Active corrosion protection of super-hydrophobic corrosion inhibitor intercalated Mg–Al layered double hydroxide coating on AZ31 magnesium alloy
2020-04-29XinWngChunJingYuxingChnXiushungWngGngZhoXingZhngLingWuXioyingLiuBiqinDongYuxinZhng
Xin Wng, Chun Jing, Yuxing Chn, Xiushung Wng, Gng Zho, Xing Zhng,Ling Wu, Xioying Liu, Biqin Dong, Yuxin Zhng,∗
aState Key Laboratory of Mechanical Transmissions, College of Materials Science and Engineering, Chongqing University, Chongqing 400044, P.R. China
bAcademy of Dazu Rock Carvings, Chongqing 402360, P.R China
c Research Institute of Petroleum Engineering Technology, Shengli Oilfield Company, Sinopec, Dongying 257000, P.R. China
dEngineering Research Center for Waste Oil Recovery Technology and Equipment of Ministry of Education, Chongqing Key Laboratory of Catalysis and New Environmental Materials, College of Environment and Resources, Chongqing Technology and Business University, Chongqing 400067, P.R. China
e Guangdong Provincial Key Laboratory of Durability for Marine Civil Engineering, Shenzhen University, Shenzhen 518060, P.R. China
Abstract Magnesium alloys, the advanced lightweight structural materials, have been successfully applied in the manufacturing field. Unfortunately,their poor corrosion resistance restrains the potential wide applications. In this work, anti-corrosion coatings were fabricated via the insitu growth of the corrosion inhibitors intercalated magnesium-aluminum layered double hydroxide (Mg–Al LDH) on AZ31 magnesium alloy and then post-sealing it by a super-hydrophobic coating. SEM, XRD, EDS, FTIR, XPS and contact angle test were conducted to analyze physical/chemical features of these coatings. Potentiodynamic polarization curves and electrochemical impedance spectroscopy were recorded to assess the anti-corrosion performance of prepared coatings. Surprisingly, Mg–Al LDH with molybdate intercalation and lauric acid modification achieves the excellent corrosion inhibition performance (99.99%) due to the multicomponent synergistic effect such as the physical protection of Mg–Al LDH, the corrosion inhibition of molybdate and super-hydrophobic properties of lauric acid. This work presents a scientific perspective and novel design philosophy to fabricate the efficient anti-corrosion coating to protect magnesium alloys and then expand their potential applications in other field.
Keywords: Magnesium alloy; Superhydrophobicity; LDH films; Corrosion inhibitor; Corrosion resistance.
1. Introduction
Magnesium alloys are advanced lightweight structural materials, which have been applied in aerospace, automobile and electronic industries [1–3]. Unfortunately, they are easily corroded for low potentials, restraining their widespread applications. Consequently, experts are dedicated to improving the anti-corrosion performance of magnesium alloys. To date,various methods of protecting magnesium alloys have been proposed and developed. One is metallurgical methods including alloying [4], adding rare earth elements [5] and homogenizing microstructure[6],based on the alloy itself.However, this method is not effective in inhibiting galvanic corrosion. Another method is to fabricate protective coatings on the surface of the alloy, impeding the contact of the corrosion mediums and magnesium alloys. The application of organic coatings on the metal surface is a simple, popular and conventional method of inhibiting corrosion [7]. However, traditional organic coatings play an only role of physical barriers and even cause severe localized corrosion when the coatings become not intact [8]. Therefore, self-healing coatings with strong adhesion to substrate are required.
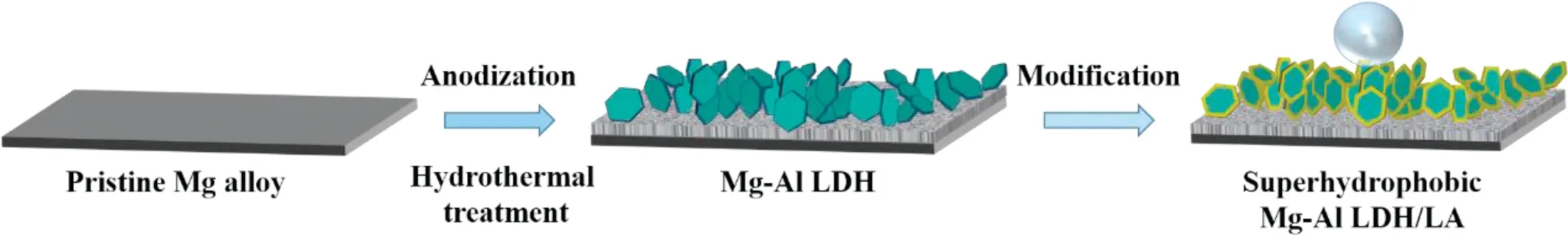
Schematic 1. Schematic illustration of the fabrication process of the hydrophobic Mg–Al LDH coating on AZ31.
Self-healing ability can be obtained by introducing corrosion inhibitor. Lately, nano-containers with high loading capacity and ion-exchange capacity have attracted extensive attention as corrosion inhibitor carriers [9,10]. Layered double hydroxides are a kind of two-dimensional layered structural material, composed positively charged laminates and interlayer anions [11]. In recent years, LDHs have become a research hotspot in metal corrosion protection for its unique inherent ion-exchange property [12,13]. Anion corrosion inhibitors can be accommodated in LDHs galleries and cannot be released unless triggered by corrosion phenomena. At the same time of releasing, aggressive anionic species could be captured by LDHs. In other words, LDHs loaded with corrosion inhibitor can conditionally release anionic corrosion inhibitors and entrap aggressive anions [14].
In-situ prepared LDH coatings can effectively protect metal substrate. Uniform and dense Zn–Al LDH coatings with nitrate intercalation were prepared via electrochemical deposition and doubled the corrosion resistance [15]. In-situ grown Mg–Al LDHs coating intercalated carbonate inhibits anodic dissolution, reducing corrosion current density from 59.4 μA cm−2to 4.53 μA cm−2[16]. Other researches show that LDH coating can enhance the corrosion resistance of metals [17,18]. Nevertheless, the methodologies of preparing LDH directly on metal are relatively complex. Anodizing and micro-arc oxidation are another type of surface treatment method for protecting metal, which suffers from the micropores and cracks,leading to limited protection.Recently,LDH coatings can be easily prepared using the oxides on the metal surfaces and the combination of LDH coatings and oxide coatings provide stronger protection [19–26]. Moreover, various inhibitor ions have been intercalated into LDH coatings to further improve corrosion resistance, such as molybdates and vanadates [27–29].
