Granulocyte colony-stimulating factor-producing squamous cell carcinoma of the tongue exhibiting characteristic fluorine-18 deoxyglucose accumulation on positron emission tomography-computed tomography:A case report
2020-05-13HiroakiShimamotoYukaHirotaYoshihisaKashimaNaoyaKinoshitaMisakiYokokawaTohruIkedaHiroyukiHarada
Hiroaki Shimamoto, Yuka Hirota, Yoshihisa Kashima, Naoya Kinoshita, Misaki Yokokawa, Tohru Ikeda,Hiroyuki Harada
Yuka Hirota, Human Pathology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo 113-8549, Japan
Tohru lkeda, Oral Pathology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo 113-8549, Japan
Abstract
Key words: Tongue cancer;Granulocyte colony-stimulating factor;Hyperlukocytosis;Positron emission tomograph;Case report
INTRODUCTION
Sudden hyperleukocytosis has a range of underlying etiologies, including inflammatory diseases, acute leukemia, chronic myelocytic leukemia (CML),granulocyte colony-stimulating factor (G-CSF)-producing tumors, non-CML myeloproliferative diseases, bone marrow metastases of malignant tumors and administration of G-CSF agents.G-CSF is a cytokine produced in inflammatory environments, primarily by macrophages, fibroblasts, endothelial cells, but also to a lesser extent by other cells.In addition, it induces differentiation and proliferation of neutrophils in the bone marrow.Fluorine-18 deoxyglucose (FDG) positron emission tomography-computed tomography (PET/CT) is recognized as a useful modality for tumor imaging.FDG accumulation correlates with bone marrow and peripheral neutrophil counts.Here we report a rare case of G-CSF-producing squamous cell carcinoma of the tongue that grew rapidly and through PET/CT exhibited FDG accumulation not only in the primary lesion and metastatic lymph nodes but also in the spleen and bone marrow throughout the body.
CASE PRESENTATION
Chief complaint
A 58-year-old woman with an enlarged mass on the tongue was examined in our department.
History of illness
The patient noticed a growth of the mass 1 mo prior to the examination.
Personal and family history
The patient had a history of hypertension and mitral regurgitation.
Physical examination
A 30 mm × 40 mm pedunculated tumor with partial necrosis was observed on the right side of the tongue (Figure 1A).The mass was growing rapidly.On admission to the hospital, the tumor continued to rapidly grow outward, filling the oral cavity to the extent that the patient was unable to close her mouth (Figure 1B).
Laboratory examinations
Blood test results from the initial examination revealed a leukocyte count of 21380/μL, neutrophil of 84%, and C-reactive protein (CRP) of 0.99 mg/dL, all of which were considered high levels.Upon hospital admission, serum G-CSF level was measured at 331 pg/mL, interleukin (IL)-6 at 14.6 pg/mL and blood test revealed a leukocyte concentration of 24510/μL, neutrophils at 83%, and CRP at 0.99 mg/dL(Table 1).
Imaging examinations
In PET/CT, extensive FDG accumulation was observed in the tongue (Figure 2A) and bilateral cervical lymph node, while elevated FDG accumulation was also observed in the spleen and bone marrow throughout the body (Figure 2B and C).No distant metastases were observed in PET/CT.
Pathological examination
Upon histopathologic examination of tissue, large carcinoma cells with high proliferation potency were confirmed in the majority of the tumor.The majority of the tumor comprised of poorly differentiated squamous cancer cells.Immunohistochemical staining with G-CSF antibodies on biopsy tissue and specimens resected during surgery revealed both to be G-CSF positive (Figure 3).
FINAL DIAGNOSIS
G-CSF producing squamous cell carcinoma of the tongue.
