Human equivalent dose of oral 4-aminopyridine differentiates nerve crush injury from transection injury and improves post-injury function in mice
2020-04-29ChiaGeorgeHsuHassanTalukderLiYueLoelTurpinMarkNobleJohnElfar
Chia George Hsu , M A Hassan Talukder , Li Yue, Loel C. Turpin, Mark Noble, John C. Elfar
1 Center for Orthopaedic Research and Translational Science, Penn State Hershey College of Medicine, Milton S. Hershey Medical Center, Hershey,PA, USA
2 Department of Medicine, Aab Cardiovascular Research Institute, University of Rochester School of Medicine and Dentistry, Rochester, NY, USA
3 Department of Orthopedics, The Warren Alpert Medical School of Brown University/Rhode Island Hospital, Providence, RI, USA
4 Department of Neuroscience, The University of Rochester Medical Center, Rochester, NY, USA
5 Department of Biomedical Genetics, The University of Rochester Medical Center, Rochester, NY, USA
Abstract 4-Aminopyridine (4-AP), an FDA-approved drug for the symptomatic treatment of multiple sclerosis, is used to improve neuromuscular function in patients with diverse demyelinating disorders. We recently demonstrated that local, transdermal or injectable forms of 4-AP improve myelination, nerve conduction velocity, muscle atrophy, and motor function after traumatic peripheral nerve injury in mice. While oral 4-AP is most commonly used in the clinic, it is unknown whether human equivalent oral doses of 4-AP have effects on traumatic peripheral nerve injury differentiation, myelination, muscle atrophy, functional recovery, and post-injury inflammatory processes in animals.Mice with sciatic nerve crush or denervation injury received oral or intraperitoneal 4-AP (10 μg) or vehicle alone and were examined for pharmacokinetics, motor function, muscle mass, intrinsic muscle force, nerve morphological and gene expression profiles. 4-AP showed linear pharmacokinetics and the maximum plasma 4-AP concentrations were proportional to 4-AP dose. Acute single dose of oral 4-AP administration induced a rapid transient improvement in motor function that was different in traumatic peripheral nerve injury with or without nerve continuity, chronic daily oral 4-AP treatment significantly enhanced post crush injury motor function recovery and this effect was associated with improved myelination, muscle mass, and ex vivo muscle force. Polymerase chain reaction array analysis with crushed nerve revealed significant alterations in gene involved in axonal inflammation and regeneration. These findings provide convincing evidence that regardless of the route of administration, 4-AP can acutely differentiate traumatic peripheral nerve injury with or without nerve continuity and can enhance in vivo functional recovery with better preservation of myelin sheaths, muscle mass, and muscle force. The animal experiments were approved by the University Committee on Animal Research (UCAR) at the University of Rochester (UCAR-2009-019) on March 31, 2017.
Key Words: 4-aminopyridine; electron microscopy of nerves; functional recovery; gene expression; muscle force; muscle mass; oral administration;pharmacokinetics; sciatic nerve crush injury; sciatic nerve denervation injury
Introduction
4-Aminopyridine (4-AP) is a broad-spectrum potassium channel blocker and FDA-approved drug for the symptomatic treatment of multiple sclerosis (Egeberg et al., 2012; Jensen et al., 2016). 4-AP has been shown to improve neuromuscular function in patients with diverse demyelinating disorders(Lundh et al., 1979; Hansebout et al., 1993; Sanders et al.,2000; Wirtz et al., 2009). In addition to improving nerve conduction (Sherratt et al., 1980; Targ and Kocsis, 1985),4-AP enhances neurotransmitter release (Lundh, 1978), synaptic transmission (Jankowska et al., 1982), and contractile strength of the muscle (Agoston et al., 1982; Smith et al.,2000). The efficacy, safety, and tolerability of 4-AP are well documented in demyelinating disorders (Uges et al., 1982;Davis et al., 1990; van Diemen et al., 1993; Pratt et al., 1995;Goodman and Stone, 2013).
Traumatic peripheral nerve injury (TPNI) represents a major public health problem that often leads to significant functional impairment and permanent disability (Robinson,2000). After never injury, the distal stump of the injured nerve undergoes a series of molecular and cellular changes, and a complex interaction exists between macrophages,fibroblasts, Schwann cells, inflammatory and anti-inflammatory cytokines during Wallerian degeneration (Allan and Rothwell, 2001; Stoll et al., 2002; Burnett and Zager, 2004;Campbell, 2008; Gaudet et al., 2011; Dubovy et al., 2013;Menorca et al., 2013). Schwann cells and macrophages in the injured nerves express pro-inflammatory cytokines and these in turn can induce the transcription of several enzymes(Terenghi, 1999; McDonald et al., 2007). Differential expression of genes has been reported after sciatic nerve crush and transection injuries in rats (Gillen et al., 1995; Bosse et al., 2001; Li et al., 2013). However, gene expression profile during peripheral nerve injury and regeneration in mice remains largely unknown and the effect of 4-AP on the gene expression profile of injured nerve is still lacking.
We previously demonstrated that chronic daily treatment with variable doses of 4-AP, either intraperitoneal, transdermal or local, enhances global functional recovery of the affected limb, promotes remyelination of the nerve, improves the nerve conduction velocity, and attenuates neurogenic muscle atrophy in a mouse model of sciatic nerve crush injury, in addition to its acute diagnostic utility (Tseng et al.,2016; Clark et al., 2019; Noble et al., 2019; Yue et al., 2019).The oral formulation is the most commonly used form of a drug because it is easy, preferred by the patient, and sustained slow-release preparation is available for prolonged duration of action. However, it is unknown whether human equivalent oral doses of 4-AP have any effect in TPNI. It also remains unknown whether the beneficial effect of 4-AP on muscle mass and force (Yue et al., 2019) depends on the nerve continuity and how gene expression is altered in the injured nerves. This study was designed to address these gaps by testing the acute effect of human equivalent oral dosage of 4-AP on TPNI with or without nerve continuity, and the chronic effect of oral 4-AP treatment was also investigated for functional recovery, muscle atrophy, nerve morphology,and gene expression profile.