Super-hydrophobic surfaces, existing widely in nature,have water-repellent and self-cleaning properties. Inspired by the ‘lotus effect’, artificial hydrophobic coatings were prepared on metal surfaces to keep the solution containing the corrosion media away, improving the protective effect on metal substrates [30–33]. The surface microstructure and chemical composition of the substance determine its wettability. Generally, an artificial super-hydrophobic surface is prepared by firstly forming a microscopic rough surface and subsequently modifying it with low-surface-energy substances[34]. However, it is hard to obtain micro/nanostructures. LDH coating has rough surface possessing the nest-like hierarchical structure and owns abundant hydroxyl groups which can react with carboxyl, making it easier to fabricate a hydrophobic coating on. Super-hydrophobic Zn–Al LDH coatings were successfully prepared by the modification with stearic acid,resulting in better corrosion resistance than single Zn–Al LDH[35,36]. Other substances have also been used to prepare super-hydrophobic LDH coatings, for instance, aliphatic carboxylate [37–39].
It can be summarized that super-hydrophobic LDH coatings can greatly enhance the corrosion resistance of metal substrates. LDHs can be intercalated with various inhibitors,serving as nano-reservoirs. Besides, LDH coatings can be modified from hydrophilic to hydrophobic. There is some progress to prepare high performance in-suit grown superhydrophobic LDH coatings. However, few attempts have been made to study the synergistic effect of interlayer inhibitor ions and surface modification materials.
In this work, different kinds of super-hydrophobic Mg–Al LDH coatings were in situ fabricated on the surface of magnesium alloy (Scheme 1). The effect of the types of lowsurface-energy substances on the microstructure and the wettability was investigated. The chemical compositions of obtained coatings were analyzed by X-ray diffractometer(XRD),Fourier transform infrared (FTIR) and X-ray photoelectron spectroscopy (XPS). Moreover, the impact of different kinds of interlayer inhibitor and low-surface-energy substances on the anticorrosion behavior was systematically studied by electrochemical measurement. The results show that interlayer ions and low surface energy materials should match with each other for better corrosion inhibition.
2. Experimental methods
2.1. Materials
AZ31 magnesium alloy were used as substrate in this study, mainly containing Mg and having trace elements: Al,Zn, Mn, Ca, Si, Cu. All chemicals used in this experiment were purchased from Aladdin and were used as raw materials.Deionized water acted as solvent in this experiment.
2.2. Pretreatment of AZ31 alloys
The specimens were polished with SiC papers up to 2000 for removing oxide. Subsequently, the polished substrates were ultrasonically cleaned in absolute ethyl alcohol and dried at room temperature.
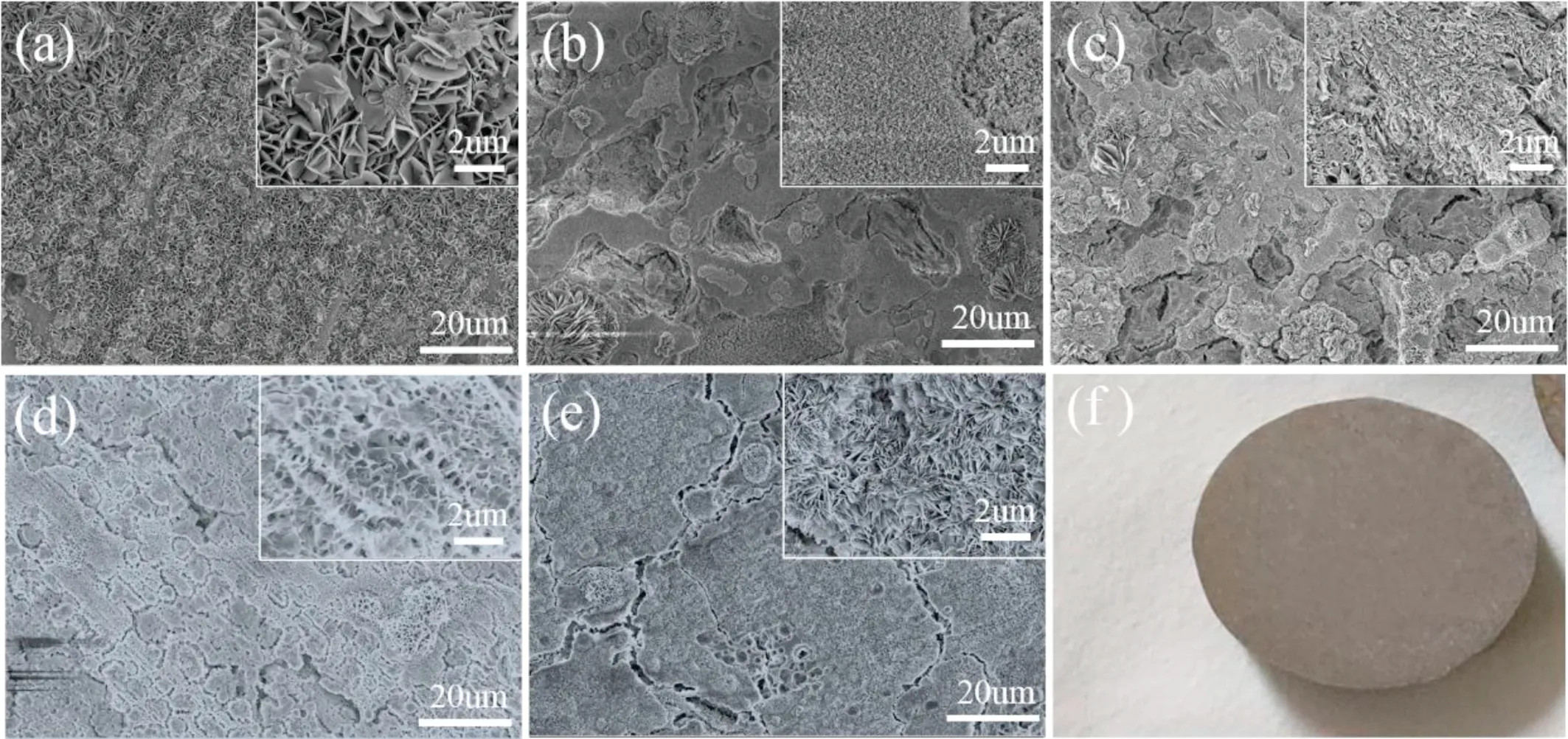
Fig. 1. SEM micrographs of Mg-Al-LDH coating modified by (a) SS, (b) LA, (c) MA and lauric acid modified Mg–Al LDH coating intercalated with(d) vanadate and (e) molybdate; (f) digital graph.
2.3. Synthesis of LDH coatings
The LDH coating was prepared by firstly anodizing under a direct voltage of 20V for 30min in the electrolyte containing 0.18M NaOH and 0.05M NaAlO2and then hydrothermally treating at 398K for 12h in Teflon-lined stainless steel autoclaves containing 0.1M NaNO3solution (pH = 7). Replace 0.1M NaNO3 solution by 0.1M Na3VO4and 0.1M Na2MoO4solution to prepareandintercalated Mg–Al LDH.For clarity of discussion,these samples were labelled in the following manner ‘Mg–Al–A LDH’, where ‘A’ represents anion used in hydrothermal reaction.