TREATMENT
Right modified radical neck dissection type I, left supraomohyoid neck dissection,and partial glossectomy were performed.On postoperative day 1, the leukocyte count was 15230/μL, neutrophil level was 90%, CRP level was 9.68 mg/dL, and serum GCSF level was 63.3 pg/mL, with decreases observed in leukocyte count and serum GCSF levels compared with preoperative measurements.On postoperative day 18, CT revealed the presence of recurrence in the neck, accompanied by multiple metastases in the lungs (Figure 4).At this point, the patient exhibited leukocytes at a concentration of 77620/μL, 84% at neutrophil, CRP at 5.66 mg/dL, and serum G-CSF at 663 pg/mL.On postoperative day 23, the patient was commenced on cetuximab(first dose of 400 mg/m2followed by 250 mg/m2) and cisplatin (80 mg/m2).Following primary systemic therapy, blood levels temporarily decreased to a leukocyte concentration of 58820/μL, CRP at 2.89 mg/dL, and serum G-CSF at 268 pg/mL.Disease progress was unable to be halted;however, blood levels eventually rose to a leukocyte concentration of 145180/μL, CRP at 13.2 mg/dL, IL-6 at 104 pg/mL, and serum G-CSF at 1520 pg/mL (Table 1).
OUTCOME AND FOLLOW-UP
The patient died from primary disease on postoperative day 45.
DISCUSSION
Sudden hyperleukocytosis has a range of underlying etiologies, including inflammatory diseases, acute leukemia, CML, G-CSF-producing tumors, non-CML myeloproliferative diseases, bone marrow metastases of malignant tumors and administration of G-CSF agents.
G-CSF is a cytokine that promotes the proliferation and differentiation of granulocytes, and is produced by macrophages, monocytes, and fibroblasts.Although serum G-CSF level is elevated in infectious diseases and blood diseases, malignant tumors themselves rarely produce G-CSF and are called G-CSF-producing tumor which is one of paraneoplastic syndromes that are induced by malignant tumors producing various bioactive substances[1].Hugheset al[2]reported the presence of malignant tumor that demonstrated leukocytosis in G-CSF-producing tumor.Later,Asanoet al[3]were the first to report on G-CSF-producing lung cancer.
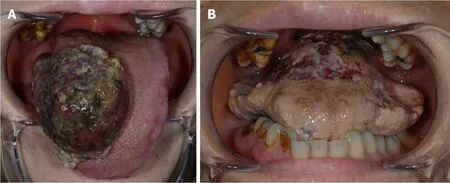
Figure 1 lntraoral view on the first visit (A) and administration (B).
There are four criteria for the diagnosis of G-CSF-producing tumors:Increase in leukocyte count, elevation in G-CSF levels, decrease in leukocyte count and G-CSF levels following treatment, and production of G-CSF by tumor cells[3].This final criteria (G-CSF production by tumor cells) can be assessed by observing G-CSF staining through immunohistochemical staining[4];however, this is said to be difficult due to weak antigenicity and significantly small amount of G-CSF protein, which is stored in cells for a short time before being released[5].In the case presented here, GCSF was confirmed in both the biopsy tissue and resected specimen.Furthermore, the other criteria were satisfied, based on which G-CSF-producing squamous cell carcinoma was diagnosed.
In the present case, PET/CT revealed FDG accumulation in the primary lesion metastatic lymph nodes, spleen, and bone marrow throughout the body.The relationship between G-CSF and bone marrow FDG-PET findings has previously been reported[6].In their study, chemotherapy patients receiving G-CSF agents exhibited significantly higher standardized uptake values compared with the control group.Furthermore, another study has shown a strong correlation between FDG accumulation in the bone marrow during PET imaging and leukocyte count in the peripheral blood, particularly neutrophils[7].The authors stated that this result reflected increased bone marrow metabolism through the stimulation of granulocytes and other substances[7].
It is thought that G-CSF increases granulocytic hematopoiesis in the bone marrow,where elevated glucose metabolism results in FDG accumulation[6];we consider that the diffuse FDG accumulation observed in the bone marrow in the present study was due to the same mechanism G-CSF administration induces extramedullary hematopoiesis, strengthening the hematopoietic capacity of the spleen, resulting in FDG accumulation.In the present case, G-CSF produced by the tumor is considered to have accumulated in both the bone marrow and spleen.It is important therefore not to misdiagnose diffuse FDG accumulation in the spleen and bone marrow as metastatic foci.
