Mechanisms of action of neuropeptide Y on stem cells and its potential applications in orthopaedic disorders
2020-04-30
Jian-Qun Wu, Department of Orthopedics and Traumatology, Huadu District People’s Hospital,Guangzhou 510800, Guangdong Province, China
Nan Jiang, Bin Yu, Division of Orthopaedics and Traumatology, Department of Orthopedics,Nanfang Hospital, Southern Medical University, Guangzhou 510515, Guangdong Province,China
Nan Jiang, Bin Yu, Guangdong Provincial Key Laboratory of Bone and Cartilage Regenerative Medicine, Nanfang Hospital, Southern Medical University, Guangzhou 510515, Guangdong Province, China
Abstract Musculoskeletal disorders are the leading causes of disability and result in reduced quality of life.The neuro-osteogenic network is one of the most promising fields in orthopaedic research.Neuropeptide Y (NPY) system has been reported to be involved in the regulations of bone metabolism and homeostasis,which also provide feedback to the central NPY system via NPY receptors.Currently, potential roles of peripheral NPY in bone metabolism remain unclear.Growing evidence suggests that NPY can regulate biological actions of bone marrow mesenchymal stem cells, hematopoietic stem cells, endothelial cells, and chondrocytes via a local autocrine or paracrine manner by different NPY receptors.The regulative activities of NPY may be achieved through the plasticity of NPY receptors, and interactions among the targeted cells as well.In general,NPY can influence proliferation, apoptosis, differentiation, migration,mobilization, and cytokine secretion of different types of cells, and play crucial roles in the development of bone delayed/non-union, osteoporosis, and osteoarthritis.Further basic research should clarify detailed mechanisms of action of NPY on stem cells, and clinical investigations are also necessary to comprehensively evaluate potential applications of NPY and its receptor-targeted drugs in management of musculoskeletal disorders.
Key Words:Neuropeptide Y; Bone marrow mesenchymal stem cells; Hematopoietic stem cells; Fracture; Osteoporosis; Osteoarthritis
INTRODUCTION
As a substantial portion of the whole body, skeletal muscles are integral for locomotion and metabolic health[1].Musculoskeletal disorders, such as fracture,delayed/non-union, osteoporosis, and osteoarthritis, are the primary causes of disability and lead to reduced life quality.Skeletal metabolism and homeostasis are accurately regulated by neural signal networks between nervous systems and bone cells.
Recently, neuropeptide Y (NPY) has emerged as one of the major regulators of bone metabolism and homeostasis.NPY, a 36-amino-acid peptide first isolated from the porcine brain by Tatemoto[2]in 1982, is widely distributed in the central nervous system (CNS) and peripheral nervous system[3].It is produced by nerve endings, and acts as a critical molecule in the interactions between nerves and the osseous system through central- and peripheral-mediated pathways[4,5].NPY can maintain the homeostases of bone, blood vessels, and immune systemviadifferent NPY receptors[6,7].Previous studies have indicated that NPY can stimulate proliferation,promote osteoblastic differentiation, and prevent apoptosis of bone marrow mesenchymal stem cells (BMSCs) though Y1R[8].In addition, NPY is also able to facilitate neuroprotection, restore bone marrow dysfunction, and thus mediate bone marrow microenvironment[9].Aside from BMSCs, treatment with NPY caused a decreased number of osteoclasts by promoting mobilization of hematopoietic stem cells (HSCs)viaY2R and Y5R.Furthermore, NPY can accelerate endothelial cell (EC)proliferation and capillary tube formation though Y1R, Y2R, and Y5R[9,10].As NPY possesses capacities of regulating different types of cells, it determines the relative rates of bone formation and resorption process, which is critical for prevention against bone structure damage and bone metabolic disorders[11].
In recent years, stem cell therapy is a burgeoning field in regeneration and restoration of the impaired musculoskeletal tissues[12].Enrichment and differentiation of stem cells have been confirmed for the benefits in bone regeneration and maintenance of bone homeostasis.As both experiments and clinical investigations have revealed definite efficacy of stem cells in treatment of fractures, osteoporosis,cartilage, and ligament injuries[13-15], it has become an attractive avenue of research for therapeutic applications in musculoskeletal disorders.
This article reviews the current knowledge of NPY, potential roles of NPY receptors,and interactions among NPY, NPY receptors, and stem cells, primarily BMSCs and HSCs.In addition, the potential influences of NPY on biological functions of ECs and chondrocytes, which are also involved in the development of many orthopaedic disorders, are also discussed.Furthermore, the potential applications and efficacy of NPY and its receptor-targeted drugs in the treatment of fracture, osteoporosis, and osteoarthritis are also summarized.
NPY AND RECEPTORS
Recent studies have shown that NPY participates in many physiological and psychological processes, such as inhibition of vascular smooth muscle contraction and alleviation of anxiety and depression[16,17].Till now, five primary NPY receptors (Y1R,Y2R, Y4R, Y5R, and y6R) have been found in mammals, and all of them belong to the superfamily of G protein-coupled receptors[18].Different receptors are distributed differently in the human body, play different biological functions, and have different affinities for NPY (Y2R > Y1R > Y5R, Y4R = y6R)[19].Y1R and Y2R are reported to be involved in bone homeostasis.Y4R is distributed in both brain and peripheral tissues[20].Y5R, predominant in the CNS, consistently co-localizes with Y1R[21].y6R,also observed in rat genome, seems to be non-functional in humans[22].