2.4. Chemical modification
To prepare super-hydrophobic coatings, sodium stearate(SS), lauric acid (LA) and myristic acid (MA) were used to bring the surface energy down. A sample prepared in the previous step was vertically placed in the stainless Teflonlined autoclave containing 15mL 0.01M low surface energy materials ethanol solution and 15mL deionized water and hydrothermally treated at 413K for 12h. These specimens were labelled as Mg–Al–A LDH/SS, Mg–Al–A LDH/LA or Mg–Al–A LDH/MA.
2.5. Characterization
Micromorphology of the treated sample surface was observed by scanning electron microscope (FIB/SEM, ZEISS AURIGA). The crystallographic structure of the coatings was measured by X-ray diffraction (XRD; Rigaku D/Max 2500X,Japan) at a scanning rate of 4° min−1within the range of 2θ = 5–80°. The chemical components of these coatings were confirmed by Fourier Transform Infrared Spectrometer (FTIR, Nicolet 5DXC), Energy Dispersive Spectrometer(EDS, Oxford Instruments Isis 300) and X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250Xi). The static contact angles of specified samples were tested through an optical angle meter (HARKE-SPCA, China) to evaluate hydrophobicity.
Evaluation the corrosion resistance of prepared coatings by electrochemical measurement (including the electrochemical impedance spectra and the polarization curves) in a threeelectrode system, consisting the working electrode (the exposed area is 1 cm2), saturated calomel electrode (SCE) as reference electrode,and a platinum as counter electrode.Electrochemical experiments were performed in 3.5 wt% NaCl solution and all at open circuit potential. Impedance measurements were recorded from 10 mHz to 100kHz at a 10mV rms sinusoidal perturbation.The polarization curves were recorded at a scan rate of 2mV s−1.
3. Results and discussion
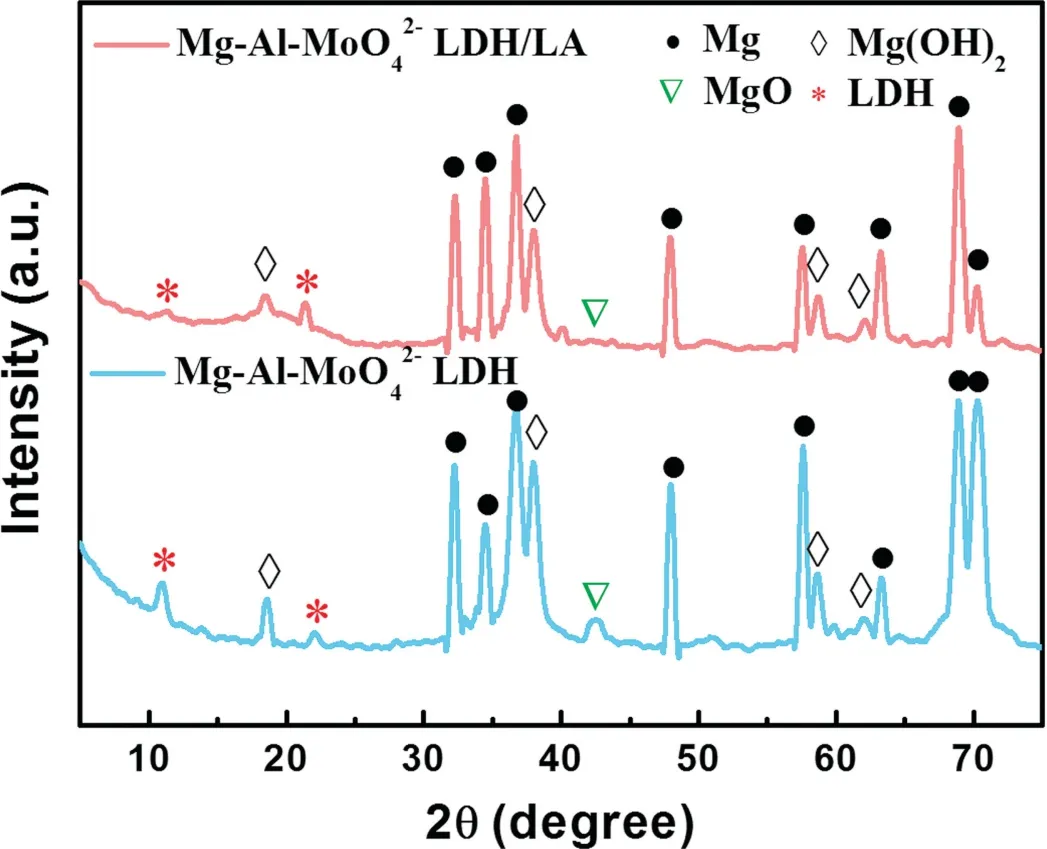
Fig. 2. XRD patterns of different coatings.
Fig. 1a–e shows the morphology of the as prepared coatings with different anions and modified by different low surface energy materials. According to Fig. 1a, the LDH nanosheets are perpendicular to the substrate and cross each other, evenly covering the whole substrate surface. This result is consistent with the previously reported literature [40].When Mg–Al–LDH modified by LA, the morphology of the coating changes significantly. The morphology of the Mg–Al–−LDH/LA coating displays a large number of irregular pores. Some of the lamellar LDHs aggregate to form a flower shape. Most of the flaky LDH cannot be easily observed. The pores are covered with finer particles. Under low magnification, the morphology of Mg–Al–LDH/MA is similar to Mg–Al–LDH/LA. But it still maintains the plate-like structure of LDH according to Fig. 1c. The microstructure of Mg–Al–LDH/LA and Mg–Al–LDH/LA are similar, but totally differ from Mg–Al–LDH/LA. As the Fig. 1d shows, nest-like substance form on the substrate. Fig. 1e displays the Mg-Al-LDH/LA coating’s morphology with thinner and abundant LDH sheets covering on the surface of substrate. The optical images of all kinds of samples are the same, as shown in Fig. 1f.
Fig. 2 displays the XRD results of Mg–Al–LDH coatings before and after modified by LA. For Mg–Al–LDH,the peaks at around 32°,34°,36°,47°,57°,68°and 78° are due to the diffraction of Mg alloy and the intensity of the peaks is particularly strong. Mg(OH)2generates diffraction peaks at around 18°, 37°, 58° and 63°. The peaks observed at about 11° and 22° correspond to (003) and (006)plans, confirming a typical layered structure of LDH. The results proved that LDH was successfully prepared on the substrate using anodic oxidation and hydrothermal process.Although the XRD patterns of the two coatings are similar, the characteristic diffraction peaks intensity decreases in Mg–Al–LDH/LA, demonstrating the shielding effect of organic matter towards the bottom LDH coating [41].
