Gut microbiota and nutrient interactions with skin in psoriasis: A comprehensive review of animal and human studies
2020-04-25GiovanniDamianiNicolaLuigiBragazziThomasMcCormickPaoloDanieleMariaPigattoSebastianoLeoneAlessiaPacificoDanicaTiodorovicSvevaDiFrancoAnielloAlfieriMarcoFiore
Giovanni Damiani,Nicola Luigi Bragazzi,Thomas S McCormick,Paolo Daniele Maria Pigatto,Sebastiano Leone,Alessia Pacifico,Danica Tiodorovic,Sveva Di Franco,Aniello Alfieri,Marco Fiore
Giovanni Damiani,Thomas S McCormick,Department of Dermatology,Case Western Reserve University,Cleveland,OH 44106,United States
Giovanni Damiani,Paolo Daniele Maria Pigatto,Clinical Dermatology,IRCCS Istituto Ortopedico Galeazzi,Milan 20122,Italy
Giovanni Damiani,Paolo Daniele Maria Pigatto,Department of Biomedical,Surgical and Dental Sciences,University of Milan,Milan 20122,Italy
Nicola Luigi Bragazzi,Postgraduate School of Public Health,Department of Health Sciences,University of Genoa,Genoa 16132,Italy
Sebastiano Leone,Department of Medicine,Division of Infectious diseases,“San Giuseppe Moscati” Hospital,Avellino 83100,Italy
Alessia Pacifico,San Gallicano Dermatological Institute,IRCCS,Rome 00144,Italy
Danica Tiodorovic,Dermatology Clinic,Medical Faculty,Nis University,Nis 18000,Serbia
Sveva Di Franco,Aniello Alfieri,Marco Fiore,Department of Women,Child and General and Specialized Surgery,University of Campania "Luigi Vanvitelli",Naples 80138,Italy
Abstract
The intestinal tract(i.e.,the gut),is where the body's nutrients are absorbed,and is simultaneously inhabited by numerous microbes.An increasing body of literature suggests a crucial role for the gut microbiome in modulating systemic inflammatory disease.Psoriasis is a chronic systemic inflammatory disease and its pathogenesis is related to the interaction between genetic susceptibility,immune response and environmental triggers.The omics era has allowed physicians to assess different aspects of psoriasis pathogenesis such as the microbiome,infectome,and autoinfectome.Furthermore,diet appears to play an important role in modulating disease activity,perhaps by influencing gut microbes.Given these observations,we aimed to summarize the current knowledge regarding skin-microbiome-gut-nutrients and psoriasis.
Key words:Gut;Microbiota;Nutrients;Endotypes;Exposome;Psoriasis
INTRODUCTION
Psoriasis is alternatively regarded as an inflammatory[1],pruritic[2],autoimmune[3,4],or even autoinflammatory[5]systemic chronic disease affecting not only the skin but the whole body,providing a potential explanation for the numerous comorbidities discovered in relation to this disease[6-12].The biomarker research performed in the past decades identified substantial inadequacies in describing the wide spectrum of psoriasis[13-15],and failed to explain the complexity of psoriasis endotypes[16].The genetic background of psoriasis patients reveals that deficiency of the interleukin 1(IL-1) receptor antagonist and deficiency of the IL-36 receptor antagonist are the only recognized mutations[17],conversely other psoriatic forms are considered complex diseases,due to the intricate interaction between inherited susceptibility alleles and environmental triggers[18].In addition,epigenetic modifications seem to play a pivotal role in developing psoriasis,establishing environmental triggers as potential modulatory factors[19]as well as in response to anti-psoriatic therapies[20].These new findings provide a rationale for observed treatment loss-of-response and biologically justify switching to alternative treatments in patient management[21-24].Furthermore,an increased body of evidence suggests a crucial role for the gut microbiome in modulating psoriasis;linking the skin and gut microbiome[25].The gut is the site where nutrients are absorbed and at the same time is inhabited by nutrient-modifying microbes.The Skin-Microbiome-Gut-Nutrient interaction is still only partially understood;therefore,this review aimed to summarize the current knowledge in this field.