Materials and Methods
Animals
The experimental design and animal protocol was approved by the University Committee on Animal Research (UCAR)at the University of Rochester (UCAR-2009-019) on March 31, 2017 and the experiments were performed according to the guidelines of UCAR. A total of 193 ten-week-old female C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME,USA) weighing 20-25 g were used in this study and mice were housed at the animal facility according to UCAR guidelines. We used female mice because they are easier to house together even after surgery than male mice and female mice have a much lower risk of fighting or mutilating wounds -which is a key reason for early post-surgical demise in mice(Tseng et al., 2016; Clark et al., 2019; Yue et al., 2019).
Pharmacokinetics of 4-AP
Since clinically relevant serum concentrations of 4-AP are in the range of 0.5-1 μM (Shi and Blight, 1997; Smith et al.,2000), it was necessary to measure 4-AP concentration in the mouse serum. To determine the pharmacokinetic parameters of 4-AP (Sigma-Aldrich, St. Louis, MO, USA), 20 μg 4-AP was administered directly into the stomach of awake mouse through oral gavage or 10 μg 4-AP was injected intraperitoneally (IP). Whole blood samples were collected in clean microcentrifuge tubes without anticoagulants at specified time points by cardiac puncture and kept at room temperature for 1 hour. The blood samples were collected after 30, 60, 120, and 180 minutes of oral 4-AP administrations and after 10, 20, 40, 60, and 120 minutes of IP 4-AP administrations. Serum was separated after centrifugation of the clotted blood sample at 1000 ×gfor 10 minutes and stored in -20°C until use. For the removal of serum protein, 150 μL of acetonitrile was added to 50 μL of serum, vortexed for 1 minute, and then centrifuged at 3000 ×gfor 10 minutes at 4°C.
The relevant concentration of 4-AP in the serum following a single oral or IP administration was determined by a modified liquid chromatography coupled with tandem mass spectrometry (LC/MS/MS) assay (Caggiano and Blight, 2013;Burnett et al., 2015). This method simultaneously determined 4-AP and 3,4-diaminopyridine (3,4-DAP; Sigma-Aldrich)(as an internal control) in the mouse serum with a chromatographic run time of 3.5 minutes. Briefly, the supernatant layer was filtered through 0.2 μm syringe filter (Millex-FG,Millipore, Burlington, MA, USA) and 10 μL of the solution was analyzed with LC/MS/MS. Liquid chromatography separation was performed isocratically at 800 μL/min on the Waters Atlantis HILIC column at 25°C. The mobile phase was 5 mM ammonium acetate in 10/90/0.2 (v/v/v) water/acetonitrile/formic acid. The mass spectrometry consisted of a Thermo Quantum Access Max Triple Quadrupole (GenTech Scientific, Arcade, NY, USA). The ion spray voltage was set at 4500 V with the source temperature at 500°C. The sheath gas pressure and auxiliary gas pressure were set at 65 psi (pounds per square inch) and 5 psi respectively. LC/MS/MS analysis was carried out with argon as the collision gas. Ion transitions were 95/78 for 4-AP and 110/93 for 3,4-DAP. 4-AP was quantified with a calibration curve (0.01-20 μM), and was qualitatively assessed by comparison of analytical response for 4-AP with that of the internal standard, 3,4-DAP. The within-day and between-day precision was established by assaying quality control samples prepared at 0.01 μM (lower limit quantitation) and at 10 μM (higher limit quantitation)for three analyses with error within 15%.
Sciatic nerve crush and denervation injuries, 4-AP treatment, and tissue harvesting
Sciatic nerve crush injury was performed as previously described (Elfar et al., 2008; Yue et al., 2019). Briefly, after IP ketamine (60 mg/kg)/xylazine (4 mg/kg) (McKesson, Irving, TX, USA) anesthesia, hair clipping and aseptic animal preparation, a lateral skin incision along the length of the femur was made, and right sciatic nerve was bluntly exposed through the iliotibial band and crushed proximal to the tibial and peroneal divisions using a smooth forceps with a metal calibration ring to standardize pressure for 30 seconds. For permanent denervation (complete transection or cut) injury,a 10 mm segment of the sciatic nerve was removed and the proximal stump was buried in the gluteal muscle to prevent any re-innervation. The wound was closed and subcutaneous buprenorphine (0.05 mg/kg) was given for postoperative analgesia immediately after surgery and every 12 hours thereafter for the next 3 days.
For the acute single-dose diagnostic effect of oral 4-AP at 3 days post-injury, sciatic function index (SFI) was measured before and 30 minutes after the single dose of 4-AP (10 μg/mouse) oral gavage. For the chronic effect of 4-AP treatment, a daily dose of 10 μg 4-AP consisted of oral gavage(control: water) or IP injection (control: saline) immediately after injuries and continued until sacrifice. Before each day’s 4-AP dose, mice were subjected to gait analysis. For the chronic 4-AP treatment groups, mice with crush injury were sacrificed at 14 days post-injury and mice with denervation injury were sacrificed at 28 days post-injury.
Unless otherwise mentioned, mice were given IP injection ketamine (60 mg/kg)/xylazine (4 mg/kg) mixture on the sacrifice day and blood samples, nerves, and muscles were then collected/harvested under deep anesthesia. Tibialis anterior(TA), extensor digitorum longus (EDL), and gastrocnemius-soleus muscles were harvested forin vitroandex vivostudies.
