Anti-fungal properties of Puroindoline A against Aspergillus glaucus
2020-05-08WenjingYuanPingpingTianAngLyuYangyongLyuWeiZhangShanWeiYuansenHu
Wenjing Yuan,Pingping Tian,Ang Lyu,Yangyong Lyu,Wei Zhang,Shan Wei,Yuansen Hu*
College of Biological Engineering,Henan University of Technology,Zhengzhou 450001,China
Keywords:
Puroindoline A
Aspergillus glaucus
Membrane integrity
Mitochondrial membrane potential
DNA damage
ABSTRACT
Aspergillus glaucus can grow in low moisture grain,and is one of the main fungi responsible for agricultural product losses.Puroindoline A(PINA)is a tryptophan-rich alkaline adiponectin that can effectively inhibit numerous plant bacteria and fungi.However,the mechanism of PINA against A.glaucus remains unclear.Herein,we found that recombinant PINA (rPINA) could inhibit A. glaucus mycelia growth on salt Czapek dox agar (SCDA) medium and spore germination on Czapek dox(CD)medium.Scanning electron microscopy revealed that incomplete morphological characteristics of both A.glaucus spores and mycelia occurred following rPINA treatment.Laser scanning confocal microscopy(LSCM)showed that rPINA could enter the interior of spores.Flow cytometry and propidium iodide(PI)staining illustrated membranes of spores were severely damaged,especially after treatment with 0.9 mg/mL rPINA for 12 h,and spores with intact membranes were reduced by 30.7%.Additionally,rPINA reduced the mitochondrial membrane potential(MMP)by 5,5′,6,6′-tetrachlorr-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide(JC-1)staining,and caused DNA damage by 4′,6-diamidino-2-phenylindole(DAPI)staining.These results indicated that rPINA may damage cell membranes and DNA structure and reduce MMP,thereby inhibiting the growth of A.glaucus.The antifungal mechanism has been demonstrated in this study, and results show that rPINA has application potential in preventing postharvest loss in the agricultural industry.
1.Introduction
Mildew can lead to loss of grain yield and deterioration of grain quality during storage [1].A.glaucusis one of the main causes of mildew during grain storage, and is used as an early warning fungus for grain storage safety due to its growth under low-moisture conditions [2,3].Current mildew prevention methods mainly depend on low temperature,drying and chemical agents [4].However, low temperature and drying treatments are expensive,and chemical treatment usually results in fungicide residues that may be harmful to human health and the environment[5].Therefore, finding safe and effective antimicrobials to replace chemical fungicides in controlling this harmful pathogen is a research priority[6].A number of promising approaches,including postharvest application of cinnamic acid [7], antagonistic yeasts [8,9], and antagonistic yeasts combined with silicon [10] have been successfully used to control postharvest diseases in fruits and grain.
Puroindoline protein (Puroindoline A and Puroindoline B) is a tryptophan-rich alkaline adiponectin that can effectively inhibit the growth of numerous plant pathogens,and exert potent activity against bacteria and fungi.Puroindoline A has obvious antifungal activity due to its five disulfide bonds and a specific tryptophan-rich domain(Trp-Arg-Trp-Trp-Lys-Trp-Trp-Lys)[11-15].The unique tryptophan-rich domain underpins the mechanism by which PINA determines grain hardness and is the feature responsible for antimicrobial activity[16,17].Recent evidence has shown that PINA has a negative effect on bacteria includingStaphylococcus aureusandAgrobacterium tumefaciens[18,19],and also on targets fungi includingAscochyta pisi,Fusarium culmorum,Botrytis cinereaandCandida albicans[20-23].Transgenic crops over-expressing PINA displayed significantly increasing resistance to plant diseases[13,24].However,there are no reports on Puroindoline proteins targetingA.glaucus.
Our previous research indicated that rPINA possessed strong inhibitory activity againstAspergillusflavus[25].The objective of the present study was to investigate the inhibitory effects of rPINA againstA.glaucus in vitro,and further ascertain its underlying mechanism against this species.Our results not only illuminated the antifungal mechanism of rPINA,but also provided a scientific basis for molecular breeding of storable wheat and the development of new grain preserving fungicides.
2.Materials and methods
2.1.Strain and culture
TheA.glaucuswas offered by Prof.Cai Jingping(Henan University of Technology,China)and cultivated on potato dextrose agar(PDA)medium at 28±2°C.The spore concentration was adjusted to 5×107spores/mL using a hemocytometer.
