姜黄素通过活化SIRT1拮抗CCl4诱导的小鼠肝脏纤维化
2020-04-20申昌军张帅李浩鹏
申昌军 张帅 李浩鹏

[摘要] 目的 探討沉默信息调节因子1(SIRT1)在四氯化碳(CCl4)致小鼠肝纤维化肝脏中的活性变化及姜黄素对肝纤维化的干预作用与分子机制。 方法 选取C57BL/6小鼠24只,随机分为正常组、模型组和姜黄素治疗组,每组8只。腹腔注射CCl4制备肝纤维化模型,建模后姜黄素治疗组给予0.5%羧甲基纤维素钠姜黄素悬液灌胃;模型组及正常组给予0.5%羧甲基纤维素钠灌胃,6周后处死小鼠。HE染色检测小鼠肝脏病理变化情况;检测各组血清谷丙转氨酶(ALT)、谷草转氨酶(AST)、白细胞介素-6(IL-6)、肿瘤坏死因子-α(TNF-α)、SIRT1活性;转化生长因子-β1(TGF-β1)、α-平滑肌肌动蛋白(α-SMA)、Ⅰ型胶原(CollⅠ)mRNA表达,及Cleaved-caspase3、CollⅠ蛋白表达情况。 结果 模型组血清中ALT、AST、IL-6、TNF-α的表达量均显著高于正常组(均P < 0.01),而治疗组血清ALT、AST、IL-6、TNF-α表达量均低于模型组,差异均有高度统计学意义(均P < 0.01);模型组肝脏组织细胞中SIRT1的酶活性低于正常组,差异有高度统计学意义(P < 0.01)。治疗组SIRT1的酶活性明显高于模型组,差异有高度统计学意义(P < 0.01)。模型组肝脏组织中TGF-β1、α-SMA的mRNA表达及CollⅠ表达量显著高于正常组,Cleaved-caspase3显著低于正常组,差异均有高度统计学意义(均P < 0.01),治疗组TGF-β1、α-SMA的mRNA表达及CollⅠ表达量低于模型组,Cleaved-caspase3显著高于模型组,差异均有高度统计学意义(均P < 0.01)。 结论 姜黄素对CCl4诱导的肝纤维化具有抑制作用,其机制可能是通过上调SIRT1活性,减轻炎性反应,抑制肝星状细胞的活化,拮抗肝脏纤维化。
[关键词] 肝脏;纤维化;姜黄素;炎性反应;沉默信息调节因子1
[中图分类号] R285.5 [文献标识码] A [文章编号] 1673-7210(2020)03(c)-0011-05
[Abstract] Objctive To investigate the activity of silencing information regulator 1 (SIRT1) in the liver of carbon tetrachloride (CCl4) induced liver fibrosis in mice, and to investigate the effect of curcumin on liver fibrosis and its molecular mechanism. Methods Twenty-four C57BL/6 mice were randomly divided into normal group, model group and Curcumin treatment group, with 8 mice in each group. Hepatic fibrosis model was prepared by intraperitoneal injection of CCl4. After the modeling, the curcumin treatment group was given 0.5% Carboxymethyl Cellulose Sodium Curcumin Suspension by gavage. The model group and the normal group were given 0.5% Sodium Carboxymethyl Cellulose by gavage, the mice were killed after 6 weeks. HE staining was used to detect the pathological changes of mouse liver. The levels of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and activity of SIRT1 were detected. Transforming growth factor β1 (TGF -β1), α-smooth muscle actin (α-SMA), collagen typeⅠ (Coll Ⅰ) mRNA expression, and Cleaved - caspase3, Coll Ⅰ protein expression. Results The expression levels of ALT, AST, IL-6 and TNF-α in serum of the model group were all significant higher than those of the normal group (all P < 0.01). The expression levels of ALT, AST, IL-6 and TNF-α in the treatment group were all lower than those in the model group, and the differences were highly statistically significant (all P < 0.01). The enzyme activity of SIRT1 in the liver cells of the model group was lower than that of the normal group, and the difference was highly statistically significant (P < 0.01). The enzyme activity of SIRT1 in the treatment group was significantly higher than that in the model group, and the difference was highly statistically significant (P < 0.01). Model group in the liver tissue, TGF-β1, α-SMA mRNA and Coll Ⅰ expression of amount of expression were significantly higher than those of normal group, Cleaved-caspase3 was significantly lower than that of normal group, with highly statistically significant differences (all P < 0.01). The mRNA expression of TGF-β1, α-SMA and Coll Ⅰ expression quantity in the treatment group were lower than those in the model group, Cleaved-caspase3 was significantly higher than that of model group, with highly statistically significant differences (all P < 0.01). Conclusion Curcumin has an inhibitory effect on CCl4-induced liver fibrosis, and the mechanism may be to up-regulate the activity of SIRT1, thereby reducing the inflammatory response, inhibiting the activation of hepatic stellate cells, and antagonizing liver fibrosis.