G-CSF increases leukocyte count but does not directly cause inflammatory responses.Some tumors produce inflammatory cytokines such as IL-6, IL-1, and tumor necrosis factor-a along with G-CSF, which causes fever and an inflammatory response[8,9].In this case, it is considered that CRP increased due to the high level of IL-6.In addition, it may cause accompanying symptoms such as hypercalcemia, but these are also considered to be related to cytokines other than G-CSF.
Interestingly, reports of G-CSF-producing tumors of the oral cavity are rare[10], with the most common location for this type of cancer being the lung[11], followed by liver[12], stomach[13], and bladder[1].There have been only 7 cases of G-CSF-producing oral cancer, including our case[10,14-18].Of 7 cases, 4 cases were tongue cancer and 3 cases were lower gingival cancer.Histopathologically, 6 cases were squamous cell carcinoma and 1 case was mucoepidermoid carcinoma.Despite chemotherapy,chemoradiotherapy and/or surgery was performed in each cases, all patients died of the disease within 12 mo (Table 2).G-CSF temporarily decreased after cetuximab administration, but did not stop progression of the disease in our case, although our case was the first cases administered cetuximab.Standard treatment for G-CSF-producing tumors has not been established, and it is considered to be necessary to accumulate and assess further case.
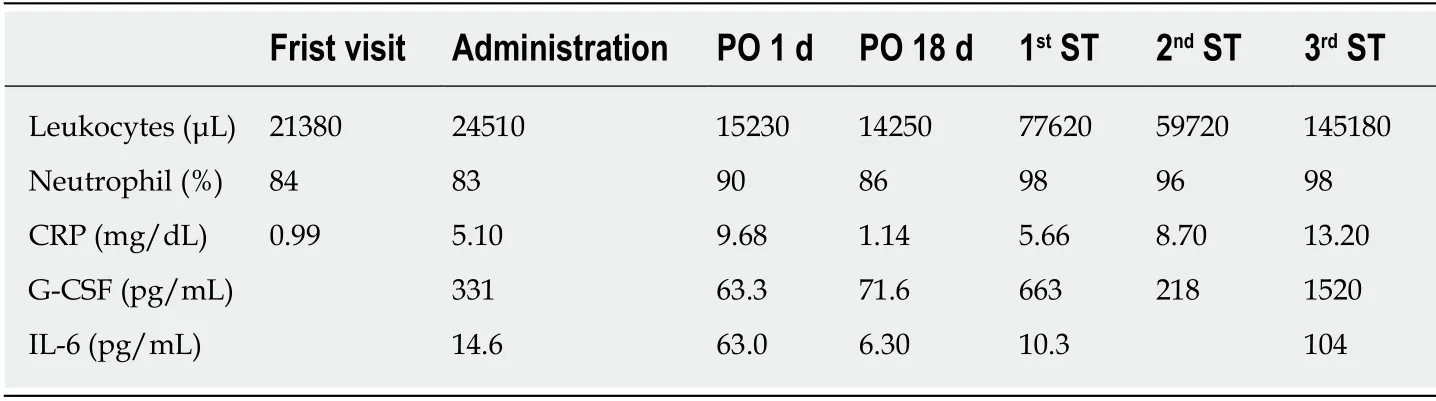
Table 1 Progression of laboratorial data
CONCLUSION
G-CSF-producing tumors progress quickly and are associated with extremely poor prognoses, as was observed in the present case.Poor prognosis is based on the fact that many G-CSF-producing tumors are undifferentiated or poorly differentiated, GCSF increased the invasive and metastatic capacity of tumors, and G-CSF-producing tumors themselves possess an autocrine pathway, although many details remain unclear[19-21].