SFI
The effects of transdermal 4-AP were evaluated by SFI, a noninvasive means to determine the directin vivofunctional recovery after sciatic nerve injury (Inserra et al., 1998;Varejao et al., 2001; Elfar et al., 2008). The SFI is measured on a scale of 0 (normal) to 100 (complete loss of function).Briefly, mice were trained to walk freely along a 77 cm by 7 cm corridor lined with white paper and individual footprints were obtained by painting each hind foot. Paw prints were measured, using two blinded evaluators, for toe spread (distance from the 1sttoe to the 5thtoe) and paw length (length from the third toe to bottom of the print). Three prints from the experimental (injured) and normal (uninjured) sides were measured, and SFI was calculated for each animal by averaging these measurements and using the following formula (Inserra et al., 1998): SFI = 118.9 ((ETS-NTS)/NTS)) -51.2 ((EPL-NPL)/NPL)) - 7.5. Where E is the experimental(injured: crush or denervated) paw, N is the normal (healthy:uninjured or control) paw, TS is toe spread, and PL is paw length. To make sure a successful crush injury, mouse with SFI lower than 80 at 1 day post-injury mice were excluded from the study.
Ex vivo muscle contraction measurement
Isolated right EDL muscle contraction was measured using an ASI muscle contraction system (Aurora Scientific, Aurora, Canada) as previously described (Liu et al., 2015; Yue et al., 2019). Briefly, EDL muscles were carefully excised and mounted between two platinum electrodes and continuously perfused with oxygenated Ringer solution in the chamber.Muscle optimal length (L0) was determined using a 1 Hz stimulation series. Stimulus output was set at 120% of the voltage that elicited maximal force. Muscles were first equilibrated using three 500 ms, 150 Hz tetani at 1 minute intervals and then subjected to a force frequency. Maximum muscle contractile force was measured at stimulation frequencies ranging from 25 to 250 Hz. To obtain specific force values,absolute force was normalized to muscle cross-sectional area determined by EDL weight and length (Hakim et al., 2011).
Real-time reverse transcription-polymerase chain reaction
Sciatic nerves were harvested from the injured site to the tibial and peroneal division and were homogenized in TRIzol.The RNA was purified using RNeasy Mini Kit (QIAGEN,Hilden, Germany) according to the manufacture’s protocol and total RNA concentration was measured by NanoDrop ND-1000 spectrophotometer (ThermoFisher, Waltham,MA, USA). cDNA was synthesized from 1 μg RNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA).To detect the expressed genes of the nerve injury, a trauma nervous system mouse 96 well-plate (Bio-Rad) was used.The list of genes and detailed information primers used are in Additional Table 1. Quantitative polymerase chain reaction (PCR) reactions were performed using PerfeCTa SYBR Green Fast Mix (Quantabio, Beverly, MA, USA) in a CFX96 real-time PCR machine (Bio-rad). Changes in gene expression of nerve injury were normalized to GAPDH and the uninjured, water-treated group. Results were considered significant when genes showed at least 2-fold differential expression levels andPvalue was < 0.05 between groups.
Transmission electron microscopy and morphometric analysis
The crushed portion of sciatic nerves were excised and fixed for 24 hours in a combination fixative of 2.5% glutaraldehyde/4.0% paraformaldehyde buffered in 0.1 M sodium cacodylate and post-fixed 1.5 hours in buffered 2.0% osmium tetroxide, dehydrated in a graded series of ethanol up to 100%, then transitioned into propylene oxide, and EPON/Araldite epoxy resin overnight. The next day, the nerves were embedded in fresh epoxy resin and polymerized for 2 days at 60°C. One micron sections were cut and stained with toluidine blue to assess the myelin before thin sectioning at 70 nm using an ultramicrotome and diamond knife. These sections were placed onto formvar/carbon slot grids, stained with uranyl acetate and lead citrate and digitally imaged using a Hitachi 7650 transmission electron microscope (Hitachi, Tokyo, Japan) with an attached Gatan Erlangshen digital camera (Gatan, Inc., Warrington, PA, USA) at 3000× magnification. The sections and images were randomly selected by staffs in Transmission Electron Microscopy Core. Images were analyzed using ImageJ (NIH, Bethesda, MD, USA).Three images from each mouse were analyzed, containing a total of approximately 20-40 axons per animal. The area and inner and outer perimeter of axons were measured with diameters calculated from these measurements and myelin thicknesses were calculated by the difference between these measurements. G-ratio was calculated by the ratio of the inner axonal diameter to the outer diameter.
Statistical analysis
All data are expressed as mean ± standard error of the mean(SEM). One-compartmental pharmacokinetic analysis of the serum concentration-time data was performed using PKSolver (version 2.0, China Pharmaceutical University,Nanjing, China) to determine a complete pharmacokinetic profile. Data were analyzed using either two-tailed Student’st-test for paired data from the same experiment or unpaired data from different experiments. SFI time-course and muscle force data were analyzed by one-way analysis of variance followed bypost hoc Ttests using Bonferroni correction for multiple comparisons. Acute SFI, muscle weight, axon morphology, and PCR array data were analyzed by two-way analysis of variance followed bypost hoc Ttest using Tukey correction for multiple comparisons, after confirmation of normally distributed data sets. Values ofP< 0.05 were considered to be statistically significant.
Results
Pharmacokinetics of oral and IP administration of 4-AP
Our results confirmed the straightforward sample preparations with small sample volume requirements for this assay,and this method proved useful for determining a pharmacokinetic profile of 4-AP with the dosage utilized. Figure 1A and B show the serum 4-AP concentration versus time profile for 20 μg oral 4-AP (n= 4-5 mice/time point) and 10 μg IP 4-AP (n= 3-4 mice/time point) administrations, respectively. In the oral 4-AP experiment (Figure 1A), the time to maximum blood concentration (Tmax) was 30 minutes and the predicted maximum concentration (Cmax) of 4-AP was 1.48 ± 0.09 μM. The mean residence time that 4-AP stayed in mice was 61.2 ± 1.74 minutes. In the IP 4-AP experiment(Figure 1B),Tmaxwas 15.5 ± 3.41 minutes, the predictedCmaxof 4-AP was 0.98 ± 0.07 μM, and mean residence time was 32.8 ± 3.17 minutes. Because serum 4-AP levels with oral 20 μg 4-AP were higher than reported in clinical use,we used 10 μg 4-AP for both oral and IP experiments for identical experiments. Selected pharmacokinetic parameters after oral and IP administration of 4-AP are shown in Additional Table 2.