[Key words] Liver; Fibrosis; Curcumin; Inflammation; Silencing information regulator 1
酒精性肝病、慢性肝炎、自身免疫性肝病等多种慢性肝病,往往导致肝纤维化病变。针对肝纤维化,目前临床上尚无特异性治疗方法阻断甚至逆转疾病进程。因此,预防和逆转肝纤维化仍然是众多肝脏疾病研究的热点及攻克的主要目标。姜黄素是姜黄根茎中提取的一种黄色酸性酚类物质,具有抗肿瘤、抗氧化、抗炎等多种药理活性[1]。近年来,姜黄素的拮抗纤维化作用日益受到人们的关注,但其拮抗纤维化的分子机制仍不清楚。本研究拟在已有研究的基础上,通过CCl4诱导的小鼠肝纤维化模型,明确姜黄素抑制肝纤维化的作用及分子机制。
1 材料与方法
1.1 动物、主要试剂及仪器来源
SPF级雄性C57BL/6小鼠24只,6~8周龄,体重18~20 g,购自空军军医大学实验动物中心(合格证号:SYXK(军)2012-0022)。肿瘤坏死因子-α(TNF-α)(货号:h052)、白细胞介素-6(IL-6)(货号:H007)、谷丙转氨酶(ALT)(货号:C009-2-1)、谷草转氨酶(AST)(货号:C010-2-1)均从南京建成生物工程研究所购买。姜黄素(分析纯,国药集团化学试剂有限公司,批号:F20050102);SYBR Green MasterMix(美国应用生物系统公司,批号:A8605),Trizol试剂(美国Invitrogen公司,批号:AHF1813A),7900HT型荧光定量PCR仪(美国应用生物系统公司)。
1.2 动物分组及模型制备
C57BL/6小鼠采用22~26℃、SPF级饲养环境,光照控制12 h明暗交替,湿度50%~60%。24只小鼠采用随机数字表法分为正常组、模型组、姜黄素治疗组,每组8只(动物伦理批准号:XJYYLL-201805023)。模型组、姜黄素治疗组小鼠按10 mL/kg给予腹腔注射10% CCl4的油剂溶液(CCl4∶橄榄油为1∶3~4次/周),经病理切片证实肝纤维化模型建立成功。6周后姜黄素治疗组采用姜黄素(剂量为40 mg/100 g)与0.5%羧甲基纤维素钠混悬后按1 mL/100 g灌胃,每周3次,共6周;模型组自第7周起给予0.5%羧甲基纤维素钠1 mL/100 g灌胃,每周3次,共6周;正常组,前6周给予腹腔橄榄油稀释液,每周3次,第7周起给予0.5%羧甲基纤维素钠1 mL/100 g灌胃,每周3次,共6周。灌胃6周后,取小鼠肝脏组织进行病理切片及对血清相关指标进行检测。
1.3 检测指标
1.3.1 形态学检测 取各组固定的组织标本,常规石蜡包埋,切片,脱蜡后HE染色,镜下观察组织形态。
1.3.2 血清学指标检测 小鼠眼球采血后,静置15 min,4℃低温离心(离心半径15 cm,1500 r/min)15 min,分离血清,按试剂盒说明书测ALT、AST、IL-6、TNF-α水平。