Current evidence indicates that hypothalamic secreted NPY inhibits bone formation.Previousin vivostudies found that an increase of NPY in the hypothalamus led to a decreased volume of cancellous bone and inhibition of the osteoblasts activity[23,24].These results are similar to the finding of another study that overexpression of hypothalamus-specific NPY in the wild-type (WT) mice displayed anti-osteogenic effects[25].Likewise, both deficiency of NPY [NPY (-/-)] in mice and inhibition of central NPY signaling pathway can induce bone gain[5,26].As targeted deletion of theNPYgene in the hypothalamus has not been realized yet, current research of the potential effects of hypothalamus-specific NPY on peripheral stem cells may be conductedviathe approach of isolating BMSCs or other stem cells from mice deficient in hypothalamus-specific NPY.
It is known that both Y1R and Y2R are distributed abundantly in the CNS.As an auto-receptor, Y2R is primarily located presynaptically, and inhibits NPY expression and release[27,28].Both hypothalamus and germline deletions of Y2R produce an identically promotive bone anabolic phenotype, implying that hypothalamic Y2R may modulate bone formationviaCNS mechanism, and also protect against central NPYinduced bone loss[26,29].A previous study showed that the osteoblastic activity of the mice with double knockout ofY4RandY2Rin the hypothalamus was more obvious than those with only knockout ofY2R, demonstrating the probably synergistic role of Y4R in hypothalamic control of the bone mass[30].Nonetheless, targeted deletion ofY1Rin the hypothalamus failed to recapitulate this increased bone mass phenotype, which had been observed in systematic deficiency of Y1R [Y1R (-/-)] mice.This implies that Y1R may participate in the regulation of bone regulation by non-hypothalamic pathways[31].Interestingly, double deletions of globalY1RandY2Rin mice did not result in any additive effects on bone phenotype, suggesting that Y1R and Y2R may share a common pathway in regulation of bone homeostasis[31].As for y6R, its mRNA is only expressed in the hypothalamus but not in bone components, such as BMSCs,osteoblasts, or osteoclasts.Compared to the WT controls, significantly decreased bone density and volumes of cortical and cancellous bone were found in the mice with y6R(-/-) in the hypothalamus, which is primarily attributed to the increasing number of osteoclast precursors in y6R(-/-) mice[22].
Peripheral NPY acts directly in a paracrine fashion to maintain the bone homeostasis.Growing evidence shows that NPY is expressed in non-neuronal cells in the bone marrow microenvironment, such as osteoblasts[32], osteocytes[33], BMSCs[8,34],and ECs[35].In vitrostudies have indicated that NPY has direct effects on osteoblast lineage by inhibiting differentiation of mesenchymal progenitors and mineralization of mature osteoblasts[33,36].Overexpression of osteoblasts-specific NPY (Col2.3NPY)resulted in decreases in bone trabecular number, thickness, and volume[37], whereas changes of serological NPY levels did not reveal such effects[37].These results imply that overexpression of local NPY may cause changes of bone phenotypes.Outcomes of clinical studies have revealed that patients with craniocerebral injury had accelerated fracture healing and higher serological NPY levels, the latter of which showed a positive correlation with the severity of craniocerebral injury[38].Guet al[38]inferred that serum NPY may originate from cerebrospinal fluid, as the NPY level in the serum was almost equal to that in the cerebrospinal fluid among the patients with craniocerebral injury, and severe craniocerebral injury may cause leakage of cerebrospinal fluid[38,39].
It is definite that Y1R is expressed in osseous tissue, including adipocytes,osteoblasts, and bone marrow cells[8,33,40-42]; however, whether Y2R is expressed in the bone or not remains in debate.Some studies reported that Y2R can be found in MC3T3 and BMSCs[41,42], but not in osteoblasts[43].It is speculated that such differences may be explained by two reasons.The first reason is that Y2R exists in the bone, but may be expressed under specific conditions, and thus, it cannot always be detected.Second,potential limitations of the current techniques may also affect the detection rate.As for Y1R, bothin vitroandin vivostudies have shown that the mice with knockout of osteoblast-specificY1Rrecapitulate the bone phenotype of germline Y1R deficient mice[5,36].These findings confirm the role of Y1R in inhibiting bone formation, as well as in enlarging the negative effects of NPY on osteocytes.Although the selective deletion of Y1R from osteoblasts did not hinder the process of fracture healing, lack of systemic Y1R caused delays of endochondral fracture repair[44].Considering that Y1R plays important roles in many physiological functions, aside from the abovementioned issues, other underlying mechanisms may also account for the effects of globalY1Rdeletion on bone healing process (Figure 1).