Oral 4-AP treatment differentiates crush injury from denervation injury, and promotes in vivo functional recovery after sciatic nerve crush injury
Figure 2 shows the functional evaluation of nerve injury and recovery using SFI at different experimental conditions. Acute single dose of oral 4-AP (Figure 2A) at 3 days post-injury significantly improved SFI in crushed nerves within 30 minutes of administration from -96 ± 5.62 to -66± 4.27 (P< 0.001,n= 9 mice/group). In contrast, this effect was absent in mice with nerve denervation (SFI, -107 ± 3.68vs. -100 ± 1.84;P= 0.095,n= 10 mice/group). This immediate improvement of gait function indicated that 4-AP can distinctly differentiate an incomplete nerve injury from a complete nerve injury. Furthermore, once-daily oral 4-AP (10 μg) markedly accelerated the functional recovery (SFI) early on from crush injury as compared with water (Figure 2B)leading to demonstrable significant improvement by 3 days post-injury (water: 67 ± 7.24vs. 4-AP: 29 ± 9.31;P< 0.01,n= 9 mice/group). By the 2-week time point, SFI reached recovery levels in both groups. In contrast, there was no functional recovery following denervation injury (Figure 2C) and SFI was identical in both water and 4-AP groups even after 28 days of treatment (n= 5 mice/group). These results demonstrate that, in addition to acute diagnostic effect on motor function, daily oral 4-AP is effective in enhancing the long-term functional recovery of sciatic nerve crush injury. We also checked the effect of IP 4-AP treatment on the functional recovery following crush and denervation injuries (Additional Figure 1). Consistent with the findings of oral 4-AP, IP 4-AP also significantly improved SFI following crush injury at day 3 and day 7 (P< 0.05;n= 5-6 mice/group), but this beneficial effect was absent in mice with denervation injury (n= 5 mice/group) as reported in our previous work (Tseng et al., 2016).
Oral 4-AP treatment attenuates muscle weight loss following crush injury
Figure 3 shows TA and EDL muscle mass (mg) in contralateral uninjured limbs (uninjured) and injured limbs (crush or denervation) with or without 4-AP treatment at 14 days post-injury. Uninjured, untreated mean TA or EDL muscle mass (mg) was not different from uninjured 4-AP treated counterparts in both crush and denervation groups. Uninjured untreatedvs. uninjured 4-AP treated TA muscle mass in crush and denervation group was 39.1 ± 0.93vs. 37.8 ±0.37 (n= 6 mice/group) and 42.4 ± 0.72vs.39.6 ± 0.98 (n= 6 mice/group), respectively. Uninjured untreatedvs.uninjured 4-AP treated EDL muscle mass in crush and denervation group was 8.8 ± 0.27vs. 9.6 ± 0.28 (n= 5 mice/group) and 10.1 ± 0.86vs. 9.5 ± 0.20 (n= 5 mice/group), respectively.Both crush (Figures 3A and B) and denervation (Figures 3C and D) injuries caused a significant loss of TA and EDL muscle mass in the injured limbs compared with respective contralateral uninjured limbs. Daily oral 4-AP treatment significantly prevented muscle loss in crush injury but not in denervation injury. On day 14, mean TA and EDL muscle mass (mg) in crush watervs.crush 4-AP group was 29.2 ±0.97vs.33.1 ± 0.92 (P< 0.05;n= 6 mice/group) and 7.6 ±0.32vs. 9.0 ± 0.39 (P< 0.05;n= 6 mice/group), respectively,showing a significant protection against muscle loss in the injured limb early on. In contrast, this muscle protective effect of oral 4-AP was absent in denervation group, and mean TA and EDL muscle mass (mg) at 14 days post-injury in denervated water-treatedvs. 4-AP treated groups was 26.9 ±0.96vs.25.7 ± 0.45 (n= 5 mice/group) and 7.0 ± 0.20vs.7.2± 0.28 (n= 5 mice/group), respectively.
Oral 4-AP treatment improves ex vivo muscle force following crush injury
Figure 4 shows the effect of daily 4-AP treatment on the EDL muscle specific force and force frequency relationship at 14 days post-injury. In the crush injury group (Figure 4A), daily 4-AP treatment significantly improved the specific force (mN/mm2) of EDL muscles compared with the water-treated group and the maximal force at 250 Hz was 174.1± 8.4vs. 136.2 ± 13.3 (P< 0.05,n= 5-6 mice/group). In contrast, the muscle force enhancing effect of 4-AP treatment was absent in the denervated EDL muscle, and the mean muscle force at 14 days post-injury was comparable between water and 4-AP groups at all frequencies (Figure 4B;n=4-5 mice/group). We also checked the effect of daily IP 4-AP treatments on muscle force (Additional Figure 2). Similar to the findings with oral 4-AP treatment,ex vivoEDL muscle force was significantly enhanced in crush injury IP-treated 4-AP animals compared with the crush injury saline-treated group, and the maximal force at 175 Hz was 225.9 ± 12.6vs.119.0 ± 36.5 (P< 0.05,n= 4 mice/group). The muscle force enhancing effect of daily IP 4-AP treatment was absent in the denervated EDL muscles and the muscle force was comparable between IP 4-AP and water-treated groups (n= 3-4 mice/group). In the sham surgery group, the specific muscle force was identical between water and IP 4-AP groups at all frequencies (n= 3 mice/group).