2.2.Preparation of rPINA
The rPINA was expressed inEscherichia coliBL21(DE3)cells,and purified following the method described previously[25].
2.3.Effect of rPINA on A.glaucus spore germination
Czapek dox(CD)medium was supplemented with different concentrations of rPINA(0,0.05 and 0.09 mg/mL),andA.glaucusspore suspension was added to obtain a final concentration of 1×107spores/mL.The inoculated CD media were cultured at 28±2°C on a rotary shaker(200 r/min).Spore germination was assayed at 36 h after inoculation[6].A spore was considered germinating when the length of the germ tube was equal to or greater than the diameter of the spore.All experiments were carried out in triplicate.
After exposure to 0,0.05 and 0.09 mg/mL concentrations of rPINA for 24 h at 28±2°C with shaking at 220 r/min,A.glaucusspores were fixed with 2.5%glutaraldehyde,washed with 10 mmol/L phosphate-buffered saline(PBS),dehydrated with an ethanol gradient(30%-100%),and resuspended in tert-butanol.Spores were then freeze-dried and coated with gold in a metallizer[26].Spores not treated with rPINA were used as controls.The morphology ofA.glaucusspores was determined using an FEI Quanta 250 FEG field emission scanning electron microscope(SEM;FEI,USA).
2.4.Effect of rPINA on A.glaucus mycelia growth
The 10 mL sample of sterile salt czapek dox agar(SCDA)medium containing different final concentrations of rPINA (0, 0.090, 0.230 and 0.334 mg/mL)was poured into a 60 mm diameter sterile plate.After solidification,2 μL ofA.glaucusspores(107spores/mL)were point-inoculated into the center of the rPINA agar culture.Plates were incubated at 28±2°C for 5 days,and the inhibition zone diameter was measured after 3,3.5,4,4.5,and 5 days.The effect of rPINA on inhibitingA.glaucusgrowth was photographed with a digital camera [27].Each treatment included three replicates,and the entire experiment was repeated twice.All experiments were carried out in triplicate.
A.glaucusspore solutions were inoculated into SCD medium and cultured at 28±2°C with shaking at 70 r/min for 4 days to obtain mycelia.After exposure to 0,0.05 and 0.09 mg/mL concentrations of rPINA for 24 h at 28±2°C,A.glaucusmycelia were fixed with 2.5%glutaraldehyde and washed with PBS,and some mycelia were evenly spread on foil and dried at 40°C[28].Subsequently,mycelia were coated with gold in a metallizer.Mycelia not treated with rPINA were included as controls.The morphology ofA.glaucusmycelia was measured using an FEI Quanta 250 FEG(FEI).
2.5.Localization of FITC-rPINA in A.glaucus
FITC coupling was performed according to the manufacturer's instructions (Solarbio, Beijing, China) [29].After exposure to 0, 0.5, and 0.9 mg/mL concentrations of FITC-rPINA at 28 ± 2 °C with shaking at 220 r/min for 6 or 12 h,A.glaucusspores were washed and resuspended in 10 mmol/L PBS,then analyzed by an LSCM(Zeiss,Jena,Germany).
2.6.Effect of rPINA on membrane integrity
After exposure to 0,0.5 and 0.9 mg/mL concentrations of rPINA for 12 h at 28±2°C with shaking at 220 r/min,A.glaucusspores were washed with 10 mmol/L PBS,propidium iodide(PI)was added to a final concentration of 1 μg/mL, and a further incubation was conducted for 0.5 h at 28 ±2°C in the dark.After washing and resuspending in 10 mmol/L PBS[30],analysis was performed using an LSCM (Zeiss) and an Accuri C6 flow cytometer(BD Biosciences,San Jose,CA,USA).
2.7.Effect of rPINA on mitochondrial membrane potential(MMP)
In this process,107spores/mL spore suspensions were cultured in the CD medium and treated with 0,0.5 and 0.9 mg/mL rPINA for 12 h at 28±2°C with shaking at 220 r/min.After culturing, spores were washed with 10 mmol/L PBS and resuspended in 10 μg/mL 5,5′,6,6′-tetrachlorr-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide (JC-1), then incubated at 28±2°C for 0.5 h in the dark[31].Spores were washed with 10 mmol/L PBS and analyzed using an LSCM(Zeiss)and an Accuri C6 flow cytometer(BD Biosciences).