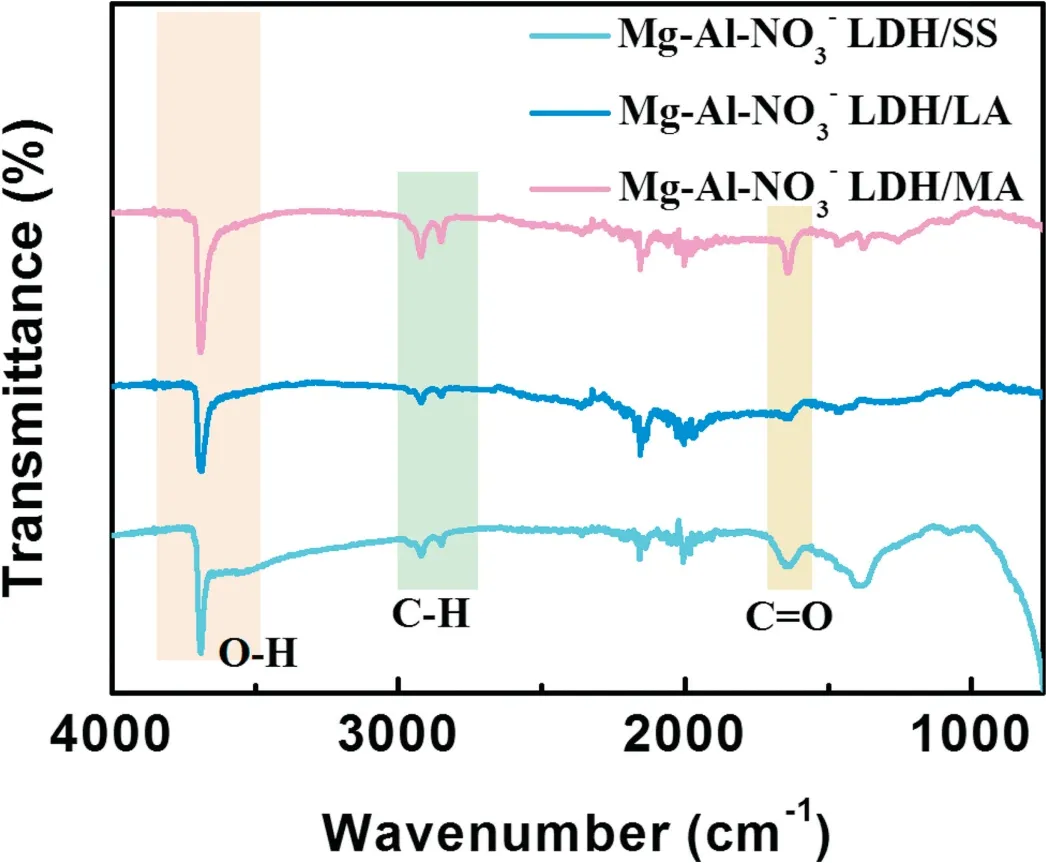
Fig. 3. FT-IR spectra of Mg–Al–NO3−LDH coating modified by SS, LA,and MA.
The absorption peak of the carboxyl group should appear at 1710 cm−1, while the results exhibits absorption at nearly 1640 cm−1. The absorption peaks at nearly 1640 cm−1may be caused by asymmetric and symmetric stretches of carboxyl group [43–45]. It can be concluded that organic low surface energy substances exist in the LDH surfaces.
In order to study the elemental composition of the coating,the Energy Dispersive Spectrometer analysis of Mg–Al–LDH/LA and Mg–Al–LDH/LA were measured and the result is displayed in Fig. 4. Both the EDS analysis show the homogeneous distribution of Mg, Al, O, confirming the presence of suitable ions that make up LDHs. N and Mo exist in the LDH structure due to the observation of nitrogen and molybdenum element in EDS mapping. Results also show the existence of carbon and oxygen element, revealing the successful modification of LDH by lauric acid. All elements are evenly distributed in the observation area, indicating the uniform composition of these coatings. EDS elemental mapping images of other coatings are provided in Fig. S2.
Fig. 5 exhibit the XPS survey spectra and high-resolution spectra the Mg–Al–LDH/LA. The XPS spectra of Mg-Al LDH/LA intercalated withandare provided in Fig. S2. Mg 1s, Al 2p, O 1s and C 1s peaks are detected from all the samples. For Mg–Al–LDH/LA,there are additional peaks of Mo 3d, suggesting the presence ofon magnesium surface. N 1s and V 2p were detected in Mg–Al–LDH/LA and Mg–Al–LDH/LA,respectively.The high resolution XPS data was collected for Mo 3d, C 1s and O 1s to get more information about the composition. For Mo 3d spectra, the fitted peaks at 231.85eV and 235.04eV are attributed Mo6+ion in Mo 3d3/2and Mo 3d5/2states. The high-resolution C 1s spectra can be divided into two peaks at 285.43eV and 284.45eV,corresponding to C=O and C–C/C–H, respectively [46,47].This result indicates the presence of the laurates on the surface after modification. After modification by lauric acid,the surface of magnesium alloy changed from hydrophilic to hydrophobic. O 1s mainly consists of three peaks shown in Fig. 5d. According to Fig. 5d, the positions of the three peaks are 531.79eV, 531.01eV, and 530.06eV, respectively due to the presence of water molecules absorbed on the surface,C=O bond and.