Oral 4-AP treatment decreases G-ratio and improves myelination of the sciatic nerve after crush injury
Figure 5 shows representative transmission electron microscopy images, mean G-ratio and myelin thickness of contralateral uninjured and injured (crushed) nerves with or without oral 4-AP treatment at 14 days post-injury. Figure 5A demonstrates an increased representation of well-preserved axons in the crush 4-AP-treated group as compared with the crush water group. While axonal myelin thickness in the uninjured nerves was similar between water and oral 4-AP groups (Figure 5B), it was significantly increased in crushed nerves 4-AP group compared with water-treated group (0.84 ± 0.04vs. 0.66± 0.04,P< 0.05,n= 6 mice/group). Consistent with these findings, we also observed small but significant reductions in G-ratio in crush 4-AP group compared with crush water group (0.69 ± 0.01vs. 0.73 ± 0.01,P< 0.05,n= 6 mice/group).The uninjured nerve G-ratio was comparable between water and 4-AP groups (Figure 5C;n= 6 mice/group).
Gene expression profile in the crushed sciatic nerve and effect of 4-AP
Figure 6 depicts heat map for the quantitative real-time PCR array analysis of gene expression in the crushed sciatic nerves at 3 days post-injury (n= 6 mice/group). The frequency of differentially expressed genes in mouse sciatic nerve was identified by a trauma nervous system 96-well panel. PCR array analysis (Figure 6) revealed significant(P< 0.05) alterations in the level of several high-expressing and low-expressing genes after crush injury. Crush injury in water group caused significant up-regulation ofIL1b,Timp1,Cd53,Lagals3,slpi, andTnfgenes and significant down-regulation ofSod1andCatgenes compared to the uninjured-water group. Interestingly, 4-AP treatment alone significantly induced an up-regulation ofParp1,Mt1,Scn10a,Sod1,Scn9a, andCatgenes in uninjured nerves compared to the uninjured-water group. Compared to the uninjured-4-AP group, while we observed a significant up-regulation ofLgals3,C1qb,slpi, andC1qagenes in the injured nerves with 4-AP treatment, there was a significant down-regulation ofMt1,Sod1andCatgenes. Compared to the crush injury-water group,IL1bgene was significantly down-regulated andLgals3,Parp1,Scn10a, andScn9agenes were significantly up-regulated in the crush inury-4-AP group. Of note, although it was not significantly different,Sod1andCatgenes in the crush injury-4-AP group was ≥ 2-fold higher than the crush injury-water group.
Discussion
The main finding of the present study is that orally administered human equivalent dosage of 4-AP improves both neuronal function and muscle atrophy in a rodent model of TPNI. We further confirm two distinct and important properties of oral 4-AP in TPNI: acute single-dose efficacy test and long-term therapeutic effects. Acute single-dose efficacy test with oral 4-AP temporarily elicited a significant improvement in the global motor function of the affected limb with crushed nerve but not in the limb with transected nerve, allowing the differentiation of these two clinically indistinguishable injuries at an early time point. Long-term therapeutic benefit with daily oral administration of 4-AP was demonstrated by improved global motor function recovery, nerve myelination, muscle atrophy, andex vivomuscle force in limbs with crushed nerves but not in limbs with complete and permanent nerve discontinuity. In addition,PCR array revealed that crush injury caused distinct up-regulation of several high-expressing and down-regulation of low-expressing genes in the injured nerve and 4-AP had significant modulatory effects on different genes in the uninjured and injured nerves. These findings provide new insights into the potential clinical use of 4-AP in TPNIs especially where an early differentiation of an injured nerve with or without axonal continuity is critical for surgical decision making surrounding the immediate need for surgical repair of nerves which are completely severed.
Traumatic peripheral nerve injuries occur along a spectrum from injuries in which some axonal continuity is maintained with demyelination (crush or compression injuries)all the way up to injuries involving complete nerve transection. Following an injury that causes demyelination, voltage-gated potassium channels are exposed and subsequent potassium leakage blocks action potential propagation causing conduction failure (Blight, 1989; Nashmi and Fehlings,2001). 4-AP is clinically used in diverse chronic demyelinating disorders because it can enhance nerve excitability by restoring conduction in demyelinated axons via potassium channel blockade. In this study, with human equivalent oral dosage, we demonstrated that 4-AP can distinctly classify a crush injury from a transection injury by supporting transient motor function recovery - an effect most likely related to nerve conduction restoration, because the time course was too rapid to be explained by an axonal regeneration. In addition to the nerve injury differentiating property, continuous daily oral 4-AP administration also promoted global limb functional improvement which was associated with improved nerve morphology and myelination (increased myelin thickness and decreased G-ratio). All of these findings are consistent with our previous studies, where chronic 4-AP treatment significantly improved motor function and nerve conduction with thicker myelin sheaths (Tseng et al., 2016;Clark et al., 2019; Mordak et al., 2019). It is apparent that improved nerve conduction and a faster communication with innervating muscle are intimately involved in 4-AP-mediated beneficial effects. Regardless of the route of administrations(oral, IP, local or transdermal), the acute single-dose efficacy in nerve injury differentiation and late long-term therapeutic benefits with 4-AP thus provide robust pre-clinical evidence for its potential use in peripheral nerve injury clinical trials.
While innervation of skeletal muscle is essential for the maintenance of muscle size, structure, and contractile function (Moresi et al., 2010), denervation results in contractile deficits and rapid muscle-fiber atrophy within the first 2 weeks post-injury (Engel and Stonnington, 1974; Day et al.,2001; Lien et al., 2008). Recently, we demonstrated that daily IP 4-AP (10 μg) treatment significantly attenuates muscle atrophy of the injured limb with increased regenerating muscle fibers and improvesex vivointrinsic contractile force of muscle. Muscle wet weight is widely used to evaluate muscle innervation after nerve injury (Sobotka and Mu, 2015). In this study, daily oral 4-AP treatment also significantly attenuated post-injury muscle loss and improvedex vivomuscle force following crush injury but not following nerve transection, further demonstrating a musculo-protective role of 4-AP against neurogenic muscle atrophy through a mechanism that may depend on nerve continuity.