PSORIASIS,DIET AND CIRCADIAN RHYTHM
Psoriasis is associated with metabolic and cardiovascular disease[6,26,27].It has been widely demonstrated that genetic and environmental factors including nutrition,may strongly influence psoriatic pathogenesis and disease progression[28,29].Increased body mass and a high fat diet may trigger as well as exacerbate psoriasis[29].Moderate to severe psoriasis is frequently associated with numerous metabolic disorders including obesity,diabetes,dyslipidemia,metabolic syndrome and non-alcoholic fatty liver disease[30].Fatty acids are increased in obese patients,leading to augmented inflammation and insulin resistance.Furthermore,obesity may also affect drug pharmacokinetics and pharmacodynamics[29].The option to treat psoriasis patients with phototherapy is affected by body mass index;obese patients require an excessive amount of photosensitizing drug which may lead to toxicities.Obesity is also an important risk factor for psoriasis,given that the relationship between obesity and psoriasis is mutually interdependent[29].Recent evidence has suggested an addition role of vitamin D in the pathogenesis of several inflammatory skin diseases including psoriasis[31].An association between low levels of vitamin D and psoriasis has been described[31].Vitamin D also participates in keratinocyte proliferation and maturation.Nevertheless,the potential value of vitamin D supplementation for psoriasis is stillunder debate[31].Dietary antioxidants including omega 3 polyunsaturated fatty acids derived from fish oil,vitamin B12,vitamin D and selenium have the capacity to decrease oxidative stress and consequently lower reactive oxygen species production,and this could be relevant in the pathogenesis of psoriasis.Based on these findings,the Mediterranean diet has been proposed to slow disease progression[32,33].All of these endogenous factors contribute to the composition of the so-called “exposome”,a measure of the whole complex of endogenous,ingested and not ingested,substances that interact with the body that are capable of perturbing,modifying and modulating vital functions[34].Exposures capable of modulating psoriasis include tobacco,which increases severity,flare frequency,and even the incidence of the psoriasis[35],alcohol[36]and pollutants[37],even if the role of both alcohol and pollutants in psoriasis is still debatable.Interestingly,the use of marijuana was recently related to secukinumab resistance in a cohort of erythrodermic patients,focusing the attention on addiction screening during intake medical history of psoriatic patients[38].Anti-psoriatic drugs are also capable of modulating taste,and consequently,the patient's diet,as recently described for both methotrexate[39]and apremilast[40].
Since the melatoninergic system was discovered in the epidermis,circadian rhythm has been regarded as a possible modulator of inflammation[41],healing[42],aging[43],neuroendocrine[44],and neoplastic conditions[45].The cyclic nature of sunlight which influences the suprachiasmatic nucleus(central clock) also influences other peripheral tissue,including skin(peripheral clock),in different ways that are slowly beginning to be understood by examining the mechanism(s) of their dysfunction[46,47].The shift in circadian rhythm may be occasional and transitory,as in the case of jet-lag during intercontinental flights[48],or in the case of the Ramadan fasting months for Muslims[49,50],or more chronic,as in night shift workers[51,52];however this effect is particularly evident in psoriatic patients.Interestingly,night-shift workers exhibited not only an increase in severity of psoriatic flares,but also an increased incidence of psoriasis,suggesting that shifting the circadian rhythm(i.e.,sleep and diet) may be a risk factor for psoriasis[52].Sunlight,in the form of narrow band UVB(NB-UVB),may also be curative for psoriatic skin,allowing a reprograming of the circadian clock and inhibition of autoimmune phenomena[53],however skin may also marginally adapt to NB-UVB,an outcome termed photoadaptation[54].Thus,sunlight is considered an integral part of the exposome.
