An in vivo confocal microscopy study: correlation between morphological changes of corneal subbasal nerve and clinical symptoms of Sjogren syndrome
2020-05-08ZhaoYangQiChen
Zhao Yang, Qi Chen
Abstract
•AIM: To observe the morphological changes of corneal subbasal nerve (SBN) plexus in patients with Sjogren syndrome (SS) by in vivo confocal microscopy (IVCM) study and analyze its correlation with clinical symptoms.
•METHODS: We collected 22 cases (44 eyes) patients with Sjogren’s syndrome (observation group) and 22 cases (44 eyes) healthy people with the same age (control group) for observation of central corneal SBN fibers diameter, number of beads, and tortuosity, and recorded tear film break up time (BUT), Schirmer’s Ⅰ test (SⅠt), tear meniscus height (TMH), dendritic cell (DC) density, the results were applied to perform statistical analysis.
•RESULTS: Under IVCM observation, the diameter of corneal SBN fibers in the observation group became thinner, the number of beads and the degree of nerve fiber tortuosity were increased compared with the control group (P<0.05). Pearson correlation analysis: the diameter of corneal SBN fibers in the observation group was significantly correlated with BUT (r=0.472, P<0.01), SⅠt (r=0.562, P<0.01), TMH (r=0.246, P=0.02) and DC density (r=-0.636, P<0.01). The number of nerve fiber beads was correlated with BUT (r=-0.621, P<0.01), SⅠt (r=-0.688, P<0.01), TMH (r=-0.438, P<0.01) and DC density (r=0.528, P<0.01). There was a significant correlation between nerve fiber tortuosity and BUT (r=-0.634, P<0.01), SⅠt (r=-0.713, P<0.01), TMH (r=-0.384, P< 0.01) and DC density (r=0.604, P<0.01).
•CONCLUSION: IVCM can effectively observe the morphology of corneal nerve plexus. In SS patients, the diameter of corneal SBN fibers becomes thinner, the number of beads increases, and the tortuosity of nerve fiber increases. Moreover, the degree of nerve fiber lesions is correlated with the severity of dry eye clinical symptoms.
•KEYWORDS:in vivo confocal microscopy; Sjogren syndrome; nerve
INTRODUCTION
Sjogren’s syndrome (SS) is a chronic inflammatory autoimmune disease mainly involving exocrine glands, in addition to the symptoms of dry eyes and mouth, it was caused by dysfunction of lacrimal gland and salivary gland epithelial cells, there are other exocrine glands and other organs involved and subsequently multiple system damage[1]. In ophthalmology, the clinical features of SS is characterized by dryness, burning and foreign body sensation, and extensive damage to the cornea and conjunctival epithelium[2]. According to rough estimation that the prevalence of SS in the world is 0.007%, while in Europe and Asia the prevalence is relatively high, reaching 0.043%[3]. For these patients, traditional examination methods include corneal fluorescein staining, schirmer’s test and comprehensive ocular surface analyze. Theinvivoconfocal microscopy, as a new non-invasive examination technique in recent years, has already been proved to be a powerful tool for theinvivoresearch of ocular surface diseases. In this research, the patients with keratoconjunctivitis sicca caused by SS were studied byinvivoconfocal microscopy, then the relationship between corneal nerve plexus changes underinvivocorneal and clinical symptom severity was analyzed, finally, we hope it can provide a theoretical basis for the clinical treatment of those patients.
SUBJECTS AND METHODS
Ethical Approval The study was approved by the Institutional Review Board of People’s Hospital of Guangxi Zhuang Autonomous Region and was conducted in accordance with the Declaration of Helsinki. All subjects were fully informed of the purpose and methods of this study and had provided written informed consent.
From January 2017 to March 2019, 22 patients (44 eyes) with SS and 22 healthy people (44 eyes) admitted to People’s Hospital of Guangxi Zhuang Autonomous Region were selected, with age and gender matching. The diagnosis of SS was made according to criteria reported by the American-European Consensus Group[4]. All SS patients didn’t receive any topical treatment before and during the study. The criteria of healthy people were included: 1) No dry eye symptoms; 2) The anterior segment of the eyeball is normal; 3) Break up time (BUT)>10s, SⅠt>10 mm/5min; 4) Previous history of ocular diseases, trauma, medication, surgery and other systemic immune diseases were excluded.