MECHANISMS OF ACTIONS OF NPY ON BMSCs AND HSCs
NPY and BMSCs
BMSCs, isolated from bone marrow, are termed as a “fibroblast-like” osteogenic cell population.With plastic-adherent culture characteristics, BMSCs express specific surface antigens CD105 and CD90 (> 95%) but not CD45 or CD34 (< 2%)[45], and are capable of differentiating into osteoblasts, chondrocytes, and adipocytes[19,46].As BMSCs play a vital role in repairing musculoskeletal tissues, fracture healing and spinal injury regeneration can be accelerated following application of BMSCs[47].Local injection of BMSCs has also yielded promising results in the treatment of bone nonunion and bone defect[48].
Currently, conflicting results still exist regarding the potential role of NPY in proliferation of BMSCs[8,36,42].Our previous study revealed that NPY can enhance proliferation and prevent apoptosis of BMSCs in a concentration-dependent manner by activating the Wnt/β-catenin signaling pathway.Such NPY-induced activities were partially blocked by PD160170, a Y1R antagonist[8], implying that NPY-induced proliferative and anti-apoptotic effects of BMSCs may be partially achieved through Y1R.Another study also found that NPY is able to stimulate proliferation of BMSCs derived from rats of different ages, and this capacity was blocked by Y5R antagonist,demonstrating an inhibitory role of Y5R in the proliferation of rat BMSCs.In addition,Iguraet al[42]found that NPY increased the proliferation of BMSCs of transgenic overexpression of Y5R in the elder rats by activating extracellular signal-regulated kinase 1/2 (Erk1/2) pathways.Aside from BMSCs, NPY can also promote the proliferation of human embryonic stem cells, which was achievedviaNPY/Y1R/Y5R by activation of ERK1/2 pathways[49].However, one study failed to find a promotive role of NPY on BMSC proliferation.Leeet al[36]reported that BMSCs isolated from Y1R(-/-) mice formed a greater number and larger size of colonies than the WT controls,implying that NPY may inhibit BMSC proliferation.
Apart from its potential influences on BMSC proliferation, NPY can also facilitate the migration of BMSCs by upregulated expression of CXC chemokin receptor 4[50],which is in accordance with the finding of our study that NPY therapy significantly increased the total migration distance and speed of BMSCs[51].
In addition to the controversy regarding the effect of NPY on the proliferation of BMSCs, the potential activities of NPY on osteogenic differentiation of BMSCs and the underlying mechanisms are also in debate.Some studies found that NPY stimulated the differentiation of BMSCs into osteoblasts, which was supported by upregulating the expression of alkaline phosphatase (ALP), collagen type I (COL-I), osteocalcin(OCN), and runt-related transcription factor 2 (Runx2) through the Wnt signaling pathway[51,52].Other studies reported that NPY inhibited osteogenic differentiation of BMSCs, evidenced by the findings of decreased ALP and OCN expression, and reduced mineralization of BMSCs as well[41].Besides, another study also found the inhibitory effect of NPY on isoprenaline-induced differentiation into osteoblasts from BMSCs[40].Interestingly, BMSCs isolated from NPY (-/-) mice display an increased ability in osteogenic differentiation, which was confirmed by increases in ALP activity,OCNgene expression, and mineralization[52].These diverse outcomes can be explained by the fact that NPY-induced osteogenic differentiation of BMSCs may be achievedviaauto-regulation mechanisms.
Growing evidence has revealed that auto-regulation mechanisms of NPY may correlate to the plasticity of its receptors.It has been noted that NPY led to upregulated expression of Y1R throughout BMSC osteogenic differentiation[36,38,41].In a recent study, Weeet al[52]found that during osteogenic differentiation of BMSCs in the WT mice, the expression of Y1R was increased while NPY was decreased.However, as in NPY (-/-) mice, Y1R expression level did not alter during differentiation,demonstrating that increased Y1R expression during BMSC differentiation may be assisted or induced by NPY.Outcomes of NPY or its receptor gene knockout animal models revealed that the presence of functional Y1R directly hindered the osteogenesis of BMSCs or bone cells, as evidenced by the finding that BMSCs isolated from Y1R (-/-) mice displayed an increased capacity of osteogenic differentiation[36].Anin vitroexperiment showed that blockade of Y1R by PD160170 facilitated osteogenic differentiation of BMSCs[53].On the contrary, Donget al[34]reported that melatonin can upregulate the expression of NPY and Y1R, and promote MSC osteoblastic differentiation.Apart from BMSCs, lack of Y1R also promoted differentiation of mesenchymal progenitor cells and activated mature osteoblasts[36].Yaharaet al[54]also noticed that inhibition of Y1R increased the ALP activity and mineralization in mouse pre-osteoblast MC3T3-E1 cells.
Aside Y1R, Y2R may also participate in osteogenic differentiation of BMSCs, which is supported by the finding that BMSCs, treated with either NPY1–36 (universal Y2R agonist) or PYY3-36 (Y2R preferring agonist), revealed significantly elevated levels of ALP activity and OCN expression.Such effects of NPY1-36 were blocked by a Y2R antagonist, BII0246, and a marked decrease of Y1R protein level was also found following treatment with exogenous PYY3–36 or NPY1–36[41].