2.8.Effect of rPINA on DNA structure
107spores/mL spore suspensions were cultured in CD medium and treated with 0, 0.5 and 0.9 mg/mL rPINA at 28 ±2°C with shaking at 220 r/min for 12 h.After culturing,spores were washed with 10 mmol/L PBS and resuspended in 1 mg/mL 4′,6-Diamidine-2′-phenylindole(DAPI)at 28 ± 2 °C for 0.5 h in the dark [32].Spores were then washed with 10 mmol/L PBS and analyzed using an LSCM(Zeiss).
2.9.Statistical analysis
All assays were performed in triplicate,and the results were presented as the mean±standard deviation(SD).Significant differences between mean values were determined by one-way analysis of variance(ANOVA)using Duncan's multiple range tests(P<0.05).Statistical analyses were performed by SPSS 13.0[26].
3.Results
3.1.Effect of rPINA on A.glaucus spore germination
In this study,the effect of rPINA onA.glaucusspore germination was investigated using optical microscopy.As shown in Fig.1,the inhibition effect of rPINA onA.glaucusspores in CD medium was significant compared with the control group;0.05 mg/mL rPINA treatment could inhibit germination of most of spores,and the inhibition effect was enhanced with increase in rPINA concentration.There were almost no germinating spores when the rPINA concentration reached 0.09 mg/mL.In addition,morphological observation ofA.glaucusspores was performed by SEM.As shown in Fig.2,most of spores were broken and depressed on the surface following rPINA treatment,relative to controls.

Fig.1.Optical microscopy images of A.glaucus spores after treatment with rPINA for 36 h(40×magnification).

Fig.2.SEM image of A.glaucus spores.
3.2.Effect of rPINA on A.glaucus mycelia
To investigate the effect of rPINA onA.glaucusmycelia growth,colony diameter was measured by the crossing method.As shown in Fig.3A,the inhibition rate of mycelia growth was 25.24%,49.53%and 59.94%for 0.090,0.230 and 0.334 mg/mL rPINA treatments,respectively.The inhibition effect of rPINA onA.glaucuswas enhanced with increase in rPINA concentration(Fig.3B).As shown in Fig.4,SEM results revealed that mycelia became shrunken and flat following rPINA treatment.
3.3.Localization of FITC-rPINA in A.glaucus
To explore whether rPINA can exert its antifungal activity through its lipid-binding ability [33] and consequent interaction with the cell membrane, localization of FITC-rPINA inA.glaucuswas investigated by LSCM.FITC is an amine-reactive fluorescent probe that labels biomolecules by forming a covalent bond between its isothiocyanate group and the primary and secondary amine groups of biomolecules,displayed with green fluorescence following excitation with a 488 nm laser.As shown in Fig.5, FITC-rPINA appeared to aggregate inA.glaucus,and spores exhibited green fluorescence,indicating that rPINA could enter the interior of spores through the membrane system.However, subsequent experiments showed that rPINA's entry into spores was not significantly correlated with rPINA concentration or treatment duration.

Fig.3.Effect of rPINA on A.glaucus mycelia growth on SCDA plates after 5 d.