Schirmer’s Ⅰ test Without local anesthesia, the filter paper was placed in the temporal conjunctival sac without contact with the cornea, the other side was placed on the outside of the lower eyelid. Patients were instructed to close their eyes and remove the filter paper strip after 5min, the wet length of the filter paper strip was recorded.
Break up Time and Tear Meniscus Height The ocular surface of the subjects was examined and analyzed by an ocular surface analyzer, then the BUT and the TMH were measured, repeat the measurement 3 times, and finally take the average value.
InVivoConfocal MicroscopyInvivoconfocal microscope (model HRT3-CM, Heidelbeg, Germany);laser light source: 670nm; scan pattern: section; scan range: 400×400 μm; magnification: 800 times; resolution: 1 μm; scanning depth: 1500 μm. Topical anesthesia was performed with Promecaine Eye Drops (Alcon, USA), the objective len was coated with Carbomer eye gel (Bausch & lomb) and placed sterile corneal contact cap, the subject’s eyelids was opened with eye speculum and their forehead and jaw were placed on the examination bracket, make the cap touch the cornea slightly and set the value to 0 when adjusting the focus on the corneal epithelium, the central cornea of the eye (5 mm2) was scanned layer by layer, finally, valuable pictures were selected to save.
Corneal Epithelial Nerve Fiber One image was selected at random and Image J software was used for it’s analysis: 1) The diameter of corneal SBN fibers: three segments of nerve fibers were randomly selected for measurement, and the average value was finally obtained; 2) Tortuosity score of nerve fibers (the Oliveira grading standard): 0 points: the morphology of nerve fibers are almost straight line; 1 points: nerve fibers partial are slight tortuosity; 2 points: nerve fibers are partial moderate tortuosity or multiple slight tortuosity; 3 points: nerve fibers are partial severe tortuosity; 4 points: nerve fibers are multiple severe tortuosity or fracture; 3) The number of nerve fiber beads: three segments of nerve fibers (100 μm per segment) were randomly selected to calculate the number of nerve beads, and the average value was finally obtained.
Dendritic Cell Density Three corneal epithelium images were captured from IVCM and the DC density was calculated, and the average value was finally obtained.
Statistic analysis was performed by using SPSS version 23.0. Data were summarized as mean and standard deviation, two samplet-test was used to estimate the differences between the two groups, Pearson correlation analysis was used to evaluate the correlation between corneal nerve fiber changes and the severity of clinical symptoms, andP<0.05 was considered statistically significant.
RESULTS
This study included 22 cases (44 eyes) patients with Sjogren’s syndrome (observation group), of which 6 cases (12 eyes) were male and 16 cases (32 eyes) were female, the mean age was 53.55±12.37 (range: 23-77) years; Healthy people 22 cases (44 eyes) (control group), of which 6 cases (12 eyes) were male and 16 cases (32 eyes) were female, the mean age was 55.23±12.23 (range: 34-68) years. There was no significant difference in age between the two groups. Compared with the control group, BUT, SⅠt and TMH were decreased and DC density was increased in the observation group (P<0.01; Table 1).

Figure 1 Nerve fiber tortuosity score (Oliveira grading standard) A: 0 points: Nerve fibers are almost straight line; B: 1 points: Nerve fibers are Partial slight tortuosity; C: 2 points: Nerve fibers are partly moderately tortuosity or multiple slightly tortuosity; D: 3 points: Nerve fibers are partly severely tortuosity; E: 4 points: Nerve fibers are multiple severely tortuosity; F: 4 points: Nerve fibers become fracture obviously.
Table 1 Clinical data

ParametersSS groupHP groupTPAge, a53.55±12.3755.23±12.23-0.640.52BUT (s)4.70±3.3213.94±4.72-10.62<0.01SⅠt (mm/5min)3.30±1.7014.57±3.79-17.98<0.01TMH (mm)0.14±0.060.24±0.07-6.63<0.01DC density (Per 100 μm2)63.07±38.8510.86±7.088.77<0.01
SS: Sjogren’s syndrome; HP: Healthy people; BUT: Break up time; TMH: Tear meniscus height; SⅠt: Schirmer’s Ⅰ test; DC: Dendritic cell.