During osteogenic differentiation of BMSCs, potential interactions may exist between Y1R and Y2R[42,43].By detecting the NPY ligand-receptor system in BMSCs derived from rats of different ages, Iguraet al[42]found that, although NPY expression increased with age, Y1R protein was upregulated whereas Y2R protein was downregulated with an increase in age.In addition, Lundberget al[43]found that the lack of Y2R signaling mediated downregulation of Y1R in BMSCs, which was proved by the finding of decreased expression of Y1R in BMSCs from Y2R (-/-) mice.They inferred that downregulation of Y1R is possibly due to the lack of feedback inhibition of NPY release and resulted in elevated levels of NPY, which in turn caused subsequent overstimulation of Y1R, following desensitization and downregulation of the Y1R population[43].Moreover, alternation of Y1R expression may have different effects on NPY.The precursor osteogenic cells (e.g., BMSCs and osteoblast precursor cells), which are able to inhibit osteogenic differentiation through Y1R, have low expression levels of Y1R.However, in osteogenic conditions (e.g., osteogenic induction medium), Y1R expression in these cells is increased, with Y2R expression decreased.
In addition to Y1R and Y2R, y6R may also be involved in the regulation of BMSC differentiation into osteoblasts.BMSCs isolated from y6R (-/-) mice showed significant reductions of ALP, osterix, and mineralizing surface, implying possibly opposite roles of Y6R to those of Y1R and Y2R in BMSC differentiation and activities[22].
Currently, some studies also reported potential mechanisms of NPY in mediation of BMSC osteogenic differentiation from another perspectives.Guet al[38]indicated that NPY can directly promote the osteogenic differentiation of MSCs by upregulating Runx2.Maet al[55]reported that the anabolic activity of osteoblasts treated with NPY may also enhance the gap junction intercellular communication (GJIC).Tanget al[56]noticed that the levels of NPY and p-ERK1/2 in fracture model rats were significantly higher than those in the controls.They also found that use of BIBP3226, a Y1R antagonist, inhibited the fracture healing process by downregulating the p-ERK expression in the fracture site, indicating that the ERK pathway may participate in the NPY-induced effects on fracture healing.Additionally, Sharmaet al[57]also observed that activation of ERK could facilitate osteogenic differentiation of human MSCs.
In summary, considering the still existing controversies regarding the role of NPY in BMSCs and still not-well-understood mechanisms, future more studies are warranted to provide more definitive evidence regarding the links between NPY/NPY receptors and BMSCs.
NPY and HSCs
HSCs, or hematopoietic stem/progenitor cells, responsible for regeneration and repopulation of all blood cell lineages, contact osteoblasts in endosteal microenvironments or sinusoidal endothelium[58].NPY receptors Y1, Y2, Y4, and Y5 were found to be highly expressed in HSCs, and NPY plays a crucial role in the proliferation and mobilization of HSCs[59,60].
Growing evidence has suggested that NPY acts directly or indirectly in the regulation of HSC proliferation.NPY can directly inhibit the proliferation of HSCs in cell cultures, as confirmed by an increased number of HSCs in G0 phase, with decreased numbers of cells in S and G2/M phases[60].Besides, the number of HSCs in NPY (-/-) mice is decreased, suggesting that NPY may protect HSCs in the bone marrow microenvironment[9,61].
In addition to potential influences on HSC proliferation, NPY and its receptors are also involved in regulating the survival and mobilization of HSCs.It was reported that NPY/Y1R can improve the survival and apoptosis of HSCs, which maintains the survival of nestin+ cells, and thus be linked to retention of HSCs[9].NPY-induced biological effects on HSCs, osteoblasts, and osteoclasts play an important role in the process of fracture healing.Nonetheless, detailed mechanisms of such effects remain largely unclear.Several studies explored the underlying mechanisms from different perspectives.Parket al[61]indicated that the effect of NPY on HSC mobilization might be achieved by increasing the expression levels of matrix metalloproteinase 9 (MMP-9)in osteoblastsviaY1R, which was strengthened by the finding that NPY failed to display a positive activity on HSC mobilization in mice with Y1R (-/-) in osteoblasts.Singhet al[59]reported that NPY3-36, a agonist of Y2R and Y5R, facilitated the mobilization of HSCs to the peripheral blood, whereas selective Y2R and Y5R antagonists hindered such activity.It has been noted that NPY can be also secreted by HSCs, indicating that HSCs may exert regulatory feedback on itself by releasing NPY[9].In ovariectomized mice, NPY therapy could help reduce the bone loss due to HSC mobilization, and result in an increased number of osteoblasts and a decreased number of osteoclasts[61].Using an ovariectomy-induced osteoporosis mouse model,Parket al[62]also found that NPY-based recombinant peptides could relieve ovariectomy-induced bone loss and may be used for osteoporosis treatment.
In summary, current evidence suggests that NPY is able to induce the rapid mobilization of HSCs into the peripheral blood, and increase the number of osteoblasts.Therefore, it is reasonable to believe that a comprehensive understanding of the role of NPY in HSCs may pave the way for its future clinical applications[63].
MECHANISMS OF ACTIONS OF NPY ON ECs AND CHONDROCYTES
NPY and ECs
NPY-positive fibers predominantly localize alongside blood vessels in bone tissue and bone marrow, and associate with EC migration, capillary tube formation, and selfrenewal[10,64,65].ECs express Y1R and Y2R and can produce, store, and respond to NPY,suggesting an autocrine regulatory mechanism of NPY in the endothelium(Figure 2)[10,35,66].