Despite extensive researches with TPNIs, the molecular mechanisms of nerve regeneration are still unclear, and currently no therapeutic options exist that can promote nerve regeneration and enhance functional recovery. Recently we demonstrated that 4-AP treatment can regulate several muscle atrophy genes to attenuate neurogenic muscle atrophy(Yue et al., 2019). Although we identified several genes in the crushed nerve with different functional categories, it is beyond the scope of this study to discuss all genes. Moreover,there is a large group of genes with unknown function that need further investigation. Our interest was mainly focused on genes involved inflammatory and immune responses, cell differentiation, nerve damage and regeneration, and antioxidant function. Interleukin-1 beta (IL-1β) is a pro-inflammatory cytokine encoded byIL1bgene. Schwann cells and macrophages in the injured nerve express IL-1β (Terenghi,1999), and IL-1β can induce the production of toxic mediators, and promote inflammation and cell death (Dubovy et al., 2013). However, it is also reported that increased IL-1β could be detrimental or beneficial to nerve regeneration and functional recovery depending on the timing and degree of inflammation (Guenard et al., 1991; Wyss-Coray and Mucke,2002; Temporin et al., 2008; Dubovy et al., 2013).IL1bgene was significantly down-regulated by 4-AP treatment in the crushed nerves compared to crush injury-water group. Tumor necrosis factor is a pro-inflammatory cytokine and it is encoded byTnfgene.Tnfgene was significantly increased only in crush injury-water group. This finding is consistent with increased tumor necrosis factor protein expression within 3 days of sciatic nerve crush injury in mice and rats(George et al., 2004, 2005). Tissue inhibitor of metalloproteinases 1 is a glycoprotein encoded byTimp1gene. We observed significantly increasedTimp1gene expression only in crush injury-water group. Tissue inhibitor of metalloproteinases is an endogenous inhibitor of the matrix metalloproteinases and it is reported to play an important role in the differentiation and function of myelin-forming Schwann cells in nerve regeneration (Kim et al., 2012). Galectin is an endogenous glycoprotein encoded byLgalsgene. Galectin-1 is present in both central and peripheral nervous system (Hynes et al., 1990) and promotes axonal regeneration and recovery of locomotor activity after spinal cord injury (Quinta et al.,2014). It is also demonstrated that galectin-1 is expressed in regenerating sciatic nerves and plays an important role in the initiation of axonal growth after nerve injury (Horie et al., 1999; Horie and Kadoya, 2000). We observed significantly increased expression ofLgals3gene in the crushed nerves and 4-AP treatment further increased this expression compared to crush injury-water group. Ploy(ADP-ribose)polymerase 1 is a nuclear protein that acts at the center of cellular stress (Kauppinen, 2007; Sriram et al., 2015) and it is encoded byParp1gene. Ploy(ADP-ribose) polymerase 1’s primary function is to repair DNA and it has been implicated in many neurological diseases. Oxidative stress causes DNA damage and ploy(ADP-ribose) polymerase 1 activation is associated with DNA repair, cell death and inflammation (Sriram et al., 2014). We observed that 4-AP treatment alone significantly increasedParp1gene in uninjured-4-AP group compared to uninjured-water group and there was no further increase inParp1gene in crush injury-4-AP group.Parp1level in crush injury-4-AP group remained significantly elevated compared to crush injury-water group.Oxidative stress is one of the main causes of nerve damage after injury (Lanza et al., 2012). Antioxidant enzymes Cu,Zn-superoxide dismutase and catalase are encoded bySod1andCatgenes, respectively. Crushed nerves in water group had significantly down-regulated anti-oxidativeSod1andCatgenes compared to uninjured-water group. In contrast,4-AP treatment induced an up-regulation ofSod1andCatgenes in the uninjured nerve and it also modestly attenuated their down-regulation in crushed nerves compared to crush injury-water group. In animal models, Cu, Zn-superoxide dismutase and catalase are well reported to play an important role in peripheral nerve injury and regeneration (Varija et al., 2009; Fisher et al., 2012; Lanza et al., 2012). While the exact impact of these different genes on TPNI requires further investigations, these differentially regulated genes with crush injury and 4-AP suggest that these genes have functional significance in nerve degeneration and/or repair. Our findings with improved nerve morphology and post-injury functional recovery at least indicate that 4-AP may play an important immunomodulatory role in limiting post-injury inflammatory processes (Chandy et al., 1984; Espejo and Montalban, 2012).
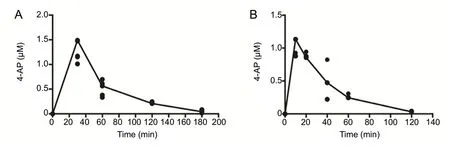
Figure 1 Serum concentrations of 4-AP from LC/MS/MS analysis.
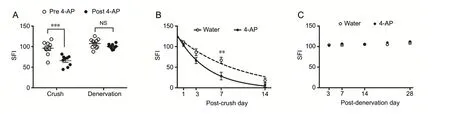
Figure 2 Effect of oral 4-AP (10 μg) administration on the motor functional recovery following sciatic nerve crush or denervation injury.
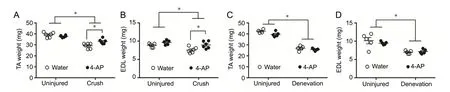
Figure 3 Effect of daily oral administration of 10 μg 4-AP or water on the muscle mass on day 14 following crush or denervation injury.