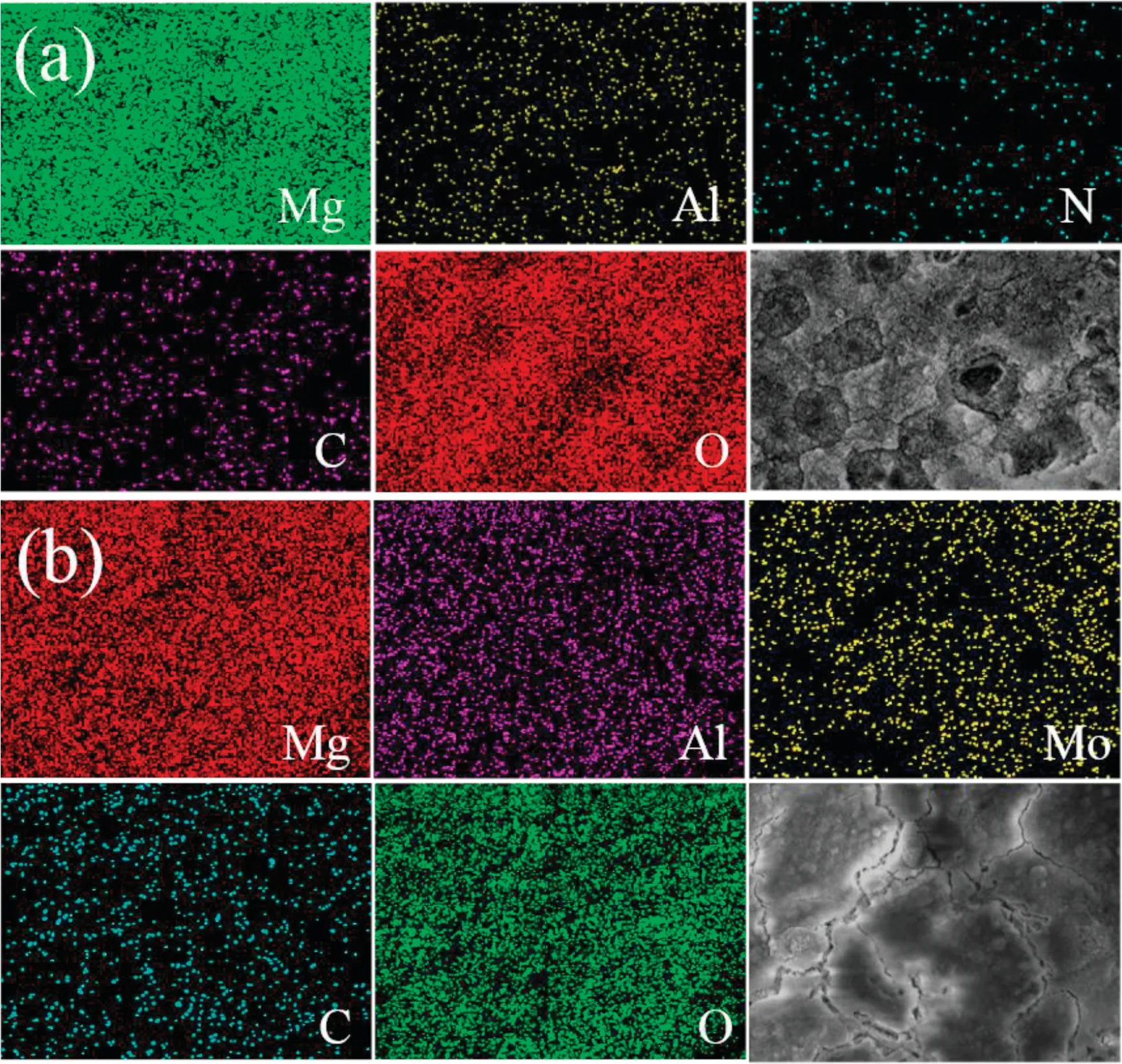
Fig. 4. Elemental mapping images of (a) Mg–Al–LDH/LA and (b) Mg–Al–LDH/LA.
In order to study the hydrophobicity of the prepared coatings, the static water contact angle of coatings was tested.The bare AZ31 Mg alloy is hydrophilic and its water contact angle is about 44.5°. After modification, the water contact angles of the Mg–Al–LDH/SS, Mg–Al–LDH/LA,Mg–Al–LDH/MA are 139.4°, 148.6° and 145.2°, as displayed in Fig. 6. Clearly, all the modified LDH coatings possess hydrophobic. Mg–Al–LDH/LA possesses the highest CA, due to the presence of LA and sufficient roughness resulted from uniform hierarchical micro/nanostructure.The results of the three samples’ contact angle are consistent with the SEM image. Chemical composition and structure determine the hydrophobicity of substances [48]. The following Cassie-Baxter equation can be used to understand why the prepared coatings are hydrophobic [49]:

In Eq. (1) above, fsrepresents the proportion of the solid surface area spread by water; θ and θ0is the water contact angle on the experimental sample surface and the bare sample surface respectively. According to the Eq. (1), the contact angle, θ, increases as the air fraction increases. This indicates that the more air trapped on the surface, the more hydrophobic the surface is. The large fraction of air area can prevent the invasion of the corrosion medium and improve the anticorrosion performance.
To evaluate instantaneous corrosion rate of Mg alloy with different coatings, potentiodynamic polarization curves were recorded. Fig. 7 displays polarization curves of various samples. To quantitatively study, corrosion current density is obtained by extrapolating the linear cathodic branches and anodic branches [50]. And the relevant electrochemical data obtained from polarization curves are listed in Table 1.
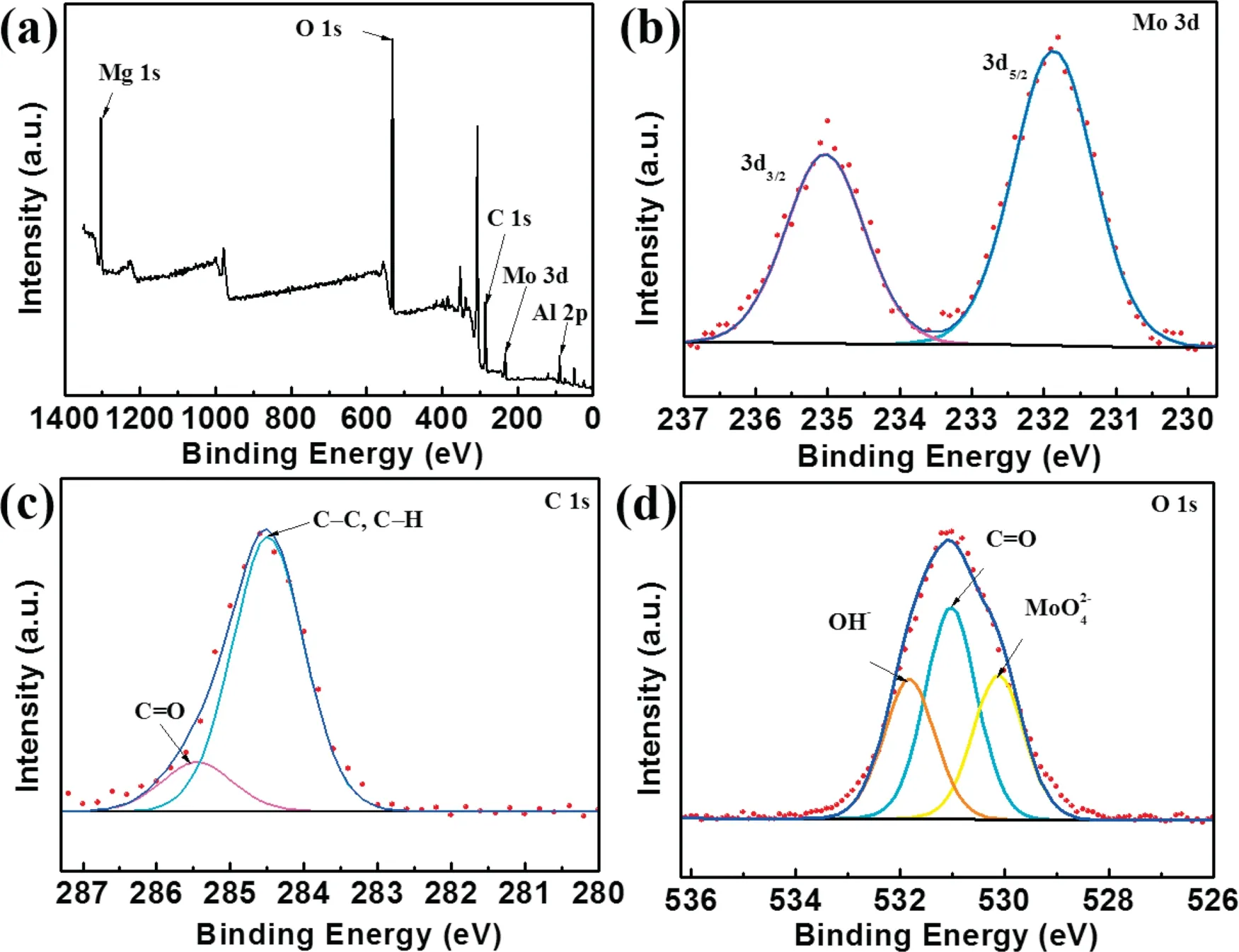
Fig. 5. (a) XPS survey spectra and XPS high-resolution spectra of (b) Mo 3d, (c) C 1s, (d) O 1s for the Mg–Al–MoO42−LDH/LA.