In vitrostudies have revealed that NPY can promote the migration and capillary tube formation of human ECsviaY1R, Y2R, and Y5R[10,65].Previous studies also indicated that NPY-induced angiogenic effect on ECs was achieved primarily through Y2R[65,67].Furthermore, NPY can regulate the angiogenic process by influencing the proliferation of ECs, which was achieved mainlyviaY5R[10].NPY3-36, an agonist of Y2R and Y5R, was found to be able to reduce EC contact and increase vascular permeability, and selective Y2R and Y5R antagonists restored the vascular integrity[59].Interactions may exist among BMSCs, HSCs, and ECsviaNPY.It has been observed that NPY originating from platelet lysate caused an decreased angiogenic activity of human adipose stromal cells, which may be linked to reduced expression of vascular endothelial growth factor (VEGF) and a lower intracellular calcium level[68].As a ubiquitous and potent peptide, VEGF is involved in many angiogenic cascades.Increased levels of VEGF facilitate angiogenesis and osteoblastic differentiation of BMSCs[69], and the level of VEGF secreted by BMSCs is elevated following treatment with NPY[51].Wanget al[50]found that NPY promoted endothelial differentiation and tube formation of BMSCs, the latter of which is involved in all the stages of EC angiogenesis, including survival and proliferation, migration, tube formation, and maturation of blood vessels[70].
NPY and chondrocytes
Although chondroblasts and osteoblasts share the same progenitor reservoir (BMSCs),specific effects of NPY on chondrocytes have not been clarified.However, anatomical studies have indicated that NPY-positive sympathetic nerve fibers are located in cartilage, and chondrocytes can also secret NPY[32].
NPY can promote the proliferation of both chondrocytes and articular cartilage, and the effect of NPY on chondrogenesis may be achieved through autocrine mechanismsviaY1R[32].A new study found that intra-articular injection of NPY caused more severe osteoarthritis phenotypes, which was evidenced by more severe cartilage degradation and fibrillation, whereas a combination of NPY with BIIE0246 (Y2R antagonist) but not BIBO3304 (Y1R antagonist) significantly alleviated the above negative effects,indicating that Y2R may play an important role in NPY-induced chondrocyte hypertrophy and cartilage matrix degradation[71].In addition, NPY-induced chondrocyte hypertrophy was also marked by increased levels of col10a1 (a biomarker indicating hypertrophy of chondrocytes), MMP-13, and ADAMTS-5 through activation of mTORC1 in a Y2R-dependent manner[71].Chenet al[72]found that NPY markedly augmented the expression of Col2a1 (a biomarker indicating proliferation of chondrocytes), Col10a1, and OCN in the murine chondrogenic cell line ATDC5.And inhibition of Y1R partly hindered the capability of NPY, demonstrating that Y1R may participate in NPY-induced activities (proliferation, chondrogenesis, and mineralization) in ATDC5 cells[72].It is known that Runx2 and Osterix are biomarkers for chondrocyte differentiation and cartilage mineralization.In the Y1R (-/-) mice, the expression levels of Runx2 and Osterix increased in the long bones[36], both of which facilitated the formation of cartilage callus.Based on current findings, potential strategies to solve NPY-related hypertrophy of chondrocytes and cartilage degradation may be a research hotspot in the future.
APPLICATIONS OF NPY IN ORTHOPAEDIC DISORDERS
Fracture
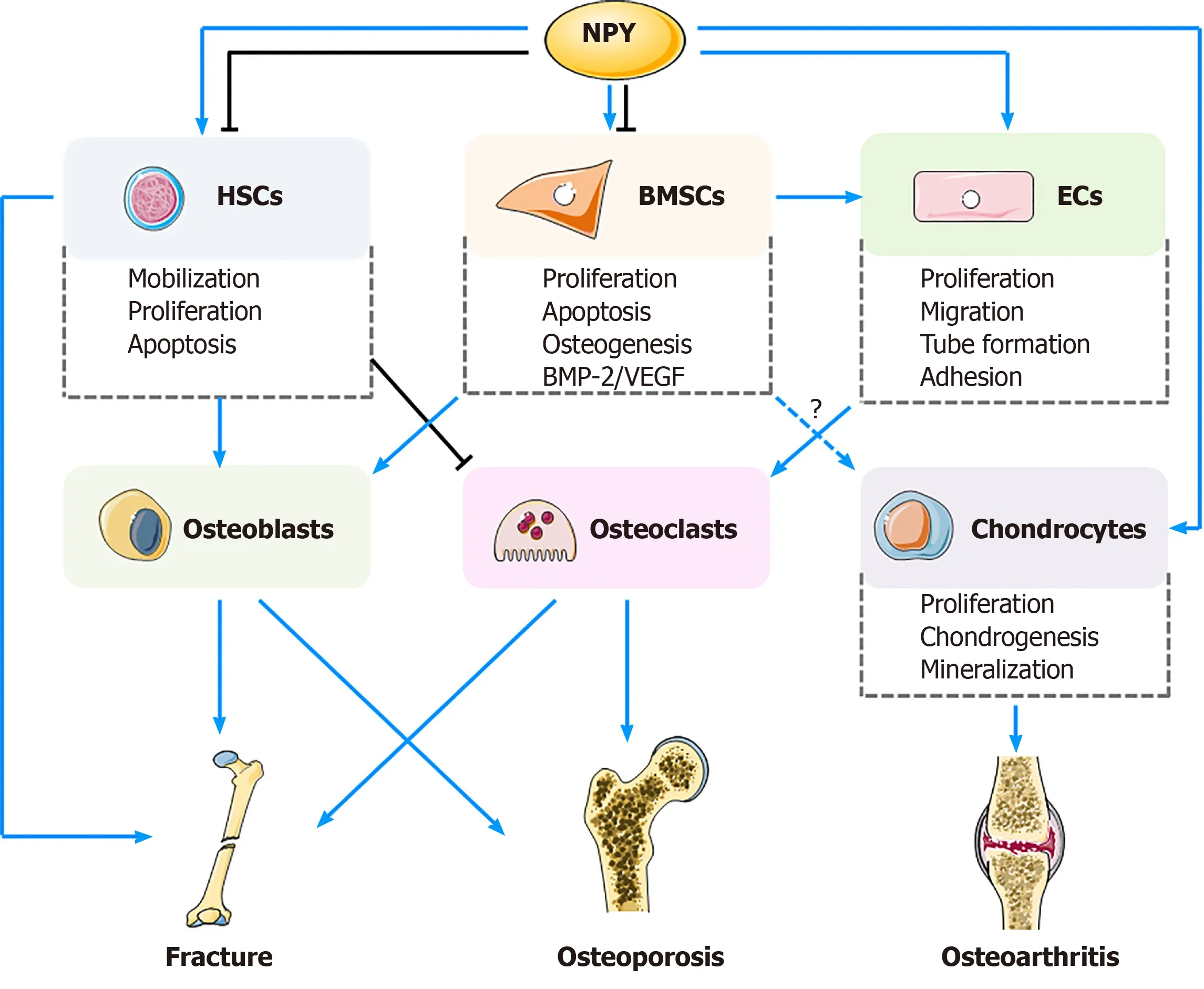