1.3.3 酶活性试剂盒检测肝脏组织沉默信息调节因子1(SIRT1)活性 肝脏组织低温裂解离心(4℃低温离心,离心半径15 cm,1500 r/min,15 min),收集上清。按照试剂盒说明书检测SIRT1酶活性。
1.3.4 实时荧光定量PCR法检测肝脏组织中纤维化相关分子TGF-β1、Ⅰ型膠原、α-SMA的表达 采用Trizol一步法提取肝脏组织中总RNA,定量后逆转录合成cDNA,进行实时荧光定量PCR反应,引物序列分别为:甘油醛-3-磷酸脱氢酶(GAPDH)上游5′-TTCCTTCCTGGGCATGGAGTCC-3′,下游5′-TGGCGTAC-AGGTCTTTGCGG-3′;转化生长因子-β1(TGF-β1)上游5′-CCACCTGCAAGACCATCGAC-3′,下游5′-CT-GGCGAGCCTTAGTTTGGAC-3′;Ⅰ型胶原(CollⅠ)上游5′-TGACTGGAAGAGCGGAGAGT-3′,下游5′-AT-CCATCGGTCATGCTCTCT-3′;平滑肌肌动蛋白α-SMA上游5′-CCCAGACATCAGGGAGTAATGG-3′,下游TCTATCGGATACTTCAGCGTCA-3′。扩增条件为:95℃预变性10 min,95℃变性10 s,60℃退火延伸20 s,循环40次,按照2-△△Ct的相对定量方法计算。
1.3.5 蛋白印迹法(Westen blot)检测肝脏组织中Cleaved-caspase3、CollⅠ的表达 肝脏组织,低温组织匀浆,进行十二烷基磺酸钠-聚丙烯酰胺凝胶电泳,转膜,室温封闭2 h,分别加入Cleaved-caspase3、Call Ⅰ一抗(1∶1000),4℃孵育过夜后加入辣根过氧化物酶标记的二抗(1∶3000),37℃孵育40 min。化学发光,凝胶图像分析系统分析数据。
1.4 统计学方法
使用SPSS 19.0软件进行数据处理,计量资料采用均数±标准差(x±s)表示,三组间比较采用单因素方差分析,两组之间比较采用两样本独立样本t检验(LSD-t检验)。以P < 0.05为差异有统计学意义。
2 结果
2.1 小鼠肝脏病理变化情况
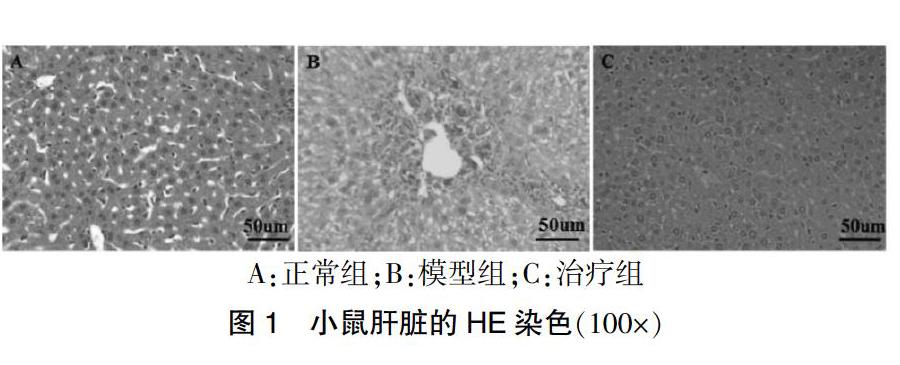
HE染色显示正常组(图1A)鼠肝细胞结构清晰,小叶及汇管区无异常。而模型组(图1B)小鼠注射CCl4后,光镜下观察到明显的小叶结构紊乱,肝细胞肿胀,气球样变可见,明显肿胀变形,伴点状、片状坏死及炎症细胞浸润,大量胶原沉积,在汇管区之间、汇管区与中央静脉之间形成纤维间隔。治疗组(图1C)经过干预后肝细胞轻度肿胀,细胞结构清楚,胶原沉积量降低程度明显。
A:正常组;B:模型组;C:治疗组
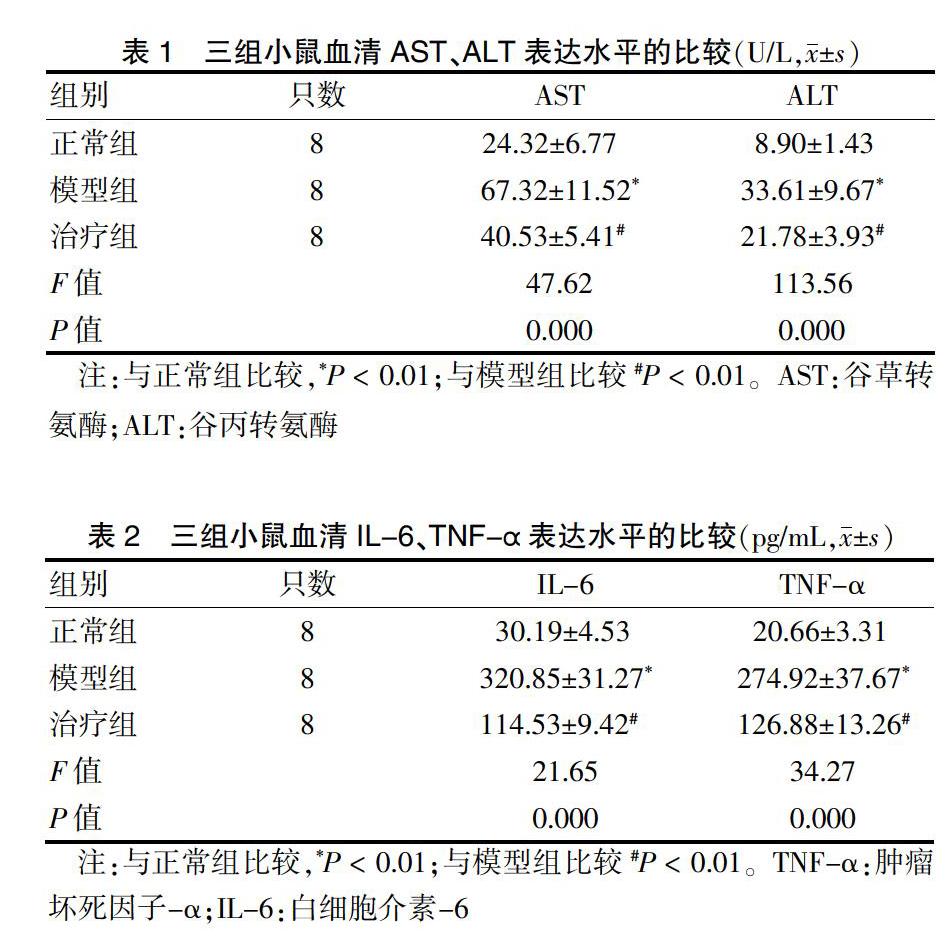