In Table 1, Ecorr, icorr, bcand barepresent the corrosion potential, the corrosion current density, the cathodic and anodic Tafel slopes, respectively. The Ecorrand icorrvalues of Mg substrate are −1.57V vs. SCE and 15.29 μA cm−2, showing poor corrosion resistance. After Mg–Al–LDH was obtained on the substrate, the sample presents a smaller corrosion current density and bcvalues significantly decreases,implying that Mg–Al–LDH coatings hindered the cathodic reaction. After further modification by low surface energy substance, modified samples present a lower corrosion current density compared with Mg–Al–LDH. Among superhydrophobicintercalated Mg-Al LDH coatings,the corrosion current of Mg–Al–LDH/LA is minimal under the same corrosion condition. This suggests that Mg–Al–LDH/LA coating has better corrosion protection effect. The protective effect of coatings can be quantified by corrosion inhibition efficiency (ηp), which is calculated by Eq. (2) [51]:

Fig. 6. The water static contact angle of Mg–Al–LDH coating modified by (a) SS, (b) LA, and (c) MA.
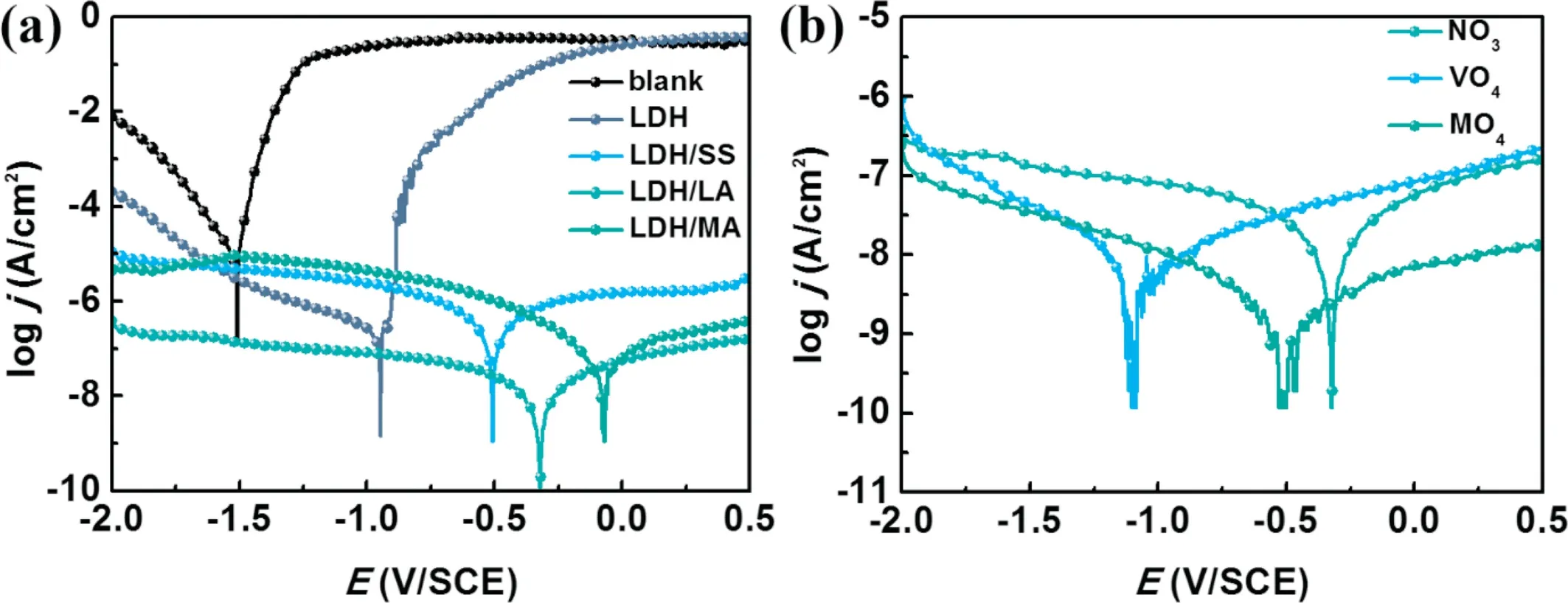
Fig. 7. Potentiodynamic curves of (a) different coatings and (b) Mg-Al LDH/LA coatings intercalated with different anion in 3.5wt.% NaCl.
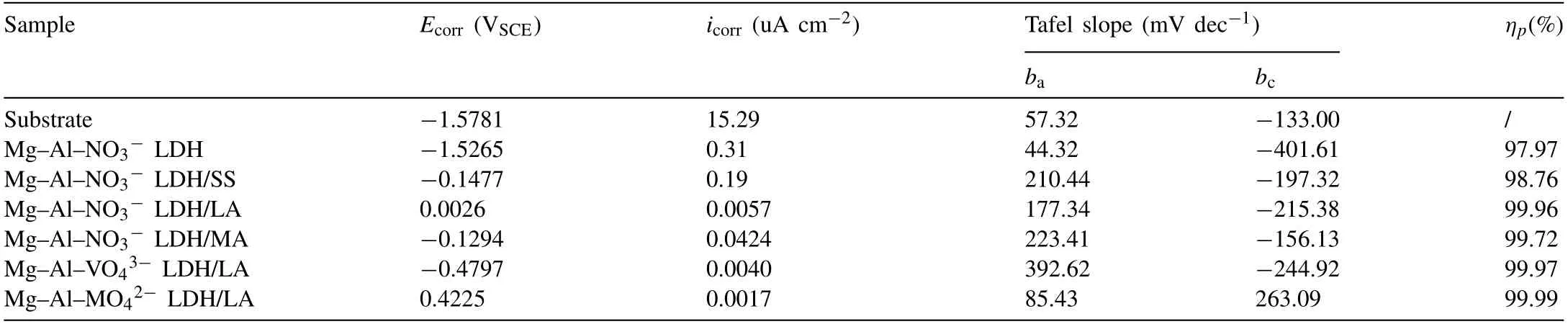
Table 1 Electrochemical parameters estimated from the polarization data in Fig. 7.