Figure 2 Neuropeptide Y exerts potential regulatory effects on biological functions of bone marrow mesenchymal stem cells,hematopoietic stem cells, endothelial cells, and chondrocytes, and their potential applications in management of fracture healing,osteoporosis, and osteoarthritis.
Fracture healing process can be divided into inflammatory, reparative, and remodeling stages.During the initial phase, BMSCs are recruited to the fracture site and differentiate into chondrocytes simultaneously, and the level of NYP in the peripheral blood is increased[56].NPY- and Y1R-positive BMSCs and osteoblasts can be found in the new osseous tissue[64].Innervation of NPY occurs in a spatio-temporal dependent manner in the inflammatory and remodeling stages[73], demonstrating different functions of NPY among different fracture stages.Previous studies found that the volumes of cortical and cancellous bones in NPY-deficient mice were significantly increased, which may result from elevated osteoblast activities and upregulated expression of Runx2 and Osterix[23,24].Lundberget al[43]reported that,compared with the WT controls, Y2R (-/-) mice presented with greater bone mass,which may correlate with the reinforced abilities of BMSCs, thus being able to produce more mineralized extracellular matrix as well as increased expression of ALP and OCN.As mentioned previously, NPY can directly increase the number and viability of BMSCs by promoting their proliferation and inhibiting apoptosis[8], together with increased osteogenic differentiation and BMP-2 expression[51].Aside fromin vitroandin vivoexperiments, clinical observations also confirmed the role of NPY in fracture healing process.Guet al[38]reported that, compared with those with a single fracture,patients with a fracture and accompanying traumatic brain injury had higher serological levels of NPY and accelerated fracture healing process.This finding can be explained by the fact that NPY can directly promote osteogenic differentiation of MSCs[38].Besides, Y1R antagonist play a direct role in regulation of BMSCs.Liuet al[53]noted that treatment with PD160170, a Y1R antagonist, promoted osteogenic differentiation of BMSCs, with higher expression of COL-I, OCN, and Runx2.Thein vivooutcomes also showed increases in bone volume/total volume (BV/TV), bone mineral density, and bone trabeculae number after PD160170 intervention[53].However, considering the still limited evidence from clinical investigations, more studies are necessary.In short, NPY can induce proliferation and angiogenic and osteogenic differentiation of BMSCs, facilitating bone regeneration.
VEGF is one of the most important components in angiogenesis, which is regarded as an essential factor that directly influences the fracture healing process[74].BMSCs can promote vessel sprouting and vascularization, which is considered to be important for bone formation, whilst ECs can accelerate bone repair by facilitating the recruitment of osteoclast precursors and stimulating the osteoclastogenic process[75].NPY can also facilitate vessel sprouting, adhesion, migration, proliferation, and capillary tube formation in ECs[10,59,65].Also, during the process of angiogenesis, NPY receptors are indispensable.For example, Leeet al[67]observed that NPY-induced aortic sprouting andin vivomatrigel capillary formation were decreased by 50% after deletingY2Rgene or using its antagonists, implying the vital role of Y2R in angiogenesis.During the angiogenesis process, continuous nutrients and cytokines are transported to the fracture site, providing synergistic effects in fracture healing.