PSORIASIS,NUTRIENTS AND SKIN CANCER
The possible influence of nutrients on gene expression and clinical progression or remission of psoriasis is not fully explored and may represent the main challenge in approaching complementary therapy for psoriasis.It is reported that many dietetic factors may exert beneficial effects,while others can aggravate inflammatory and immune networks,thus leading to psoriasis comorbidities[55].Previous studies have reported the positive effects of low-energy diets,vegetarian diets,formula diet weight loss programs,gluten-free or very low-calorie carbohydrate-free diet.It is believed that certain vitamins(e.g.,A,E and C),and oligo-elements(e.g.,iron,copper,manganese,zinc,and selenium) are anti-oxidants,leading to a reduction in oxidative stress and decreased production of reactive oxygen species[55].In fact,the disruption of cell redox signaling and involvement of oxidative stress in the pathogenesis of psoriasis was previously suggested,indicating that the potential therapeutic use of dietary antioxidants in psoriasis may represent a novel complementary strategy[56].Although limited data exist regarding the role of specific diet regimens in psoriasis,the main goal for clinicians is to reduce cardiac risk factors and obesity-related comorbidities.
Interestingly,psoriatic patients displayed a higher risk of cancer compared to the general population;furthermore,this increase is not fully explained by anti-psoriatic immunosuppressive therapies,so several real-life or ecological studies have suggested an intimate relation with diet[57].This theory is supported by the evidence that different foods modify microRNA expression in psoriatic patients[28].The influence of a restrictive caloric diet was documented to be beneficial in relapsing plaque psoriasis,and may also decrease the risk of cancer[58-60].Some lipid components,such as omega-3 polyunsaturated fatty acids may protect cells against UV-induced DNA damage by increasing the expression of tumor-suppressor protein p53,thus promoting cell cycle arrest and preventing melanoma development[61-63].Solid cancers in psoriasis have been reported,especially those linked to alcohol and smoking.A higher risk of non-melanoma skin cancers,especially squamous cell carcinoma has been shown,possibly as a result of previous exposure to 8-methoxypsoralen-ultraviolet-A(PUVA),cyclosporin,tumor necrosis factor-inhibitorsand/or methotrexate[64-66].Consideration of malignancy risk associated with individual treatments and personal nutritional phenotype may help clinicians to make optimal therapeutic decisions for individual patients.
MOUSE MODEL OF PSORIASIS AND DIET
The "omics" era has provided researchers with new powerful techniques(e.g.,metagenomics) and has elucidated a myriad of dysregulated immune responses that are heavily associated with the gut microbiota.Previous research suggested that ingested nutrients heavily affect the body's microbial composition and community.This has led to experimental approaches in mouse models of psoriasis.In recent years,nutrition and microbial influence has been heavily implicated in psoriasis onset and disease severity.The contribution of nutrients in mouse models has provided valuable insight into human disease regulation.For instance,12-O-tetradecanoylphorbol-13-acetate(TPA),a known inflammatory signal transducer,can induce psoriasis-like skin lesions in mice,while lesions and proinflammatory cytokine expression were significantly reduced in TPA-induced psoriasis by tangerine-derived nutrient flavonoids: Nobiletin(Nob) and 5-hydroxy-6,7,8,3′,4′-pentamethoxyflavone(5-HPMF)[67].In addition,obesity,a result of poor nutrition,has been shown to exacerbate the severity of psoriasiform dermatitis in imiquimod-induced rodent models[68].Collectively,these results support the need to elucidate nutritional impact on psoriasis severity.Research has suggested that monounsaturated fatty acid-rich diets,such as the Mediterranean Diet have anti-inflammatory effects and slow the progression of psoriasis in patients[33].Poor nutrition has been associated with dysregulated metabolic functions and even more so,has been shown to be critical in skin disease metabolic homeostasis compared to healthy individuals.Previous research has shown evidence for biochemical skin barrier restoration through topical administration of solenopsin,a compound of fire ant venom chemically similar to ceramides,and its derivates by reducing inflammatory markers and improving acanthosis in KC-Tie2 mice,an established rodent-model of psoriasis[69].