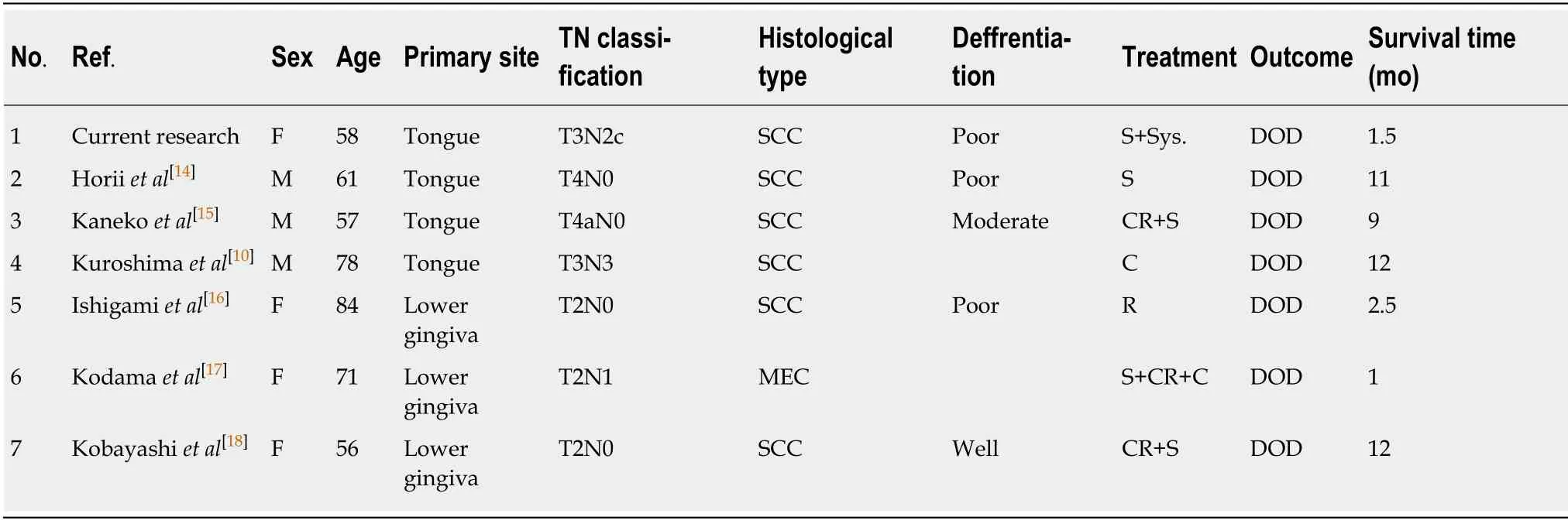
Table 2 Cases of granulocyte-colony stimulating factor-producing tumor of the oral cavity
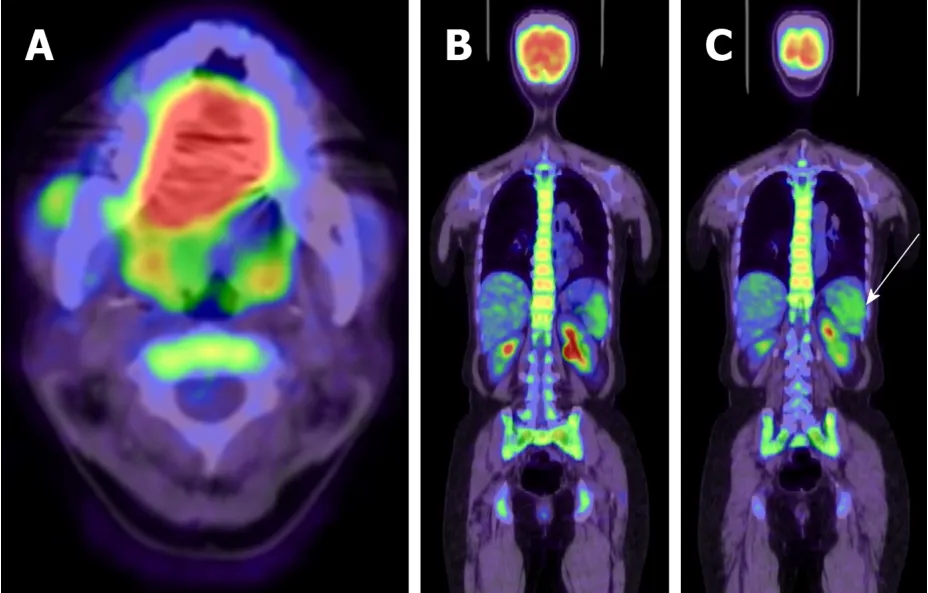
Figure 2 Positron emission tomography-computed tomography scan.