Osteoporosis
Osteoporosis, a disorder characterized by progressive bone loss and thus an increased risk of fracture, often results from menopausal loss of estrogen in women[76].Currently,the primary methods to investigate the effects of NPY system on osteoporosis include examining bone phenotypes in gene knockout models and using NPY receptor antagonists.
Increasing number of studies indicated that Y2R is essential in medicating bone gain and loss.For example, mice lacking Y2R globally or specifically in the hypothalamus are characterized by greater bone loss compared to the WT controls, indicating that Y2R is critical in protection against bone loss[77,78].In addition, ovariectomized mice injected with JNJ-31020028, a Y2R antagonist, showed increases in the whole-body bone mineral density, vertebral trabecular bone volume, and trabecular thickness[79](Table 1).NPY3-36, an agonist of Y2R and Y5R, strongly lowered the expression level of receptor activator of Nfkb ligand (RANKL) and upregulated the basal levels of osteoprotegerin (OPG) in BMSCs, suggesting that Y2R may induce bone formation by affecting the expression of RANKL and OPG in BMSCs[40,41].These results also hint that selective pharmacological manipulation of Y2R may be a potential strategy for anabolic treatment of osteoporosis.
Aside from Y2R, Y1R may be also involved in regulation of bone homeostasis.Sousaet al[80]found that in mice, oral administration of BIBO3304, a Y1R antagonist, led to increased rates of mineral deposition in both cortical and cancellous bones, which may result from an increased number of osteoblasts following direct actions of NPY/Y1R on osteoclasts[80](Table 1).Although there is still a lack of enough evidence to support the belief that NPY can improve the status of osteoporosisviadirect regulations of BMSCs, based on the potential role of Y1R in osteogenic differentiation of BMSCs, it can be speculated that inhibition of Y1R may be a potential anabolic strategy for prevention of bone loss.
Nonetheless, other studies displayed different outcomes.Tanget al[56]reported that,compared with the controls, the rats treated with the Y1R antagonist BIBP3226 showed remarkable reductions in volumes of callus bone and tissue, and BV/TV as well.This demonstrates a potential negative effect of Y1R antagonist on bone healing (Table 1).Similarly, Sousaet al[44]also observed a lower rate of bone healing in Y1R (-/-) mice,with a decreased volume of bone callus and a weaker bone strength.Osteoclasts of hematopoietic origin also express high levels of NPY and Y1R[33].Since osteoclasts are differentiated from HSCs, NPY can effectively alleviate ovariectomy-induced bone loss by decreasing the number of osteoclasts in bone marrow microenvironment through HSC mobilization, and increasing the number of osteoblasts as well[61].These findings imply that NPY may act as a potential indicator of HSC mobilization, and supplementation of NPY may provide a therapeutic effect for bone loss.
Osteoarthritis
Stem cells can provide preventive and regenerative effects in early stages of osteoarthritis[81].Neurotransmitters, such as substance P (SP) and calcitonin generelated peptide (CGRP), modulate osteo-chondrogenic differentiation of mesenchymal progenitor cells during endochondral ossification in limb development[82].Guoet al[83]found that fracture- related pain in a rat model was partially relieved by an NK1(substance P receptor) antagonist LY303870.In a rat knee model evaluating SP coupled with self-assembled peptide hydrogels, Kimet al[84]observed that cartilage regeneration and regenerative properties were markedly improved by recruiting MSCs.However, to date, few studies have addressed the role of NPY and its receptors in chondrocytes and their potential applications in osteoarthritis therapy.
One of the most frequent symptoms of osteoarthritis is pain of the affected joint,which associates with irritation of sensory nerve endings following pathological changes in the subchondral bone and synovial lining, with neuropeptide-containing nerves in the joint tissue as an important regulatory element[85,86].NPY is able to bind to free nerve endings in accessory ligaments, synovium, subchondral bone, menisci,articular cartilage, and periosteum[87], and changes afferent sensitivity[88].The visual analog scale (VAS) score, used to rate pain, was negatively correlated with the mean optical density values for NPY by immunohistochemical analysis in all patients with osteoarthritis, implying that NPY may be involved in the generation of pain[89].Similarly, the mean level of CGRP in the synovial tissue of patients with severe osteoarthritis-related pain (VAS > 6) was significantly higher than that of patients with moderate to lower pain (VAS < 6)[90].In another study, higher levels of CGRP were detected in the infrapatellar fat pad in patients with knee osteoarthritis, which isimplicated as a possible cause of osteoarthritis development and related pain[91].