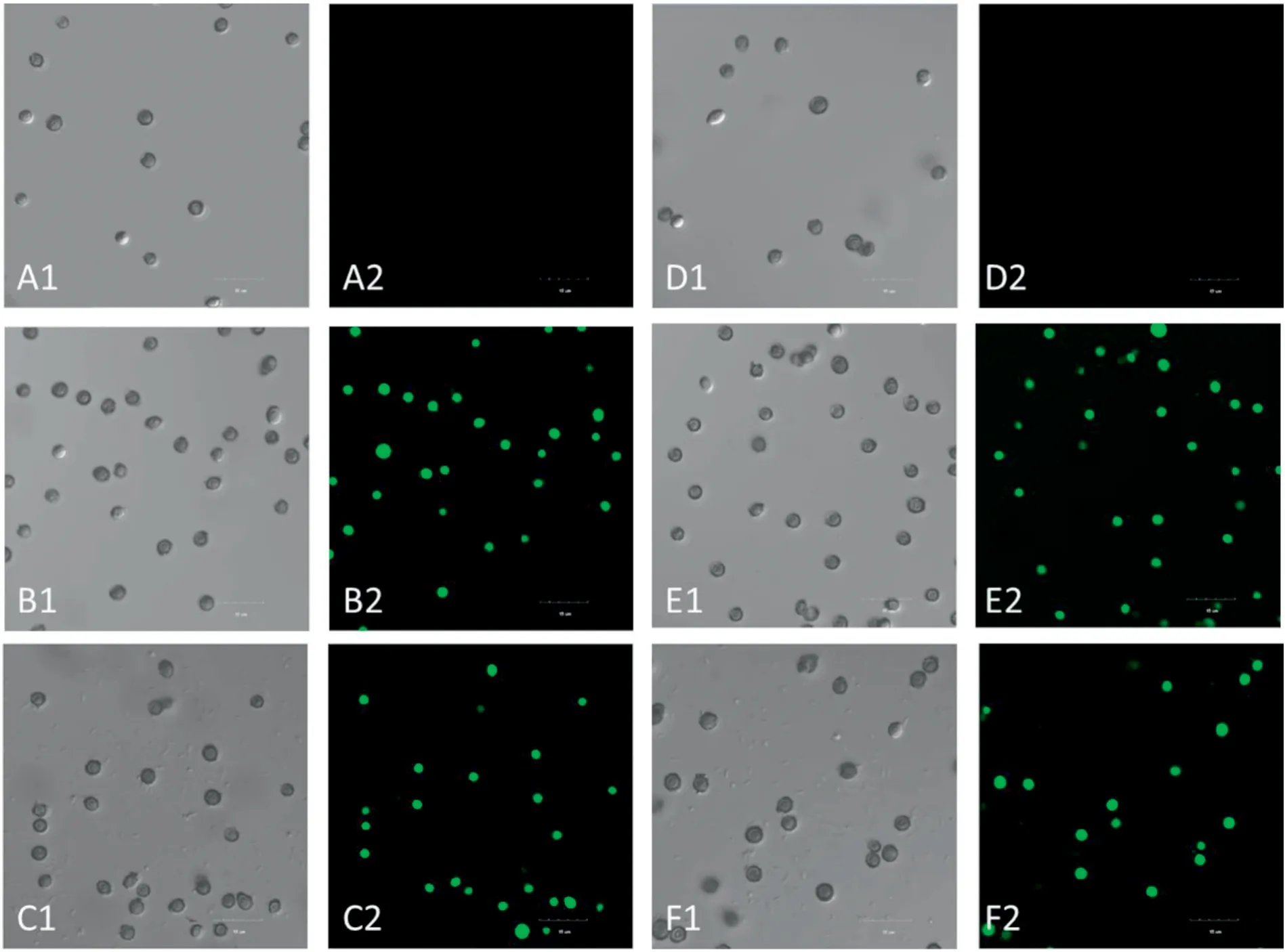
Fig.5.Localization of FITC-rPINA in A.glaucus spores analyzed by laser confocal microscopy.

Fig.6.Effect of rPINA on A.glaucus spore membrane integrity analyzed by microscopy.
3.4.Effect of rPINA on membrane integrity
PI is a membrane-impermeable dye that can be used to probe plasma membrane integrity[34].The effect of rPINA on the integrity of the cell membrane ofA.glaucuswas analyzed by LSCM and flow cytometry.As shown in Fig.6,LSCM results showed thatA.glaucustreated with rPINA exhibited red fluorescence,while the untreated group showed no fluorescence.This indicated that rPINA disrupted cell membrane integrity.Then,flow cytometry assays were applied to investigate the membrane integrity at the different concentrations of rPINA.As shown in Fig.7,after treatment with 0,0.5 and 0.9 mg/mL of rPINA, 97.6%, 74.2%, and 66.9% of spores were not stained with PI,suggesting that rPINA altered membrane integrity in a dose-dependent manner.
3.5.Effect of rPINA on MMP
Mitochondria are important because they supply ATP in cells.MMP, a sensitive indicator, was used to evaluate the energetic state of mitochondria in cells [35].The effect of rPINA on MMP inA.glaucusspores was observed by LSCM and flow cytometry using JC-1.LSCM images showed that the spore fluorescence intensity in the rPINA-treated group was significantly enhanced compared with the control group, and positively correlated with rPINA concentration (Fig.8).

Fig.7.Effect of rPINA on A.glaucus spore membrane integrity analyzed by flow cytometry.

Fig.8.Effect of rPINA on MMP in A.glaucus spores analyzed by LSCM.