PSORIASIS AND THE MICROBIOME
Several lines of evidence confirmed the relationship between skin microorganisms and psoriatic lesions,such asGroup A β-hemolytic streptococcal infectionslinked to guttate psoriasis[70].Other microorganisms includingStaphylococcus aureus,MalasseziaandCandida albicansalso appear to be involved in psoriasis pathogenesis[71].The deep inter-relationship between the mycobiome and microbiome seem to act as a diseasemodifier in psoriatic patients.Although it is well known that the gut and skin microbiome deeply interact,sparse information is available on the gut and skin mycobiome.Using high-throughput 16S rRNA gene sequencing,Alekseyenkoet al[72],found that psoriatic plaques had an abundance of the following bacteria:Corynebacterium,Propionibacterium,Staphylococcus,andStreptococcus.Bakeret al[73],found that peptidoglycan,a cell wall component of Gram-positive bacteria includingStreptococciandStaphylococci,acts as a T cell activator in psoriasis.The authors observed that dermal papillae and cellular infiltrates of guttate and chronic plaque skin lesions had higher numbers of peptidoglycan-containing cells compared to nonlesional psoriatic skin.Psoriatic dermalStreptococcal- andStaphylococcal-specific CD4+T cell lines proliferated and produced IFN-alfa in response to therespectivepeptidoglycan structures.Overall,these results suggest that peptidoglycans may be responsible for T cell activation in psoriasis.Moreover,some studies have linked gut microbiota and psoriasis.
Up to 10% of patients with inflammatory bowel disease(IBD) are diagnosed with psoriasis[74].Patients with psoriasis have a 3-fold higher risk of developing Crohn's disease as compared to the general population;and Crohn's disease patients have a 7-fold higher risk of developing psoriasis[75].Recently,Scheret al[76],using pyrosequencing,found that patients with psoriatic arthritis and patients with skin psoriasis had a decreased bacterial diversity and a reduced relative abundance of some bacterial taxa such asAkkermansia,Ruminococcus,andPseudobutyrivibrio,as compared to healthy controls.Among the risk factors for psoriatic diseases summarized in Table 1,overall,the alteration of gut microbiota may translate into physiological consequences including poor regulation of intestinal immune responses that may then affect distant organ systems[77-85].Given the gut microbiome's influence on the Gut-Skin axis,probiotic supplementation may have a promising role in the management of psoriatic patients.On this point,Guenicheet al[77],in a randomizeddouble-blind placebo-controlled clinical study,showed that oral supplementation with the probiotic strainLactobacillus paracaseidecreased skin sensitivity and increased the rate of barrier function recovery.
INFECTOMICS AND AUTOINFECTOMICS IN PSORIASIS
The term “exposome” defines all environmental factors,including infectious and noninfectious agents,to which a human is exposed over a lifetime[80].The “microbiota” is a term used to describe the 10-100 trillion symbiotic microbes harbored by each human;the “microbiome” consists of the genes that these microbes harbor[81];the“infectome” is a part of the microbiome,referring to the collection of human exposure to infectious agents;the “autoinfectome” describes a part of the microbiome that includes the infectious agents linked to the presence of autoimmune diseases[82].Figure 1 summarizes the main interactions between the exposome,microbiome,infectome and autoinfectome.Recently a systematic review,which included 933 psoriatic arthritis patients and 1611 controls,aimed to evaluate the link between infections(viral and bacterial infections) and the risk of psoriatic arthritis and reported a controversial result that exhibited a trend but failed to achieve significance[83].However,differences exist between infection,colonization and dysbiosis,as suggested by several studies highlighting a different mycobiome and microbiome in psoriasis,psoriatic arthritis and control subjects[84].In fact,a dysregulation in the ratio ofFirmicutes/Bacteroideteswas highlighted in the gut microbiome of psoriatic patients;furthermore,Actinobacteriawas reduced in the gut of psoriatic patients.Gut dysbiosis was also found to be related to skin dysbiosis as decreased beta-diversity in psoriatic skin microbiome is related to an increased risk of developing psoriatic arthritis,and skin flora are now regarded as possible sensitive and specific biomarkers to predict comorbidities in psoriatic patients[84].The skin microbiota in psoriasis patients seems to be less diverse when compared to healthy persons with a decrease inCoprococcusspecies[76],and more recentlyAkkermansia muciniphila[85].The characteristic proinflammatory mediators of psoriatic skin lesions have been reported to be the innate antimicrobial peptides and proteins(AMPs).AMPs are a diverse group of small molecules(12-100 amino acid residues) that constitute the primary effector system of innate immunity against microbes[86].Although medical history certainly plays a crucial role in psoriasis management,it has some limitations such as recall bias.In particular,it has already been demonstrated that not all infections or even dysbiosis that are not clinically evident are still capable of triggering an immune/autoimmune response.Currently,there is limited,evolving information suggesting some benefit from fecal microbiota transplantation,although durability of response is a concern and currently under investigation[87,88].
