Synaptic receptors for low pH in extracellular space: metabotropic receptors are an underestimated factor in stroke
2020-04-29SergeiV.Fedorovich,TatsianaG.Dubouskaya,TatsianaV.waseem
In the brain, extracellular pH could decrease in certain diseases. Acidification, however, is especially attributable for stroke. Lactate accumulation in the absence of anaerobic respiration is the main, but not the only, reason for lowering pH in this condition (Wemmie et al., 2013). In addition, local pH changes in the synaptic cleft may result from the release of acidic content of synaptic vesicles (Sinning and Hubner, 2013).Acidification is able to damage or even kill a neuron. The damaging effect of low pH can be mediated by a direct effect of protons on enzymes or transport systems. It can also, however, be mediated by specific receptors (Wemmie et al., 2013; Levin and Buck, 2015). The most well studied class of pH receptors is ionotropic acid-sensing ion channels(ASIC). These are ion channels which are permeable for sodium and calcium. They open in response to extracellular acidification. Activation of these receptors could lead to neuronal death (Wemmie et al., 2013).ASIC-induced neuronal death is appeared to be involved in stroke-induced brain damage. This suggestion is supported by the fact that amiloride, which inhibits ASIC, has a neuroprotective effect in stroke (Xiong et al., 2004). Amiloride is able to reduce currents through ASIC types,however, the exact structural basis for this phenomenon is still not very clear (Wemmie et al., 2013). Generally, the majority of hormones and neurotransmitters exert their action via two main types of receptors.These are ionotropic and metabotropic receptors. The function of ionotropic channels is mediated by ion channel opening and ion fluxes through plasma membranes. The function of metabotropic receptors is mediated by G-proteins and enzymatic reactions. In this case, a cellular response will be slower and it will be rather fine tuning than the “all-ornothing” effect. This seems to be the key difference between ionotropic and metabotropic receptors. Based on the knowledge in this field, the existence of both metabotropic and ionotropic receptors, which could respond to changes in extracellular pH, is possible. By now, proteins with this function have been found on plasma membrane of eukaryotic cells (Levin and Buck, 2015). The role of metabotropic receptors for low pH in regulation of activity of central nervous system cells, however, is still not very understood. Recently, we have shown that the activation of metabotropic receptor for low pH on plasma membrane of isolated neuronal presynaptic endings, most likely ovarian cancer G-protein-coupled receptor 1 (OGR1), leads to mitochondria depolarization(Dubouskaya et al., 2018).
Extracellular pH could drop to pH 5.5 during severe ischemia. Obviously, this pronounced increase in proton concentration must influence virtually all processes in cells. Indeed, strong acidification kills a neuron. A weaker lowering of pH, however, can also lead to certain deleterious consequences. We have shown that the decrease in pH even from 7.4 to 7.0 induces free radical accumulation and mitochondria depolarization in synaptosomes, isolated neuronal presynaptic endings from rat brain (Pekun et al., 2013). This fact indicates no strict threshold for low pH impact. Synapses damaged by free radicals could also underlie the loss of their functionality. We have also shown that the effects of exracellular pH on investigated processes are significantly higher than the effects of intracellular pH (Pekun et al., 2013). These findings imply an involvement of a plasma membrane receptor. A chemical signal generated by a hypothetic receptor could reach mitochondria. ASIC expression has been shown inC. eleganssynapses, but in rat synaptosomes we did not record significant alterations in sodium and calcium transport upon lowering pH (Dubouskaya et al., 2018). This fact led us to suggest that ionotropic ASIC-receptors are unlikely key players in pH-dependent oxidative stress and mitochondria depolarization in the presynaptic area.
There are a variety of molecules which are worth to discuss as potential metabotropic receptors for changes in extracellular pH. Three types of G-protein coupled receptors (GPCR) exist: GPR4, OGR1 and the product of T-cell death-associated gene 8 (Levin and Buck, 2015).Moreover, insulin related receptor linked with tyrosine-specific protein kinase was shown to be able to respond to changes in extracellular pH.Interestingly, insulin related receptor, unlike to GPCR, is able to detect alkaline pH shifts. Therefore, insulin related receptor could be ruled out(Levin and Buck, 2015). There is also a group of proteins which might serve as intracellular receptors for pH but they are out of scope of this paper.