Table 2 Corneal SBN plexus
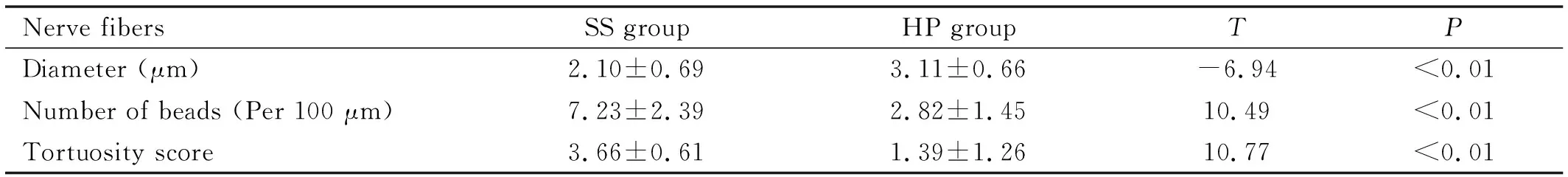
Nerve fibersSS groupHP groupTPDiameter (μm)2.10±0.693.11±0.66-6.94<0.01Number of beads (Per 100 μm)7.23±2.392.82±1.4510.49<0.01Tortuosity score3.66±0.611.39±1.2610.77<0.01
SS: Sjogren’s syndrome; HP: Healthy people; SBN: Subbasal nerve.
In the observation group, the corneal SBN fibers in the central cornea became thinner, the number of beads was increased, and the tortuosity was increased. Some corneal nerve fibers of SS patients were multiple severe tortuosity or fracture. There was significant difference between the two groups. (P<0.01; Table 2).
In the observation group, the diameter of nerve fibers was positively correlated with BUT, SⅠt, TMH and negatively correlated with DC density, the number of nerve fibers beads was negatively correlated with BUT, SⅠt, TMH and positively correlated with DC density, and the tortuosity score of nerve fibers was negatively correlated with BUT, SⅠt, TMH and positively correlated with DC density (Table 3; Figure 1).
DISCUSSION
IVCM is an optical microscope with confocal laser as light source, as a new non-invasive technique, it can observe the pathophysiological changes of ocular surface diseases at the level of living cells[5]. In the field of ophthalmology, IVCM has been used to the examination of meibomian gland, lacrimal gland, cornea, bulbar and palpebral conjunctiva, for the corneal examination, it can assess the morphology which including epithelial cells density, stromal keratocyte density, endothelial cells density, nerve plexus density, the number of beads, nerve tortuosity, nerve reflectivity, and inflammatory cells density. In addition, due to the subclinical visualization of IVCM which could detect disease at earlier stage, the application of IVCM in ocular surface disease is a powerful method to evaluate the morphologic change[6-7].

Table 3 Analysis of the correlation between nerve plexus changes and severity of clinical symptomsr (P)
BUT: Break up time; SⅠt: Schirmer’s Ⅰ test; TMH: Tear meniscus height; DC: Dendritic cell.
There are numerous nerve endings in the corneal tissue and the corneal sensory nerves originate from ophthalmic branch of the trigeminal nerve, they cross the long ciliary nerve and form cyclic annular nerve plexuses in the corneal limbus, subsequently mainly divided into three major nerve plexus in the corneal tissue: intraepithelial, subepithelial and basal nerves, for this reason, the corneal perception is very sensitive. Under normal conditions the morphology of corneal SBN plexus are regular, roughly parallel, fewer branches, less directional changes and less tortuosity. The lacrimal gland causes lacrimal gland secretion reflection of tears which was innervated by the trigeminal nerve and the facial nerve, furthermore, it also produces lacrimal gland-derived cytokines which plays an important role in maintaining corneal sensitivity and nutrient supply of corneal epithelial cells[8]. The corneal epithelium of SS patient presents morphological changes in areas of enlarged and irregular shaped cells, moreover, the density of superficial cells, wing cells and basal cells were all decreased versus heathy person[9-12].