TREATMENT OF PSORIASIS: TAKING INTO ACCOUNT INTERACTIONS WITH DIET AND MICROBIOME
Drug therapies are an important consideration for alteration of the cutaneous and gut microbiome[78,89]as well as the mycobiome;however,few studies have explored this topic,and are summarized in Table 2.
To date IL-17/IL-17RA signaling has been demonstrated to be a key component in regulatingCandidain the gut microbiome[79].Furthermore,psoriatic arthritis and IBD have genetic and environmental similarities,highlighting that microbiome dysbiosis may affect autoimmune diseases[78].T-cell activation is an important mechanism of psoriasis,and dysbiosis has been associated with the differentiation of T-cells into effector T cells with fewer regulatory T-cells resulting in changes in the levels of cytokines.In particular,Th17 inhibitors produced the best response compared to patients treated with tumor necrosis factor-α and IL-23 inhibitors[78].It is interesting to note that this Th-17 mediated response may not translate to the skin,as the skin microbiome could prevent the development of psoriatic plaques in these individuals[3,79].It is possible that transplanting fecal microbiota could improve or resolve the dysbiosis present in psoriatic arthritis[79].Fecal microbiota transplants have been used with success in IBD.In fact,Kragsnaeset al[90]are currently exploring fecal microbiota transplantation(FMT) in patients with psoriatic arthritis currently on methotrexate to examine their treatment response.Evaluating evidence of the efficacy of FMT is likely due to be complicated by various factors including antibiotic use,prior psoriasis therapy,subtype of psoriasis and comorbidities which similarly affect the gut or skin microbiome[91].Future studies are needed to identify potentialmodulators of the gut and skin microbiome,and identify which medications would be optimal for a patient's individual microbiome signature.
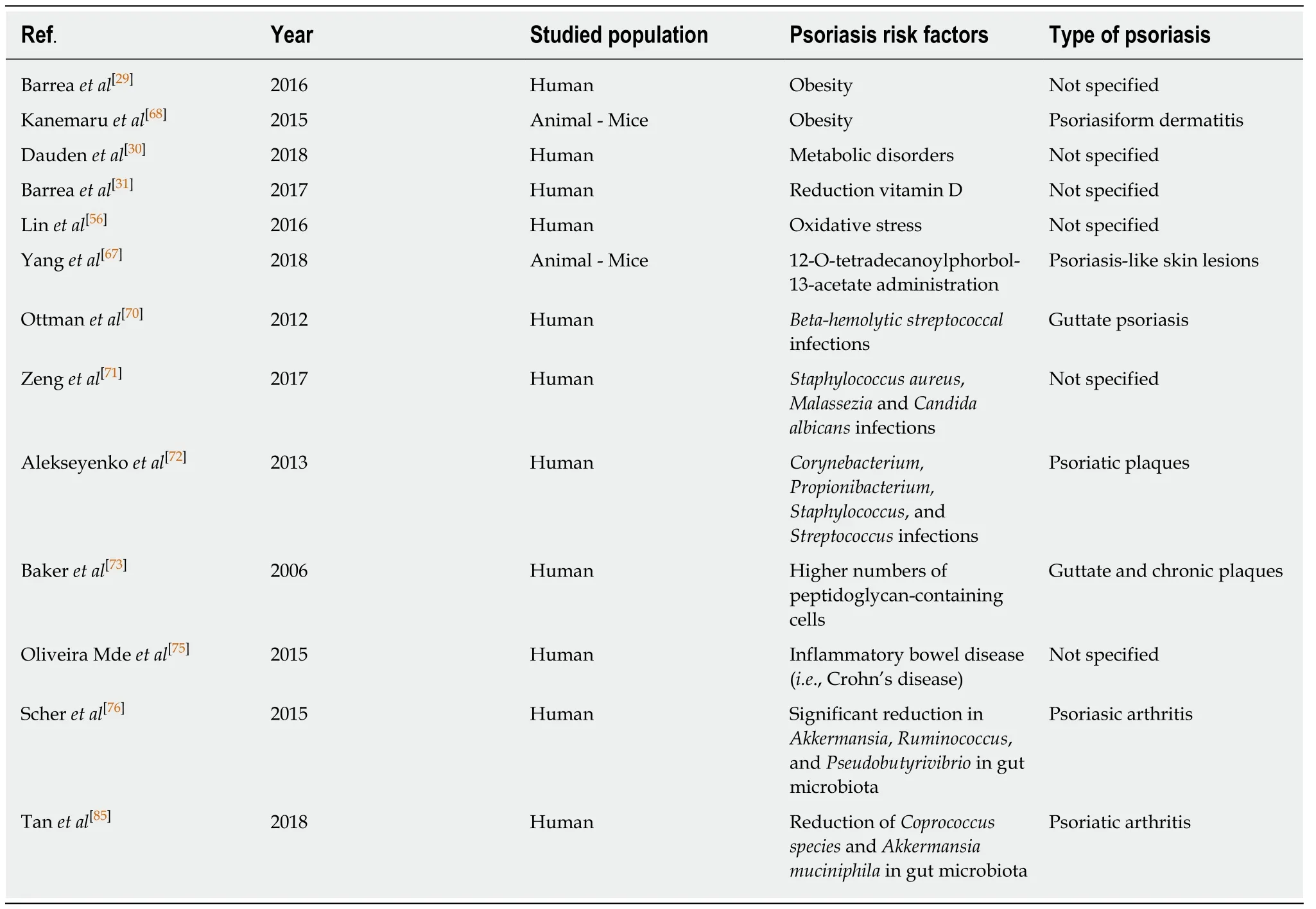
Table 1 Synthesis of psoriasis risk factors reporting reference number,first author surname,year of publication,population risk factors reported in each study and the type of psoriasis
CONCLUSION
The interaction of Microbiome-Gut-Nutrients in psoriasis is beginning to be understood with the advent of improved omics technologies and their possible integration with each other in order to more precisely separate psoriasis patient endotypes.The transition from immune-targeted therapy to precision-based therapy will be based on the mix between biological signature,the endotype,and potential specific interaction within the exposome.