Some GPCRs have been found in neurons. Notably, they are more sensitive to changes in pH than ionotropic receptors. Protonation of histidine residues in GPCRs occurs at pH 6.8 followed by activation of heteromeric G-protein. Depending on the type of G-protein the downstream activation of adenilate cyclase modulates the cyclic adenosine monophosphate level or, alternatively, activation of phospholipase C causes accumulation of inositol triphosphate and calcium release from endoplasmic reticulum. OGR1 is linked to Gq-protein and inositol triphosphate-dependent pathway of intracellular signaling. In contrast,GPR4 activates Gs-protein and cyclic adenosine monophosphate-dependent pathway of intracellular signaling (Levin and Buck, 2015).
Contemplating a role of GPCRs as pH-receptors, it is expected that deflections in mitochondria potential and reactive reactive oxygen species accumulation will be sensitive to compounds modulating cyclic adenosine monophosphate-dependent signaling or inhibitors of phospholipase C. Recently, we have shown that in rat synaptosomes the depolarization of mitochondria induced by extracellular acidification is inhibited by phospholipase C blocker U73122 (Dubouskaya et al.,2018). Furthermore, we have found two evidences for intracellular calcium stores involvement. First, changes in mitochondria potential were sensitive to thapsigargin which inhibits the calcium pump in the endoplasmic reticulum. Second, extracellular acidification itself elevated cytosolic calcium level without accompanying45Ca transport across the plasma membrane in synaptosomes (Dubouskaya et al., 2018). Therefore, in neuronal presynaptic terminals the main pH-sensor is likely to be a metabotropic receptor rather than ionotropic. Identification of a particular protein is complicated by the lack of specific inhibitor. A role for OGR1, however, is corroborated by the sensitivity of pH effects to low concentrations of copper and zinc (Ludwig et al., 2003; Dubouskaya et al., 2018).
Another intriguing question is what happens after activation of the OGR1-dependent pathway of intracellular signaling. The main physiological function of presynaptic endings is neurotransmission. This process is triggered by calcium. Calcium signaling, however, acts very locally. Exocytosis of glutamate-containing synaptic vesicles requires such a high local calcium concentration which is never reached in cytosole. The required calcium levels arise for a fraction of a second very close to the mouth of votage-gated channels (Sudhof, 2013). Calcium release from the endoplasmic reticulum upon extracellular acidification is therefore unlikely a trigger for spontaneous neurotransmitter release.This suggestion is further supported by our earlier findings that even moderate extracellular acidification down to pH 7.0 does not induce glutamate release from synaptosomes (Fedorovich et al., 2003). It is well-known that the endoplasmic reticulum is closely associated with mitochondria. Proceeding from this fact extracellular acidification would be expected to drive a redistribution of calcium between the endoplasmic reticulum and mitochondria. We have demonstrated this using fluorescent dye Rhod-2 which specifically reflects the changes in mitochondrial calcium levels (Dubouskaya et al., 2018). An increase in mitochondrial calcium levels seems to be the main reason for depolarization of this organelle with the subsequent synthesis of superoxide anion radicals.
An illustrative scheme of putative events occurred in neuronal presynaptic endings at moderate extracellular acidification (pH 7.0) is shown in Figure 1. Proton binding to OGR1 leads to Gp-dependent activation of phospholipase C, followed by inositol triphosphate synthesis. Next, inositol triphosphate binds to its receptor on the endoplasmic reticulum and induces calcium release. An excess of calcium is accumulated by mitochondria and this might trigger the subsequent depolarization and dysfunction of mitochondria. Underlying mechanisms for mitochondrial depolarization and especially for oxidative stress after strong acidification (pH 6.0) could be different (Pekun et al.,2013).
Synaptic transmission is accompanied by changes in pH within a synaptic cleft. This is caused by the release of the acidic content (pH~5.5) of synaptic vesicles. Released protons which transported from presynaptic and postsynaptic neurons during pH regulation would also contribute to these changes (Sinning and Hubner, 2013). Highly sensitive metabotropic receptors including OGR1 could provide the feedback between these small local changes in pH and presynaptic physiology.
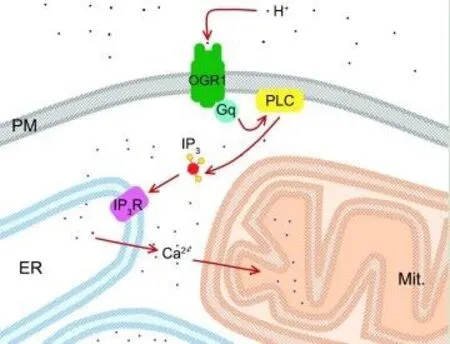
Figure 1 Putative molecular events after moderate extracellular acidification in isolated neuronal presynaptic endings (pH 7.0).