2.2 三组小鼠血清中AST、ALT表达水平的比较
与正常组比较,模型组AST、ALT表达量明显高于正常组,治疗组AST、ALT表达量均低于模型组,差异有高度统计学意义(P < 0.01)。见表1。
2.3 三组小鼠血清中IL-6、TNF-α表达水平的比较
模型组IL-6、TNF-α表达量均明显高于正常组,治疗组IL-6、TNF-α表达量均低于模型组,差异有高度统计学意义(P < 0.01)。见表2。
2.4 三组小鼠肝脏细胞中SIRT1活性的比较
正常组小鼠SIRT1相对活性为(127.62±17.34)%,模型组为(64.80±7.25)%,治疗组为(104.72±11.14)%,三组比较差异有统计学意义(F = 17.36,P = 0.012)。模型组小鼠SIRT1相对活性明显低于正常组(t = 13.57,P < 0.01);治疗组小鼠SIRT1相对活性明显高于模型组(t = 6.54,P = 0.001)。
2.5 三组小鼠肝脏组织纤维化相关分子TGF-β1、CollⅠ、α-SMA表达及Cleaved-caspase3、CollⅠ相关蛋白表达的比较模型组中TGF-β1、α-SMA、CollⅠ的mRNA及CollⅠ蛋白表达明显高于正常组;治疗组TGF-β1、α-SMA、Coll Ⅰ的mRNA及Coll Ⅰ蛋白表達明显低于模型组,模型组小鼠肝脏中Cleaved-caspase3的表达低于正常组,而治疗组Cleaved-caspase3的表达高于模型组,差异均有高度统计学意义(均P < 0.01)。见表3~4、图2。
3 讨论
损伤后的肝脏在各种刺激下,肝脏内细胞外基质(ECM)产生和降解失衡,导致过度增生与异常沉积,使肝脏结构破坏、功能异常,从而导致肝脏纤维化[2]。目前肝纤维化治疗主要是通过抗病因治疗,尚无特效的药物治疗。
临床上无论是肝脏损伤、病毒感染、药物作用、自身免疫等因素最终都会经过肝星状细胞(hepatic stellate cells,HSCs)活化发展成纤维化[3]。当肝脏受到损伤后直接或间接激活HSC,使其由静息状态转变为活化状态,导致大量ECM的形成。IL-6、TNF-α等促炎介质能通过介导炎性反应启动纤维化的形成,并可通过促进ECM的合成而促进肝纤维化的发生发展[4]。已有研究显示,TGF-β1能够刺激ECM合成,抑制其降解,是最强促纤维化细胞因子[5]。本研究结果显示,与模型组比较,正常组TNF-α、IL-6、TGF-β1表达均明显增多,而治疗组可显著降低TNF-α、IL-6、TGF-β1的表达。
此外,HSC的活化可促进ECM的大量表达,在此过程中纤维化相关因子α-SMA的生成被认为是HSC活化的主要标志物[6],而CollⅠ是ECM的主要成分,产生大量的CollⅠ是纤维化疾病的共同病理特征。Inagaki等[7]研究显示,抑制CCL4诱导的肝纤维化大鼠体内CollⅠ的表达,可减轻ECM的过度增生。在本研究中,与模型组比较,治疗组可降低ALT、AST活性,减少致纤维化因子α-SMA,CollⅠ的表达。通过模型验证,治疗组显示出良好的拮抗肝损伤和阻止纤维化的功能,且可能通过抑制HSC的活化发挥作用。