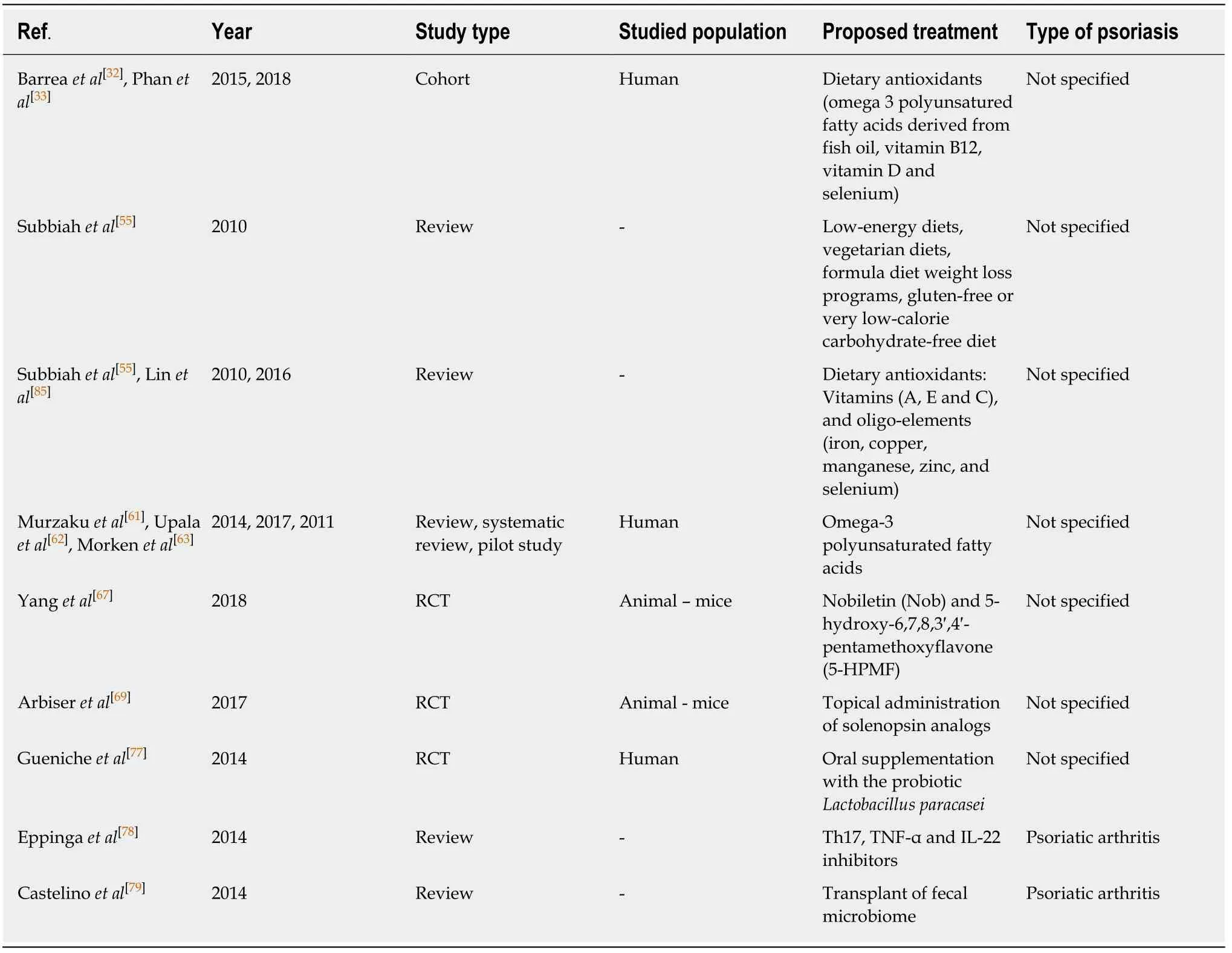
Table 2 Synthesis of psoriasis proposed treatments including reporting reference number,first author surname,year of publication,population risk factors reported in each study and the type of psoriasis
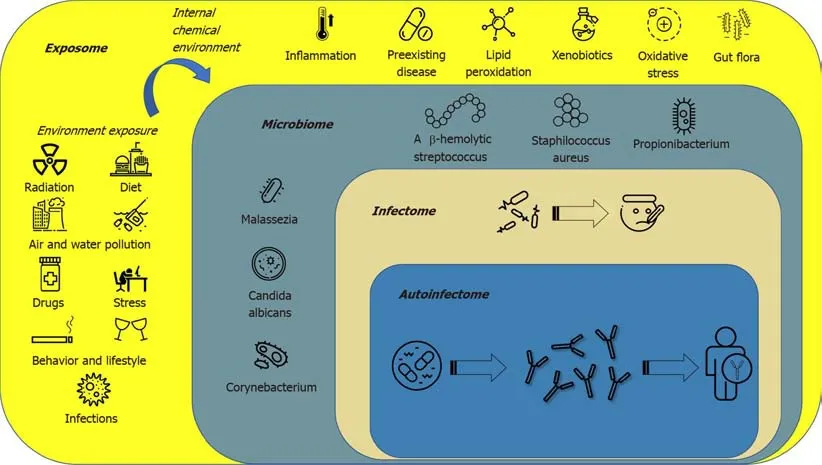
Figure 1 The figure describes the complex panorama of the exposome.
杂志排行
World Journal of Clinical Cases的其它文章
- Microbiota-gut-brain axis and its affect inflammatory bowel disease:Pathophysiological concepts and insights for clinicians
- Distal esophageal spasm: Update on diagnosis and management in the era of high-resolution manometry
- Clinical course of percutaneous cholecystostomies: A crosssectional study
- Clinical characteristics and 28-d outcomes of bacterial infections in patients with hepatitis B virus-related acute-on-chronic liver failure
- Application of hybrid operating rooms for treating spinal dural arteriovenous fistula
- Ruxolitinib add-on in corticosteroid-refractory graft-vs-host disease after allogeneic stem cell transplantation: Results from a retrospective study on 38 Chinese patients