It is shown that ionotropic pH receptors are tightly involved in neuronal death in the acute brain ischaemia when pH drops to the extreme values such as pH 5.5 (Wemmie et al., 2013). Moreover, the chronic brain ischaemia could also induce vascular dementia in addition to direct brain injury (Iadecola, 2013).
Cellular and molecular mechanisms of vascular dementia are still not clear. It is established that synapse elimination has a key role in the development of dementia in Alzheimer’s disease (Morrison and Baxter,2012). This process can be more important than neuronal death. Potentially, synapse damage induced by metabotropic receptors for low pH would also be crucial for developing vascular dementia.
Finally, there are two important questions. What is the function of metabotropic receptors for low pH in normal conditions? What is the mechanism by which they could damage cells in different pathologies accompanied by extracellular acidification? As mentioned above,calcium released from the endoplasmic reticulum is unable to induce exocytosis. However, an increase in total cytosolic calcium levels can reduce a threshold required for exocytosis. Additionally, for the first time we have shown effects of metabotropic receptors located on the plasma membrane onto mitochondria (Pekun et al., 2013; Dubouskaya et al., 2018). Therefore, an additional function of these proteins may be the adaptation of neuronal presynaptic endings metabolism to excessive acidification of synaptic cleft. These changes in pH can serve as the feedback mechanism between synaptic activity and metabolism. On the other hand, in certain pathological conditions, for instance, chronic ischaemia, continuous activation of synaptic metabotropic receptors can lead to energy depletion of neuronal presynaptic endings. Furthermore, this could be the first step to synapse elimination, neurodegeneration, and ultimately to dementia.
ASICs were shown to be important for perception of pain and fear.Inflammation is generally accompanied by acidification. Therefore, it is reasonable to suggest the existence of a mechanism informing the brain about pH changes. Processes of fear and behavior regulation are very complex. It is established that CO2inhalation leads to fear and uncontrolled panic in different animals and humans. CO2inhalation leads to acidosis which can be detected by ionotropic pH receptors,and indeed elimination or inhibition of ASICs reduces this behavioral response (Wemmie et al., 2013). Fear and anxiety which are developed via ASIC-dependent pathway could help to overcome stress situations and restore normal breathing. It is unknown whether metabotropic pH receptors are also involved in implementing any behavioral programs.
Our results suggest that metabotropic receptors for low pH including OGR1 would be a perspective target for new drugs against diseases which are accompanied by acidification, primarily vascular dementia.Unfortunately, the exploration of both ionotropic and metabotropic pH receptors is slowed down by the absence or limited number of specific inhibitors. It has recently been demonstrated that some traditional Chinese medicines target ionotropic pH-sensitive ASICs (Wemmie et al.,2013). It is possible that OGR1 inhibitors can be found among natural compounds.
We thank Dr. Daniel Gerrard for English language editing to improve the manuscript.
This work was supported by the Belorussian Republican Foundation of Basic Investigation (grant B19-001).
Sergei V. Fedorovich*, Tatsiana G. Dubouskaya,Tatsiana V. Waseem
Institute of Biophysics and Cell Engineering, Minsk, Belarus(Fedorovich SV, Dubouskaya TG)
Department of Biochemistry, Belarusian State University, Minsk,Belarus (Fedorovich SV)
Department of Pharmacology, University of Oxford, Oxford, UK(Waseem TV)
*Correspondence to:Sergei V. Fedorovich, PhD,fedorovich@ibp.org.by or sergeifedorovich@yahoo.co.uk.
orcid:0000-0003-4474-1954 (Sergei V. Fedorovich)
Received:January 8, 2020
Peer review started:January 19, 2020
Accepted:March 7, 2020
Published online:May 11, 2020
doi:10.4103/1673-5374.282249
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- The role of the TrkB-T1 receptor in the neurotrophin-4/5 antagonism of brain-derived neurotrophic factor on corticostriatal synaptic transmission
- Could non-invasive brain-stimulation prevent neuronal degeneration upon ion channel re-distribution and ion accumulation after demyelination?
- The role of exercise in brain DNA damage
- Combined effect of repetitive transcranial magnetic stimulation and physical exercise on cortical plasticity
- Should mast cells be considered therapeutic targets in multiple sclerosis?
- Neuroprotection mediated by natural products and their chemical derivatives