有研究报道[8-10],SIRT1在多种疾病中具有重要的调节作用。Cappetta等[11]发现,激活SIRT1可通过下调TGF-β/smad3信号通路发挥抗心肌纤维化实现。本研究检测了姜黄素对SIRT1活性表达的影响。结果显示,与正常组比较,模型组SIRT1的酶活性显著下调。而治疗组可显著改善CCl4介导的肝脏组织细胞SIRT1的酶活性水平。提示SIRT1信号分子可能参与姜黄素对肝脏纤维化的拮抗作用。
目前研究显示,在不同器官中,姜黄素拮抗纤维化可能有多种途径,与细胞的氧化应激、炎性反应等密切相关[12-14]。而SIRT1是生物体内具有重要意义的去乙酰化酶。研究显示,SIRT1可调控肝脏疾病的多个环节[15]。在肝癌的研究中发现,姜黄素可以抑制肿瘤进展[16]。此外,SIRT1可显著抑制高脂饮食导致的肝损伤[17-19]。而脂肪肝、肝纤维化、肝癌本身可能是相互转化[20-21]。本研究显示,姜黄素可能通过调控SIRT1进而抑制HSC的活化拮抗肝纤维化。姜黄素可以在肝脏病变的多个环节影响疾病的进展、改善结局。因此,深入研究姜黄素-SIRT1调节轴的作用机制,发现多种肝脏疾病的共同调控通路,可以为临床治疗提供新的思路。
综上所述,姜黄素可通过抑制HSC的活化拮抗CCl4介导的肝脏纤维化,在此过程中可能通过增加SIRT1的酶活性发挥作用。
[参考文献]
[1] Kao NJ,Hu JY,Wu CS,et al. Curcumin represses the activity of inhibitor-κB kinase in dextran sulfate sodium-induced colitis by S-nitrosylation [J]. Int Immunopharmacol,2016,38:1-7.
[2] Su TH,Kao JH,Liu CJ. Molecular mechanism and treatment of viral hepatitis-related liver fibrosis [J]. Int J Mol Sci,2014,15(6):10578-10604.
[3] Hu YB,Ye XT,Zhou QQ,et al. Sestrin 2 Attenuates Rat Hepatic Stellate Cell (HSC) Activation and Liver Fibrosis via an mTOR/AMPK-Dependent Mechanism [J]. Cell Physiol Biochem,2018:2111-2122.