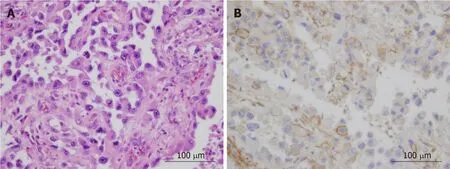
Figure 3 Hematoxylin and eosin (A) and immunohistochemical staining of granulocyte-colony stimulating factor (B) of primary tumor.
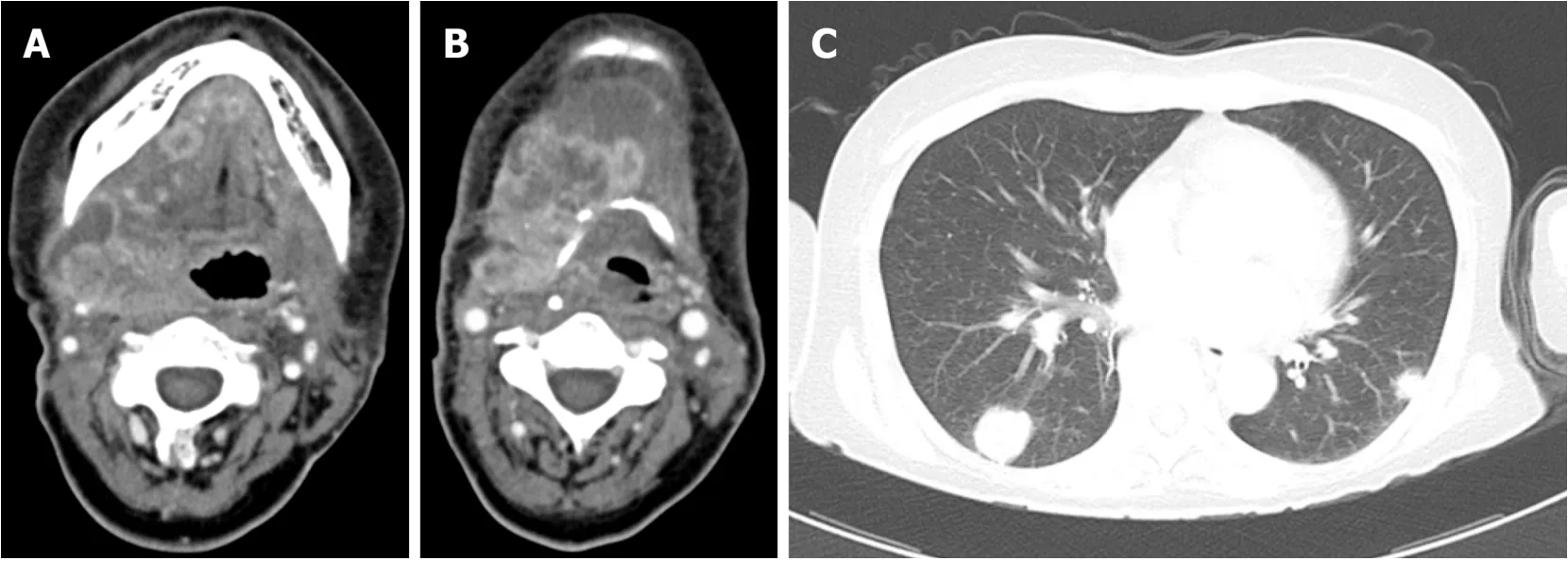
Figure 4 Computed tomography revealed the presence of recurrence in the neck (A, B), accompanied by multiple metastases in the lungs (C) on postoperative day 18.
杂志排行
World Journal of Clinical Cases的其它文章
- Nutrition management in acute pancreatitis:Clinical practice consideration
- Bone disease in chronic pancreatitis
- Role of microRNAs in the predisposition to gastrointestinal malignancies
- Recurrent anal fistulas:When, why, and how to manage?
- Removal of biofilm is essential for long-term ventilation tube retention
- Neutrophil gelatinase-associated lipocalin does not predict acute kidney injury in heart failure