Sjogren-related keratoconjunctivitis sicca was caused bygradual gland destruction and impaired lacrimal gland which was characterized by the replacement of functional epithelium with lymphocytic infiltrates, leading to aqueous tear deficiency or abnormal tear quality or fluid dynamics of the tear film, furthermore, it also includes autoimmune inflammatory lesions on ocular surface and morphological changes of corneal SBN plexus, and cytokine levels such as IL-17, TNF-α, and IL-6 in tear decrease and the involvement of different inflammatory processes cause dry eye syndrome. So it is more severe than idiopathic dry eye and the treatment modalities that work in idiopathic dry eye may not be effective in SS patients[2,13-14]. Compared with healthy people, we found that the diameter of corneal SBN fibers in SS patients became thinner and fibers irregular, branches increasing, and even reticular or fracture, indicating that there were degenerative changes in nerve fibers. The mechanical, chemical, and thermal sensitivity of SS patients and the number and density of SBN were found to be significant decrease[11,15]. It was reported by Tepelusetal[16]that the density of inflammatory dendritic cells and nerve tortuosity was increased, nerve fibers density and reflectivity was decreased in the SS patients. The corneal nerve density and reflectivity under observation of IVCM were correlated with severity of subjective dry eye symptoms, as measured by Ocular Surface Disease Index (OSDI) score. Corneal SBN plexus had fewer fibers and higher bead density in SS patients, indicating that there may be some regeneration mechanism after neural lesion and negative feedback mechanism produced by dryness of ocular surface, it results in nerve fibers sprouting, regeneration and abnormal morphology of nerve plexus[17]. Therefore, we speculate that the abnormal morphological changes of corneal SBN plexus in SS-related dry eyes can cause blink reduction, evaporation on preocular aqueous tear loss, unstable tear film, leading to the decline of tear meniscus height, on the other hand, the decrease of corneal sensitivity can cause the decrease of tear secretion reflex. Topical cyclosporine A led to an increase in corneal SBN density and decrease in inflammatory dendritic cells, improving clinical symptoms of SS patients, especially to the patients with less initial nerve damage would be more effective[18]. It shows that there may be a certain correlation between clinical symptoms and inflammation and changes of nerve fibers. McNamaraetal[19]found that the levels of endogenous tear protein and lacritin are linked to altered corneal innervation and dry eye severity in SS patients. Reduced tear lacritin levels in SS patients are highly correlated with clinical symptoms of dry eye, as well as decreased nerve fiber density and length. Lacritin and its components provide excellent diagnostic sensitivity and specificity in SS patients. It was reported by Cardigosetal[20]that the corneal SBN plexus density and length are significantly lower, and tortuosity is significantly higher in SS patients than healthy people, they presente a strong association with STI and BUT. These studies showed that the corneal SBN fibers and autoimmune inflammatory of cornea are relate to the degree of dry eye, these morphological changes could lead to dysfunction of corneal epithelial cells, edema of epithelial cells and even pathological changes, and decrease the stability of tear film. The imbalance between inflammation and immune regulation may be an important reason of tissue damage, the density of corneal dendritic cells of SS patients is higher than that of idiopathic dry eye, which indicates that the immune inflammatory is also a part that should not be ignored in the treatment of this disease[16,21].
For SS patients, the corneal subepithelial plexus are not only including the increase of branches, tortuosity and fracture, but also the change of the number of beads. Tuominenetal[22]found that the SBN plexus of cornea are sprouting and reveale abnormal morphology in SS patients, implying ongoing active neural growth. The degeneration of corneal SBN is interlinked with the increase of tortuosity of nerve fibers, the increase number of nerve beads and neural regeneration. The nerve beads may be containing active metabolic transmitters, which contribute to the recovery of corneal epithelial dysfunction, or the formation of nerve beads which means the lesion of nerve fibers and need to through inflammation to stimulate the secretion of nerve growth factor to promote it repair[17,23]. Therefore, we believe that the number of SBN fibers beads in cornea is also valuable for evaluating the severity of SS-related dry eye.
As an objective, non-invasive and convenient optical imaging technology, IVCM can clearly and intuitively observe the morphology of corneal epithelial cells, nerve fibers, stromal cells and endothelial cellsinvivo. Through the observation of corneal nerve plexus in SS patients with IVCM can further understand the severity degree of keratopathy in SS related dry eyes and provide theoretical basis for clinical treatment. As an objective device to monitor clinical treatment efficacy, IVCM may allow the possibility of tailoring treatment based oninvivocellular morphological changes, rather than only clinical changes.