The formation of the JC-1 monomer in mitochondria led to a fluorescence change from red to green in stained cells.In addition,the ratio of red to green fluorescence also reflected the strength of the MMP, and the results showed that the red-green fluorescence ratio in spores was significantly reduced with increase in rPINA concentration (Fig.9).These results indicated that rPINA could reduce MMP.

Fig.9.Effect of rPINA on MMP in A.glaucus spores analyzed by flow cytometry.
3.6.Effect of rPINA on DNA damage
To explore the effect of rPINA onA.glaucusDNA,A.glaucusspores were stained with DAPI after rPINA treatment.LSCM images showed that the fluorescence intensity of rPINA-treated groups was significantly enhanced and more concentrated or divided compared with the control group(Fig.10).These results suggested thatA.glaucusDNA was damaged by rPINA treatment[25,36].
4.Discussion
Due to the health risks of synthetic chemical fungicides,there is an increasing demand for effective and eco-friendly agents to control fungal contamination in foods.Some natural proteins found in plants and animals have potential for use as fungal control agents,which has aroused great interest and hope [33,35,37].PINA can effectively control common food fungi such asFusarium culmorum,Botrytis cinereaandA.flavusduring grain storage[18-23].However,the mechanism of action againstA.glaucusremains unclear.In the present study,we found that mycelia growth and spore germination ofA.glaucuswere inhibited by rPINA,and inhibition was enhanced with increase in rPINA concentration(Figs.1 and 3).
Substance exchange and immunity functions of the cell membrane are very important for cell growth [38,39].Recent research demonstrated that antifungal proteins and plant essential oils destroy cell membrane integrity and fluidity[40,41].Herein,PI staining showed that the cell membrane integrity ofA.glaucusspores was reduced significantly with increase in rPINA concentration (Figs.5 and 6).Similar findings were also reported previously[37].Some studies have shown that the destruction of cell membrane integrity can lead to internal dissolution,and eventually spore depression and mycelia fold deformation [42-44].This was confirmed to be the case in the present work based on SEM images(Figs.2 and 4).We therefore propose that rPINA exerts its antifungal activity by disrupting cell membrane.

Fig.10.Effect of rPINA on DNA in A.glaucus spores analyzed by LSCM.
Mitochondria play essential roles in cells,including energy production,ATP synthesis,calcium mobilisation,and apoptosis[45-48].MMP is a sensitive indicator of the energetic states of both mitochondria and cells[49],and can be used to assess the activity of mitochondrial proton pumps,electrogenic transport systems, and changes in mitochondrial permeability[50].Cells usually maintain MMP balance across the inner membrane of mitochondria via an electrochemical gradient generated by the electron transport chain[51].Some cellular functions can be disrupted once the MMP is reduced,resulting in deficient ATP synthesis,increased intimal permeability[36]and imbalanced H+gradient[52].These changes may affect the normal physiological activities of cells.For example,reduced MMP was reported in apoptoticAspergillusflavusandCandida albicans[53,54].In the present study,MMP inA.glaucusspores treated with rPINA was reduced in a dose-dependent manner(Figs.8 and 9).These results indicate that rPINA decreases the MMP inA.glaucusspores.
It has been reported that mitochondrial dysfunction can cause some pro-apoptotic factors (cytochrome C, Aif1p and Nuc1p) to flow from mitochondria into the cytoplasm and nucleus [55], where they may cause damage to DNA.To further examine whether rPINA causesA.glaucusDNA damage, DAPI assays were performed [55], and the results indicated that rPINA did indeed cause DNA damage inA.glaucusspores.
In conclusion,rPINA exerted inhibitory action againstA.glaucusby altering membrane integrity,reducing mitochondrial membrane potential,and inducing DNA damage.This study provides an understanding of the fungicidal mechanisms of rPINA againstA.glaucus, and suggests that rPINA has potential for application as a food safety additive.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by grants from the Natural Science Foundation of China(31871852,31972176),Natural Science Foundation of Henan Province(162300410047).
杂志排行
Grain & Oil Science and Technology的其它文章
- GC-MS,GC-O and OAV analyses of key aroma compounds in Jiaozi Steamed Bread
- Physicochemical and structural characteristics of the Venn components of wheat gliadin
- Influence of low temperature on lethal time extension for different life stages of Cryptolestes ferrugineus(Stephens)with strong resistance to phosphine fumigation
- Quality deterioration and improvement of wheat gluten protein in frozen dough
- Grain & Oil Science and Technology