Based on this result, in the subsequent experiment on the effect of different anions intercalated in LDH on the corrosion resistance, lauric acid was selected to modify LDH coatings.The representative polarization curves of Mg-Al LDH/LA intercalated with different anion are displayed in Fig. 7(b). The corrosion current values of all three samples are all of the same order of magnitude. Whenis replaced byor, the corrosion current density decline. It can draw a conclusion based on Fig.7(b)that Mg–Al–LDH/LA has the best corrosion resistance.
The phase angle and impedance modulus are recorded by applying sine wave perturbation signal to form practical electrochemical impedance spectroscopy (EIS), which is used to measure and analyze resistance in the corrosion process [52,53]. The Bode plots of different specimens are depicted in Fig. 8. In Bode plots, high impedance modulus at the low-frequency zone represents good corrosion resistance.The bare sample has the lowest impedance modulus. Due to ion-exchange capability of LDH, the aggressive medium(Cl−) could not easily reach at the metal surface and the impedance modulus of Mg–Al–LDH is higher than bare alloy. The impedance modulus of coatings after modification by low surface energy substance increases again.Mg–Al–LDH/LA has the highest impedance, as the Fig.8a shows.The Bode plots of Mg-Al LDH/LA intercalated with different anion are shown in Fig. 8b. The impedance modulus of super-hydrophobic LDH coating intercalated withis the highest.
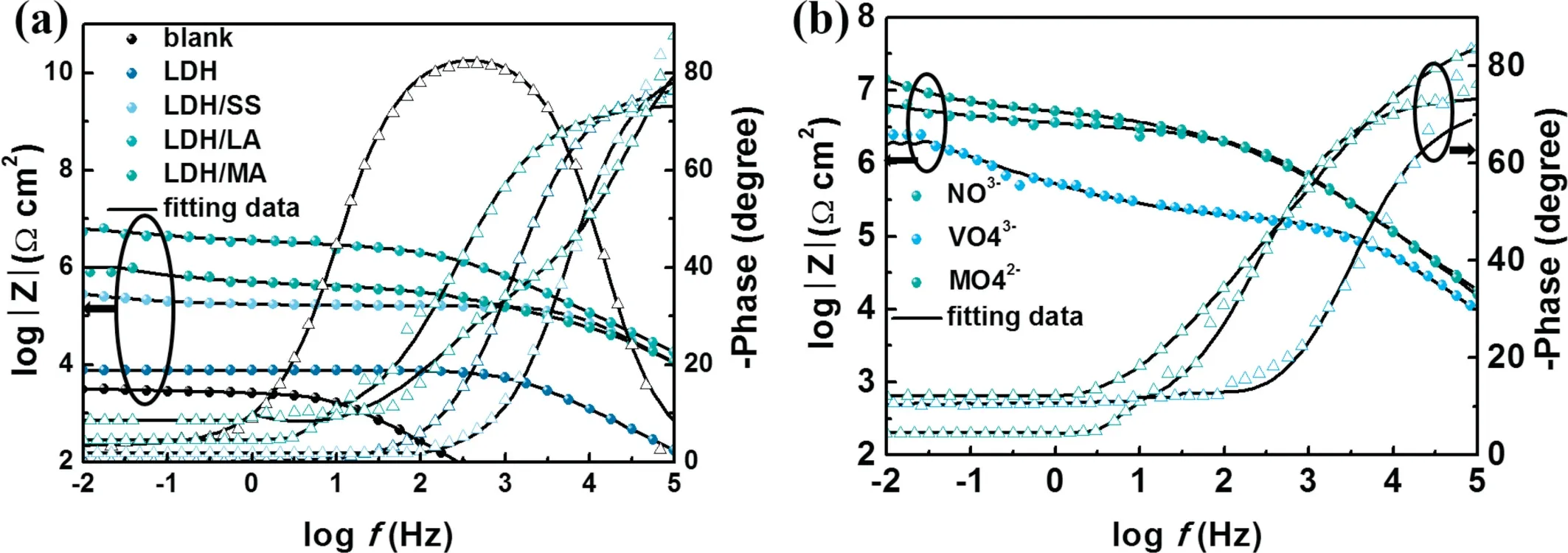
Fig. 8. EIS spectra of (a) different coatings and (b) Mg–Al LDH/LA coatings intercalated with different anion in 3.5wt.% NaCl.
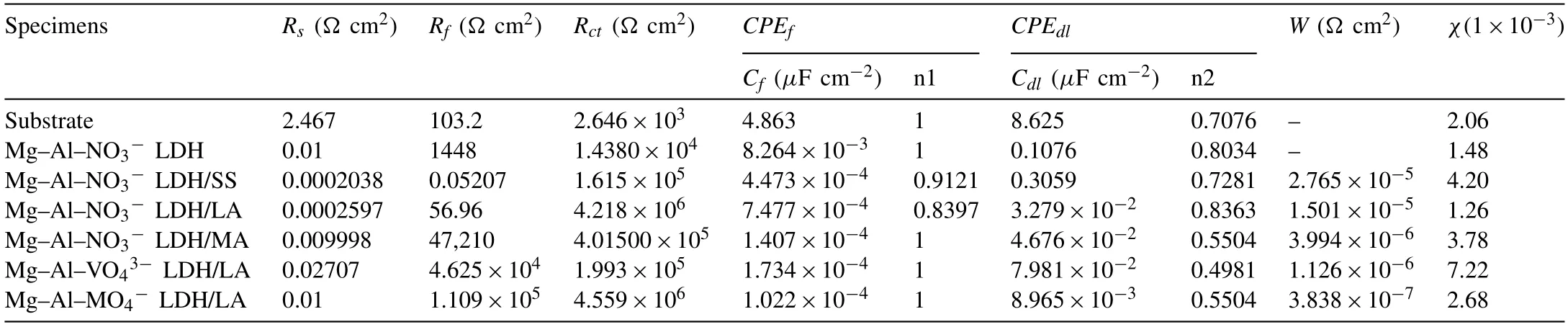
Table 2 Fitted parameters of the EIS spectra of the bare AZ31 and coated samples using equivalent circuits shown in Fig. 9.

Fig. 9. Equivalent electrical circuit used to fit the EIS spectra of different samples.
The equivalent circuit shown in Fig. 9a without Warburg impedance is used to fit the EIS data of the bare sample and Mg–Al–LDH. Another is used to fit the data of Mg-Al LDH modified by SS, LA, MA. In both circuits, Rsrepresents the solution resistance caused by electrolyte, Rctis the charge transfer resistance existing in the coating-substrate interface, and Rfshows the resistance of the protective coating formed on the AZ31 substrate. W corresponds to Warburg impedance, caused by a diffusion of the dissolved Mg2+from the substrate to the bulk solution. CPE is a non-ideal capacitive component. The chi-squared (χ2) error value represents the quality of fits and the value of χ2around 10−3or less indicates good fit. The data fitted by equivalent circuits is listed in Table 2. Typically, the corrosion process is largely dependent on the ion transport process at the interface, so the high value of Rctrepresents good corrosion resistance.Observed from Table 2, the Rctvalue of bare substrate is the lowest. The value of Rctincreases after LDH in-suit grown on substrate. After the modification, the values of Rctfurther increase by one to two order of magnitude compared with LDH without modification. Among hydrophobic coatings intercalated with nitrate, Mg–Al–LDH/LA has the especially highest values in Rct. For Mg-Al LDH/LA coatings intercalated with different anion, the Rctof coating intercalated withis the highest. In summary, Mg-Al-LDH/LA coatings render a best protection for the substrate. This result is in good agreement with polarization potentiodynamic curves.