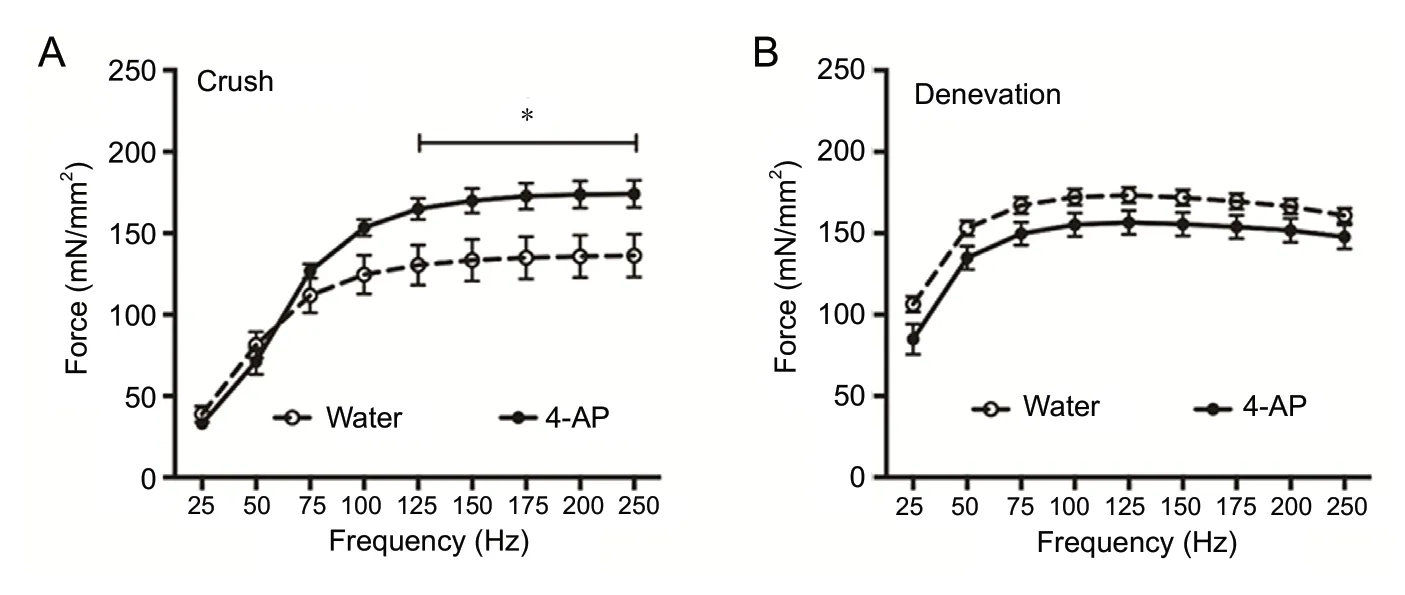
Figure 4 Effect of daily oral 4-AP (10 μg)treatment on the ex vivo contractile function in EDL muscles on day 14 following crush or denervation injury.
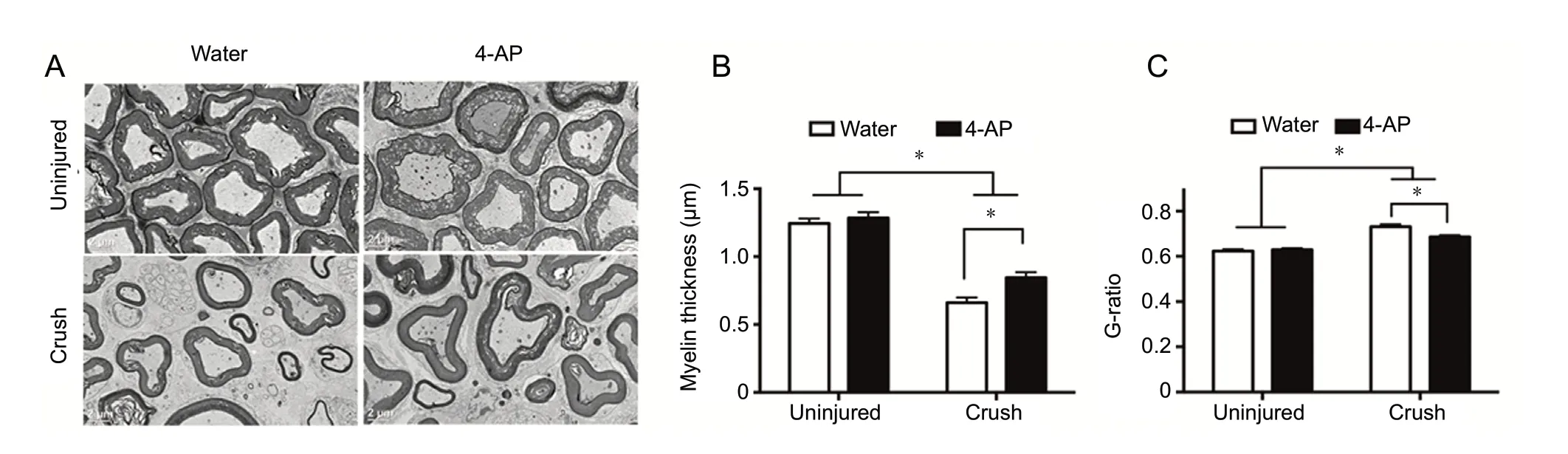
Figure 5 TEM analysis of transverse sections of sciatic nerves within the crush injury site at 14 days post-injury for the effect of oral 4-AP (10 μg) and vehicle (water) treatments on nerve myelination and G-ratio.
