiation recall with targeted therapies is not well known[1,2]. Here we report a patient treated with dabrafenib and trametinib who was diagnosed with radiation recall dermatitis after palliative radiation to bone metastasis.
An institutional review of targeted therapy–induced radiation recall by Levyet al[7]described 16 cases associated with various agents, including mechanistic target of rapamycin inhibitors (everolimus, temsirolimus), epidermal growth factor receptor inhibitors (cetuximab), histone deacetylase inhibitors, and BRAF inhibitors(vemurafenib)[7]. Dabrafenib is a BRAF inhibitor indicated for advanced melanoma and non-small cell lung cancer patients harboring the BRAF mutation. It is used with trametinib which is a MEK inhibitor[8].
Vemurafenib was the first BRAF inhibitor to be used, and in the literature, several cases of radiation recall phenomenon due to vemurafenib have been reported.Haraldsdottirel al[9]also reported radiation recall dermatitis with concomitant dabrafenib and pazopanib therapy. Their patient was treated for BRAF mutated,metastatic, non–small-cell lung adenocarcinoma of the bone and thoracic lymph nodes, and received whole-femur irradiation. Similarly, our patient was treated with tyrosine kinase inhibitors, dabrafenib and trametinib, his tibia was irradiated due to metastatic disease and he subsequently developed radiation recall dermatitis.
In previous reports, patients were diagnosed with targeted therapy-induced radiation recall dermatitis based on the following clinical findings: Acute inflammatory skin reaction with localized symptoms such as painless erythematous lesion, dry desquamation, pruritus, and patchy hyperpigmentation within a previously irradiated area, skin reaction development during targeted therapy, no other suspected medication, and negative test results for local skin infection[6,8-10]. The patient in our study also experienced localized acute inflammatory symptoms in the irradiated area without evidence of infection during dabrafenib and trametinib treatment. He did not take any other medications except dabrafenib and trametinib before the development of radiation recall dermatitis and there were no signs of skin infection.
Histological findings of sorafenib-induced radiation recall dermatitis were reported in a patient. Skin biopsy of an erythematous lesion revealed a diffuse perivascular lymphohistiocytic infiltrate with few eosinophils in the corium, and apoptotic keratinocytes and distinct vacuolization in the junction zone[11]. Our patient’s skin biopsy revealed orthokeratotic epidermis with lymphocytic infiltration.
The management of radiation recall dermatitis has included oral or topical steroids,nonsteroidal anti-inflammatory drugs, and antihistamines. It is not clear whether these agents hasten the recovery[1]. Many reports suggest discontinuing treatment with the offending agent; however, Conenet al[12]reported a patient who successfully continued vemurafenib therapy despite the occurrence of radiation recall. Similarly,we decided to continue the treatment of metastatic melanoma with dabrafenib and trametinib, and the patient still benefits from this combination therapy.
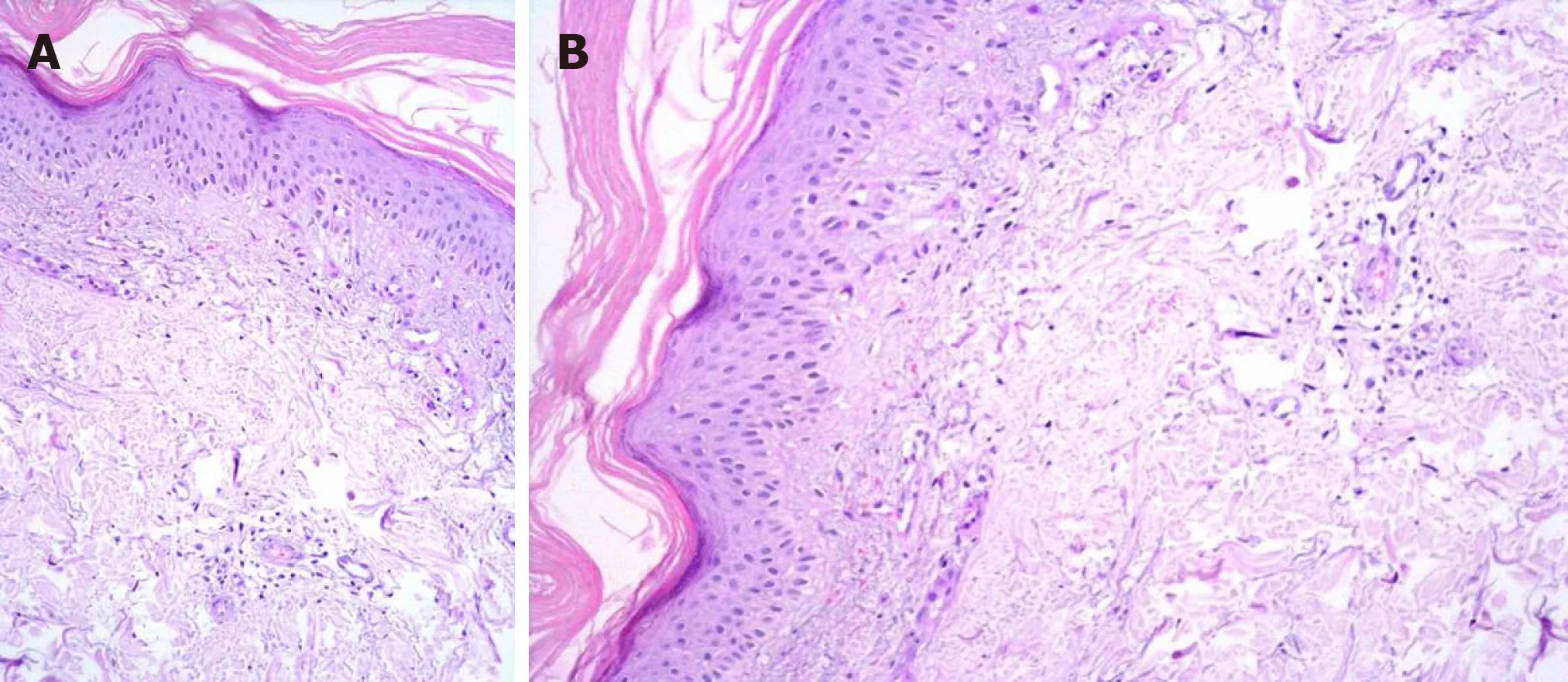
Figure 3 A dermal biopsy was performed and orthokeratotic epidermis with lymphocytic infiltration was observed in the pathologic specimen. A: Perivascular lymphocytes (Hematoxylin-eosin staining, × 20); B:Perivascular lymphocytes (Hematoxylin-eosin staining, × 20).
CONCLUSION
Here we present a case of radiation recall dermatitis after completion of palliative radiotherapy while being treated with a combination of dabrafenib and trametinib.This is a very rare toxic event, and there is insufficient data to describe prevention strategies. Increased awareness and reporting of cases will help to better explain the association between targeted therapy and the radiation recall phenomenon.
杂志排行
World Journal of Clinical Cases的其它文章
- Novel zinc alloys for biodegradable surgical staples
- Peutz-Jeghers syndrome with mesenteric fibromatosis: A case report and review of literature
- Complex liver retransplantation to treat graft loss due to long-term biliary tract complication after liver transplantation: A case report
- Successful treatment of adult-onset still disease caused by pulmonary infection-associated hemophagocytic lymphohistiocytosis: A case report
- Isolated vaginal metastasis from stage I colon cancer: A case report
- Erdheim-Chester disease with asymmetric talus involvement: A case report