For bare AZ31 substrates, corrosive media can directly contact with metal and then the corrosion process can occur relatively easily. The anti-corrosion performance can be improved through developing LDH coating on the substrate, for LDHs can capture aggressive Cl−into their interlayer preventing aggressive ions reaching at the surface of substrate. After further modification, hydrophilic surface transforms into a hydrophobic surface. Hydrophobic coating would not be wetted and further inhibit the contact of corrosive media with substrate, endowing better protection. Compared with Mg–Al–LDH coatings modified by SS and MA, Mg–Al–LDH/LA has the best corrosion resistance due to its uniform and dense microstructure and the best hydrophobicity. When nitrate is replaced by molybdate, the corrosion resistance is greatly improved.ions, a kind of inhibitor, can react with Mg2+, forming a protective deposition coating and preventing further dissolution of magnesium. The reaction can be given as follows:
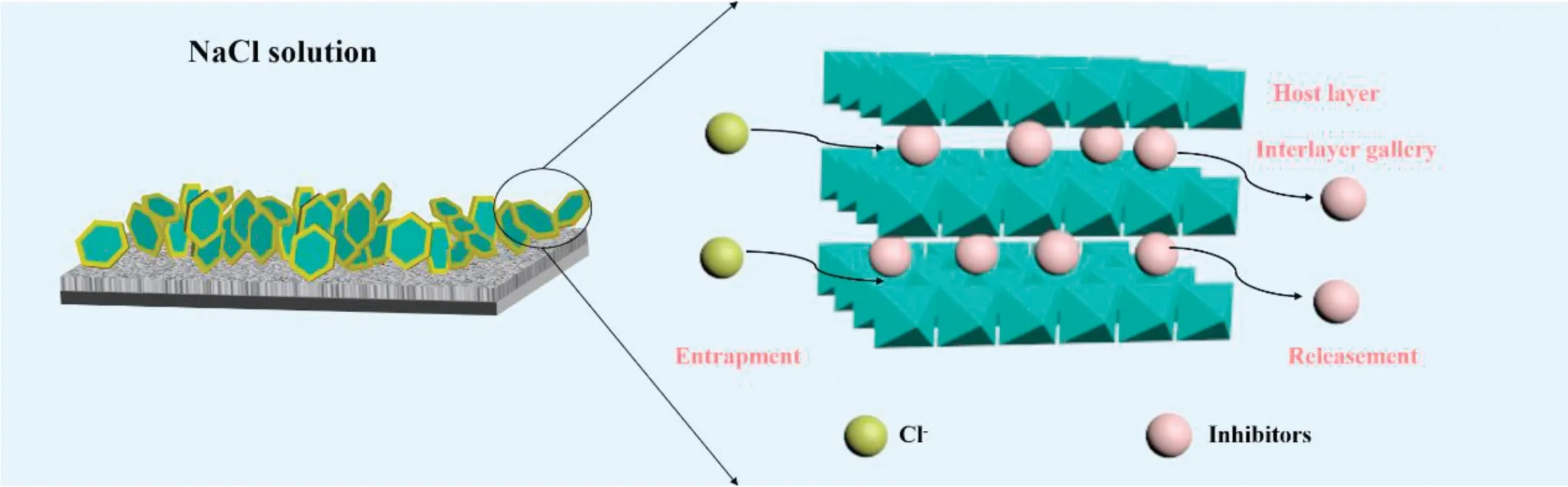
Fig. 10. Schematic illustration of the corrosion protection mechanism of superhydrophobic LDH coating.
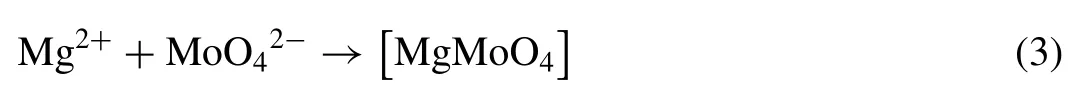
The excellent corrosion resistance of super-hydrophobic corrosion inhibitor intercalated Mg-Al LDH coatings may be due to the synergistic effect of the following (as shown in Fig. 10): (1) LDH coatings act as physical barriers, inhibiting direct contact between chloride ions and metal substrate.(2) The presence of corrosion inhibitor anion enhances the corrosion inhibition ability of LDH to magnesium alloy. (3)The fabrication of hydrophobic coating can achieve further isolation of chloride ions, which exist in aqueous solution.
4. Conclusion
In this work, different kinds of super-hydrophobic Mg-Al LDH coatings were successfully fabricated on bare magnesium alloy. The effect of LDH interlayer ion species and low-surface-energy species on the corrosion resistance performance has been studied. Hydrophilic Mg–Al–NO3−LDH coatings turn into hydrophobic after modification. And the static contact angle of Mg-Al LDH intercalated with NO3−modified by SS, LA and MA are 139.4°, 148.6° and 145.2°,respectively.Superhydrophobic LDH coatings significantly reduce the corrosion current density. The EIS and potentiodynamic polarization curves results show the good protection of Mg–Al–LDH/LA,probably due to its uniform structure and hydrophobic. The replacement offurther improve the anti-corrosion resistance of Mg-Al LDH/LA. These results show that synergistic effect of molybdate and lauric acid lead to a better corrosion resistance performance.
Acknowledgements
This work is financially supported by the Graduate Research and Innovation of Chongqing, China (Grant No. CYB18002), the National Natural Science Foundation of China (Grant No. 21576034), the State Education Ministry and Fundamental Research Funds for the Central Universities (2019CDQYCL042, 106112017CDJXSYY0001, 2018CDYJSY0055, 106112017CDJQJ138802,106112017CDJSK04XK11, 2018CDQYCL0027), the Joint Funds of the National Natural Science Foundation of China-Guangdong (Grant No. U1801254) and Fundamental Research Funds for the Central Universities (NO. 2018CDJDCD0001).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jma.2019.11.011.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Latest research advances on magnesium and magnesium alloys worldwide
- Advances in coatings on biodegradable magnesium alloys
- Stability of twins in Mg alloys – A short review
- A review on thermal conductivity of magnesium and its alloys
- Effect of Ca addition on the microstructure and the mechanical properties of asymmetric double-sided friction stir welded AZ61 magnesium alloy
- Effect of pre-deformation on microstructure and mechanical properties of WE43 magnesium alloy II: Aging at 250 and 300 °C