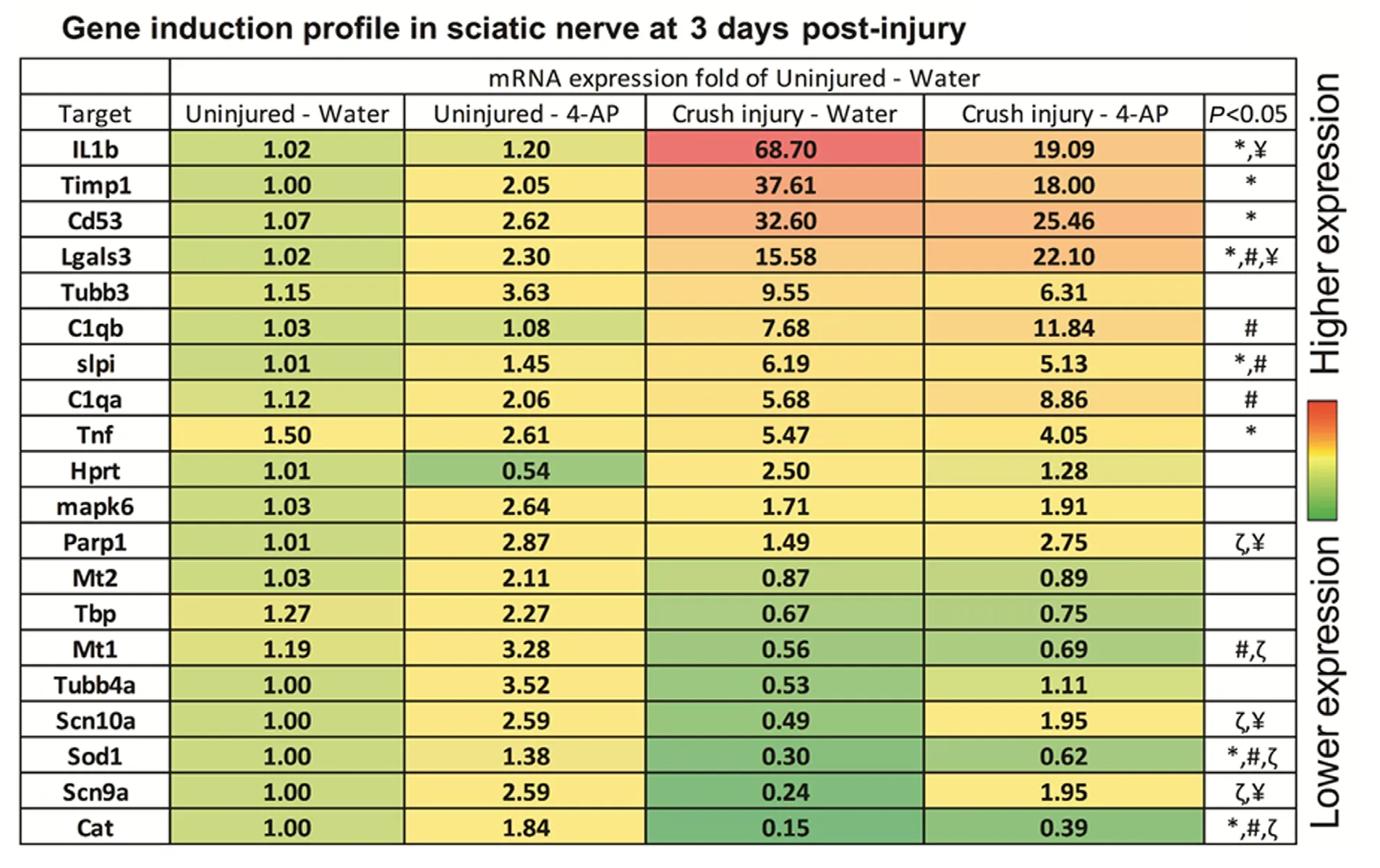
Figure 6 Heat map for quantitative real-time reverse transcription-polymerase chain reaction array data for gene expression profile in the injured (crush injury) and uninjured nerves at 3 days post-injury with or without daily oral 4-AP (10 μg) treatment.
Consistent with pharmacologic trials (Uges et al., 1982;Davis et al., 1990; Blight and Henney, 2009) and our recent study (Clark et al., 2019), we observed linear pharmacokinetics with oral and IP 4-AP where the peak serum concentrations were clearly dose dependent and the beneficial effects on the post nerve injury recovery were without side effects. Oral 4-AP both at single and daily doses conforms to our earlier pre-clinical findings with IP (Noble et al.,2019; Yue et al., 2019), transdermal (Clark et al., 2019) and/or local administration of 4-AP (Tseng et al., 2016). Taken together, we provide consistent and convincing pre-clinical evidence that 4-AP has the potential to be used in the clinic as an acute single-dose efficacy tool to differentiate TPNI patients with or without continuity, and chronically to treat TPNI patients with nerve continuity for accelerated recovery.
Author contributions:Data acquisition, analysis and interpretation,writing initial draft, and final approval: CGH; data analysis, organization and interpretation, writing initial draft, compiling, revising and editing the draft with intellectual content: MAHT; data analysis: LY; data acquisition: LCT; revising the draft: MN; concept and design of the study,funding acquisition, data organization and interpretation, revising and editing the draft with intellectual content: JCE. All authors approved the final version of the paper.
Conflicts of interest:The authors have no conflicts of interest to disclose.
Financial support:This work was supported by grants from the National Institutes of Health (NIH; K08 AR060164-01A) and the Department of Defense (DoD; W81XWH-16-1-0725) to JCE in addition to institutional support from the University of Rochester and Pennsylvania State University Medical Centers.
Institutional review board statement:The animal experiments were approved by the University Committee on Animal Research (UCAR) at the University of Rochester (UCAR-2009-019) on March 31, 2017.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files Additional Figure 1:Effect of daily intraperitoneal 4-AP (10 μg) treatment on the motor functional recovery after sciatic nerve crush or denervation injury.Additional Figure 2:Effect of daily intraperitoneal 4-AP (10 μg) treatment on the ex vivo contractile function in EDL muscles at 14 days following crush or denervation injury or sham surgery.Additional Table 1:Primers used in real-time reverse transcription-polymerase chain reaction.Additional Table 2:Selected pharmacokinetic parameters after oral or intraperitoneal 4-AP administration.
杂志排行
中国神经再生研究(英文版)的其它文章
- The role of the TrkB-T1 receptor in the neurotrophin-4/5 antagonism of brain-derived neurotrophic factor on corticostriatal synaptic transmission
- Could non-invasive brain-stimulation prevent neuronal degeneration upon ion channel re-distribution and ion accumulation after demyelination?
- The role of exercise in brain DNA damage
- Combined effect of repetitive transcranial magnetic stimulation and physical exercise on cortical plasticity
- Should mast cells be considered therapeutic targets in multiple sclerosis?
- Neuroprotection mediated by natural products and their chemical derivatives