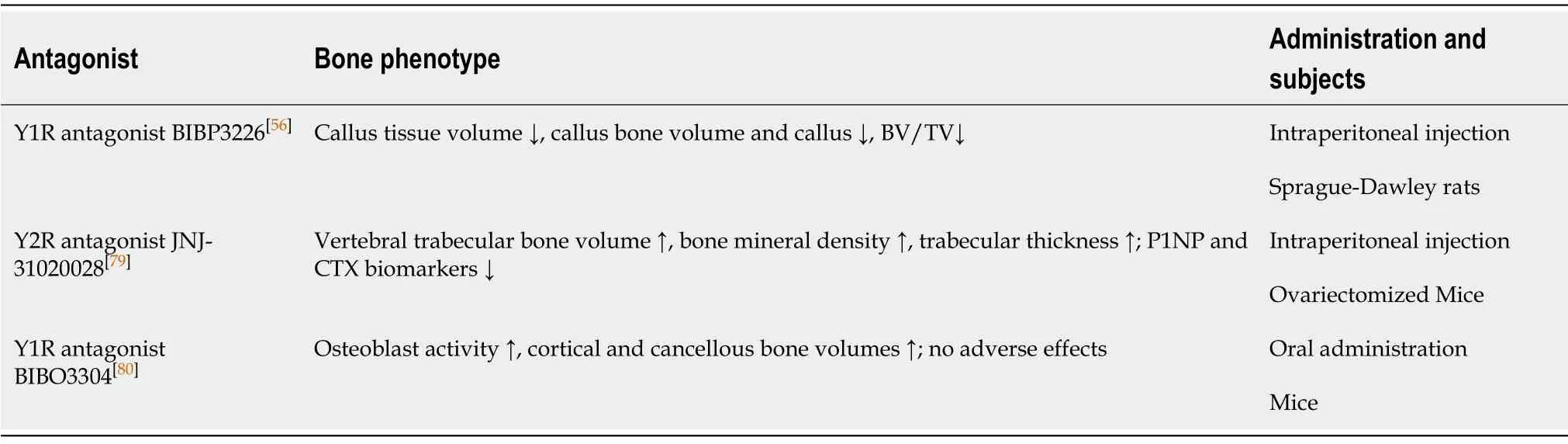
Table 1 Effects of neuropeptide Y receptor-targeted drugs on bone metabolism
Traditionally, the rationale application of MSCs for management of osteoarthritis stems from their ability to differentiate into chondrogenic lineage rather than osteoblasts, which can help improve degeneration of the cartilage, and alleviate joint damage and related pain[92].A decrease in the subchondral bone lesions may help reduce joint pain, which may be a future treatment target in relief of osteoarthritisrelated pain[93].Besides, NPY can increase the VEGF expression and promote osteoblastic differentiation and angiogenesis of BMSCs[38,51,55].As cartilage does not contain blood vessels, it may be considered a hostile environment for spreading of vascular channels[82].VEGF, a crucial regulator of angiogenesis, can induce tube formation activity into cartilage channels, likely resulting in calcification and stiffness of subchondral bone, accompanied by increased innervation and subsequent increases in nociceptors[94].Therefore, blocking NPY could be of interest in pain treatment and osteoarthritis prevention.
Inflammation plays a crucial role in the pathogenesis of osteoarthritis.Prostaglandins are the key mediators of inflammation and pain in osteoarthritis[87].NPY is considered to have pro-inflammatory effects.In a clinical study, Hernanzet al[95]found that the level of NPY in the synovial fluid of the knee joint in patients with rheumatoid arthritis was higher than the healthy controls, and NPY stimulated the expression of interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α).In a murine chondrogenic cell line, NPY was found to be able to promote proliferation and increase viable cell count of ATDC5 cells.Besides, the chondrogenic differentiation of ATDC5 can be enhanced by NPYviaupregulating Runx2 and Col10a1.In the tissue engineering field, stem cell-scaffold-NPY combinations may be used to repair cartilage and tissues.
NPY receptor-targeted drugs
As mentioned previously, Y1R and Y2R antagonists have been applied to minimize the bone loss caused by different musculoskeletal disorders.Y1R and Y2R have been identified as novel therapeutic targets for the treatment of obesity and cancer[96,97].Recently, a group of Y1R antagonists have been commercially available.Among these,BIBO3304, a Y1R antagonist, is the most potent[98].However, the effect and efficacy of BIBO3304 have been only evaluated in animals, and clinical trials are required to confirm its role[56,79,80](Table 1).
CONCLUSION
This review introduces NPY and NPY receptors, and the potential mechanisms of actions of NPY on BMSCs, HSCs, ECs, and chondrocytes.NPY exerts different biological effects in different types of cells, including proliferation, apoptosis,differentiation, migration, mobilization, and cytokine secretion.NPY and its receptors play important roles in promoting bone union and anti-osteoporosis by regulating relative cell functions.Currently, limited studies have investigated the role of NPY in chondrocytes and its efficacy in controlling pain and inflammation in osteoarthritis.Improved clinical investigations addressing the role of NPY and its receptors in orthopaedic disorders may provide new insights into the stem cell research and therapy.
ACKNOWLEDGEMENTS
The authors are grateful to Dr.Song Y, from Nanfang Hospital, Southern Medical University for her help in figure preparation.
杂志排行
World Journal of Stem Cells的其它文章
- Human mesenchymal stem cells derived from umbilical cord and bone marrow exert immunomodulatory effects in different mechanisms
- Mass acquisition of human periodontal ligament stem cells
- Perspectives on mesenchymal stem/progenitor cells and their derivates as potential therapies for lung damage caused by COVID-19
- Stem cell treatments for oropharyngeal dysphagia:Rationale,benefits, and challenges
- Senescent mesenchymal stem/stromal cells and restoring their cellular functions
- Mechanotransduction of stem cells for tendon repair