[4] O′Reilly S,Ciechomska M,Cant R,et al. Interleukin-6 (IL-6) trans signaling drives a STAT3-dependent pathway that leads to hyperactive transforming growth factor-β (TGF-β) signaling promoting SMAD3 activation and fibrosis via Gremlin protein [J]. J Biol Chem,2014,289(14):9952-9960.
[5] Khanizadeh S,Ravanshad M,Hosseini S,et al. Blocking of SMAD4 expression by shRNA effectively inhibits fibrogenesis of human hepatic stellate cells [J]. Gastroenterol Hepatol Bed Bench,2015,8(4):262-269.
[6] Kisseleva T,Brenner DA. The phenotypic fate and functional role for bone marrow-derived stem cells in liver fibrosis [J]. J Hepatol,2012,56(4):965-972.
[7] Inagaki Y,Nemoto T,Kushida M,et al. Interferon alfa down-regulates collagen gene transcription and suppresses experimental hepatic fibrosis in mice [J]. Hepatology,2003, 38(4):890-899.
[8] Gu L,Tao X,Xu Y,et al. Dioscin alleviates BDL-and DMN-induced hepatic fibrosis via Sirt1/Nrf2-mediated inhibition of p38 MAPK pathway [J]. Toxicol Appl Pharmacol,2016,292:19-29.
[9] Sodhi K,Puri N,Favero G,et al. Fructose Mediated Non-Alcoholic Fatty Liver Is Attenuated by HO-1-SIRT1 Module in Murine Hepatocytes and Mice Fed a High Fructose Diet [J]. PLoS One,2015,10(6):e0128648.
[10] Li M,Hong W,Hao C,et al. SIRT1 antagonizes liver fibrosis by blocking hepatic stellate cell activation in mice [J]. FASEB J,2018,32(1):500-511.
[11] Cappetta D,Esposito G,Piegari E. SIRT1 activation attenuates diastolic dysfunction by reducing cardiac fibrosis in a model of anthracycline cardiomyopathy [J]. Int J Cardiol,2016,205:99-110.
[12] Zhao XA,Chen G,Liu Y,et al. Curcumin reduces Ly6Chi,monocyte infiltration to protect against liver fibrosis by inhibiting Kupffer cells activation to reduce chemokines secretion [J]. Biomed Pharmacother,2018,106:868-878.
[13] Sica A,Invernizzi P,Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology [J]. Hepatology,2014,59(5):2034-2042.
[14] Zheng J,Wu C,Lin Z,et al. Curcumin up-regulates phosphatase and tensin homologue deleted on chromosome 10 through microRNA-mediated control of DNA methylation--a novel mechanism suppressing liver fibrosis [J]. FEBS J,2014,281(1):88-103.
[15] Bellet MM,Masri S,Astarita G,et al. Histone Deacetylase SIRT1 Controls Proliferation,Circadian Rhythm,and Lipid Metabolism during Liver Regeneration in Mice [J]. J Biol Chem,2016,291(44):23318-23329.
[16] Mccubrey JA,Lertpiriyapong K,Steelman LS,et al. Effects of resveratrol,curcumin,berberine and other nutraceuticals on aging,cancer development,cancer stem cells and microRNAs [J]. Aging,2017,9(6):1477-1536.
[17] Lee DE,Lee SJ,Kim SJ,et al. Curcumin Ameliorates Nonalcoholic Fatty Liver Disease through Inhibition of O-GlcNAcylation [J]. Nutrients,2019,11(11):E2702.
[18] Ding RB,Bao J,Deng CX. Emerging roles of SIRT1 in fatty liver diseases [J]. Int J Biol Sci,2017,13(7):852-867.
[19] Yin H,Hu M,Liang X,et al. Deletion of SIRT1 From Hepatocytes in Mice Disrupts Lipin-1 Signaling and Aggravates Alcoholic Fatty Liver [J]. Gastroenterol,2014, 146(3):801-811.
[20] Marengo A,Rosso C,Bugianesi E. Liver Cancer:Connections with Obesity,Fatty Liver,and Cirrhosis [J]. Annu Rev Med,2016,67:103-117.
[21] Unalp-Arida A,Ruhl CE. PNPLA3 I148M and liver fat and fibrosis scores predict liver disease mortality in the United States population [J]. Hepatology,2019,31032.
(收稿日期:2019-09-30 本文編辑:封 华)
